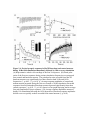
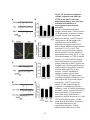
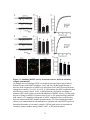
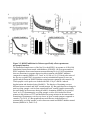
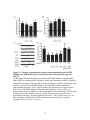
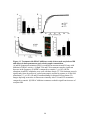
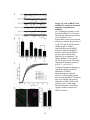
* Your assessment is very important for improving the workof artificial intelligence, which forms the content of this project
Download mecp2 and the epigenetic regulation of excitatory synaptic
Molecular neuroscience wikipedia , lookup
Metastability in the brain wikipedia , lookup
Development of the nervous system wikipedia , lookup
Behavioral epigenetics wikipedia , lookup
Neuromuscular junction wikipedia , lookup
Nervous system network models wikipedia , lookup
Central pattern generator wikipedia , lookup
Premovement neuronal activity wikipedia , lookup
Clinical neurochemistry wikipedia , lookup
Neurotransmitter wikipedia , lookup
Neuroanatomy wikipedia , lookup
Feature detection (nervous system) wikipedia , lookup
Nonsynaptic plasticity wikipedia , lookup
Pre-Bötzinger complex wikipedia , lookup
Optogenetics wikipedia , lookup
Synaptic gating wikipedia , lookup
Synaptogenesis wikipedia , lookup
Channelrhodopsin wikipedia , lookup
Neuropsychopharmacology wikipedia , lookup
Chemical synapse wikipedia , lookup
MECP2 AND THE EPIGENETIC REGULATION OF EXCITATORY SYNAPTIC TRANSMISSION APPROVED BY SUPERVISORY COMMITTEE __________________________________ Ilya Bezprozvanny, Ph.D. __________________________________ Lisa Monteggia, Ph.D. __________________________________ Ege Kavalali, Ph.D. __________________________________ Weichun Lin, Ph.D. Dedicated to my parents, Ann & Steve Cunningham and Jon Nelson, and to the rest of my family and friends for their continuing love and support. ii MECP2 AND THE EPIGENETIC REGULATION OF EXCITATORY SYNAPTIC TRANSMISSION By ERIKA DAWN NELSON DISSERTATION Presented to the Faculty of the Graduate School of Biomedical Sciences The University of Texas Southwestern Medical Center at Dallas In Partial Fulfillment of the Requirements For the Degree of DOCTOR OF PHILOSOPHY The University of Texas Southwestern Medical Center at Dallas Dallas, Texas June 2007 iii Copyright by Erika Dawn Nelson, 2007 All Rights Reserved iv ACKNOWLEDGEMENTS I would like to thank my mentor, Dr. Lisa Monteggia, for all of her support and guidance over the last 4 years. I would also like to thank Dr. Ege Kavalali for teaching me all about synaptic transmission and for his advice and expertise in the field. I would like to acknowledge all past and present members of the Monteggia lab for all of their help and encouragement. Especially Terry Gemelli and Sunbola Ashimi for their senses of humor and Megha Upadhyaya for being an awesome friend and a great cheerleader. I would also like to acknowledge all past and present members of the Kavalali lab, in particular, Tuhin Virmani, Yildrim Sara and Mert Ertunc for their extraordinary knowledge of electrophsiology and their patience in sharing some of that with me. Also, I thank Catherine Wasser for being a fortunate person to work so closely with and for being an inspirational friend. I’d like to thank the other members of my thesis committee, Ilya Bezprozvanny and Weichun Lin, for all of their helpful suggestions over the years. I must also thank our collaborators Eric Olson and Rusty Montgomery for the use of their HDAC mice and to Tina Han and Leeju Wu in the McKnight lab for their assistance in measuring DNA methylation. I would also like to thank my fellow researchers Tom Green, Imran Alibhai, Brian Potts, Victor Galanis and Herb Covington for being great people to work with and to get to know. Finally, I would like to thank the University of Texas Southwestern Medical Center for their monetary support through the University training grant. As for my family and friends, I don’t have enough thanks for my mother who is always there to calm me down after a bad day and who loves me unconditionally. To Christi Edwards, thanks for continuing to be my best friend even when I found myself too v busy to spend time with you. Thank you to John Lacy, to whom I owe my new optimistic outlook on life. Last but not least, I’d like to thank my pug, Brownie, for always making me smile and helping me to get out of bed in the mornings. You all mean so much to me… I am truly blessed to have you in my life! vi MECP2 AND THE EPIGENETIC REGULATION OF EXCITATORY SYNAPTIC TRANSMISSION Erika Dawn Nelson, Ph.D. The University of Texas Southwestern Medical Center at Dallas, 2007 Supervising Professor: Lisa Monteggia, Ph.D. Accurate regulation of gene expression is critical for normal brain function. Many human neurodevelopmental and neurodegenerative disorders are associated with mutations in genes important for controlling transcription. Mutations in one such gene, the transcriptional repressor methyl-CpG-binding protein 2 (MeCP2), lead to a form of mental retardation called Rett Syndrome (RTT). Though the MeCP2 protein is expressed ubiquitously, symptoms of RTT patients are primarily neurological, which include reduced mental capacity, autistic-like behavior and autonomic dysfunction. In addition, a mouse model with reduced MeCP2 expression specifically in postnatal, forebrain neurons recapitulates many of the phenotypes seen in human patients. These findings, among others, lead to interest in MeCP2’s function in the brain. Our research has focused on the transcriptional repression activity of MeCP2 and its role in the regulation of synapse vii function. Using mainly electrophysiological techniques, we found that the loss of MeCP2 in hippocampal neurons results in deficits in both spontaneous and evoked excitatory synaptic transmission. Using pharmacological manipulations, we were able to attribute these deficits to the loss of transcriptional repression by MeCP2. By utilizing a conditional knockout approach, we found that these effects were not due to the loss of MeCP2 during neurodevelopment and that they were primarily due to a deficiency in presynaptic vesicle release. We further extended these findings by looking at two mechanisms for controlling the repression of gene expression, DNA methylation and histone deacetylation, both of which are important for MeCP2’s function as a transcriptional repressor. Using inhibitors of DNA methyltransferases, we discovered that synaptic activity-dependent decreases in DNA methylation occur in post-mitotic neurons, and that these changes in DNA methylation can regulate spontaneous synaptic transmission. We were also able to rescue the MeCP2-dependent decrease in spontaneous activity by treating neurons with the methyl donor, S-adenosyl-L-methionine. Finally, we addressed the role of histone deacetylation in synapse function by conditionally deleting histone deacetylases (HDACs) 1 and 2 from mature hippocampal neurons. HDAC1 and 2 are present in the transcriptional repressor complex containing MeCP2. After acute knockdown of HDAC1 or HDAC2, we found deficits in excitatory synaptic transmission that mimicked the defects seen after the constitutive loss of MeCP2. In summary, we have discovered a role for the transcriptional repressor, MeCP2, and two components of its repressor complex, DNA methylation and HDACs, in the control of excitatory synaptic transmission between hippocampal neurons. viii TABLE OF CONTENTS Dedication ………………………………………………………………………………...ii Acknowledgments…………………………………………………………………………v Abstract…………………………………………………………………………………..vii Table of Contents…………………………………………………………………………ix Prior Publications………………………………………………………………………….x List of Figures…………………………………………………………………………….xi List of Abbreviations……………………………………………………………………xiv Chapter 1: Introduction...………………………………………………………………...17 MeCP2…………………………………………………………………………...20 DNA Methylation………………………………………………………………..26 Histone Deacetylation…………………………………………………………....32 Chapter 2: MeCP2-dependent Transcriptional Repression Regulates Excitatory Neurotransmission……………………………………………………….37 Introduction……………………………………………………………………....37 Results…………………………………………………………………………....40 Discussion………………………………………………………………………..46 Chapter 3: Activity-dependent Suppression of Excitatory Miniature Neurotransmission Through the Regulation of DNA Methylation………......56 Introduction……………………………………………………………………....56 Results…………………………………………………………………………....58 Discussion………………………………………………………………………..68 Chapter 4: Loss of HDAC1 and HDAC2 in Hippocampal Neurons Results in Specific Alterations in Excitatory Synaptic Transmission…………...79 Introduction……………………………………………………………………....79 Results…………………………………………………………………………....82 Discussion………………………………………………………………………..87 Chapter 5: Conclusions and Future Directions…………………………………………..96 Materials and Methods………………………………………………………………….104 References………………………………………………………………………………114 Vitae…………………………………………………………………………………….126 ix PRIOR PUBLICATIONS Nelson, E.D., Kavalali, E.T., Monteggia, L.M. (2006). MeCP2-dependent transcriptional repression regulates excitatory neurotransmission. Current Biol. 16, 710-716. Gemelli, T., Berton, O., Nelson, E.D., Perrotti, L.I., Jaenisch, R., and Monteggia, L.M. (2005). Postnatal loss of MeCP2 in the forebrain is sufficient to mediate behavioral aspects of Rett Syndrome in mice. Biol Psychiatry. 59, 468–476. Nelson, E.D., and Monteggia, L.M. (2007). Activity-dependent regulation of gene expression. Encyclopedia of Neuroscience. (In press). Elsevier. Nelson, E.D., Kavalali, E.T., Monteggia, L.M. (in revision) Activity-dependent suppression of excitatory miniature neurotransmission through the regulation of DNA methylation. Nelson, E.D., Montgomery, R., Olson, E.D., Kavalali, E.T., Monteggia, L.M. (in preparation) Loss of HDAC1 and HDAC2 in hippocampal neurons results in specific alterations in excitatory synaptic transmission. x LIST OF FIGURES Figure 1-1. Transcriptional repression by MeCP2 is relieved following synaptic activity…………………………………………………………..…………....20 Figure 1-2. Common MeCP2 mutations in Rett Syndrome……………………..……….22 Figure 1-3. DNA methylation and demethylation……………………………………….26 Figure 1-4. Histone acetylation and deacetylation……………………………………….33 Figure 2-1. Spontaneous miniature synaptic currents in cultured hippocampal neurons from MeCP2 knockout and control mice…………………………………….49 Figure 2-2. Miniature EPSC frequency is reduced in older MeCP2 KO neurons……. ...50 Figure 2-3. Total pool and readily releasable pool of synaptic vesicles from MeCP2 knockout and control neurons………………………………………………..51 Figure 2-4. Evoked synaptic responses in MeCP2 knockout and control neurons during 10 Hz field stimulation immediately followed by 1Hz recovery stimulation……………………………………………………………………52 Figure 2-5. Spontaneous miniature synaptic responses from wild type C57BL/6 and MeCP2 knockout hippocampal cultures after a 24-hour treatment with inhibitors of transcriptional repression and activation……………………….53 Figure 2-6. High and low titer lentiviral infection of dissociated hippocampal cultures………………………………………………………………………54 Figure 2-7. Spontaneous miniature excitatory synaptic currents in floxed MeCP2 neurons infected with a lentivirus expressing Cre recombinase……………..55 Figure 3-1. Inhibiting DNMT activity in neurons causes a deficit in excitatory synaptic transmission………………………………………………………...71 xi Figure 3-2. DNMT inhibition for 24 hours specifically affects spontaneous presynaptic function………………………………………………………….72 Figure 3-3. Changes in spontaneous excitatory neurotransmission after DNMT inhibition are mediated by the loss of function of the transcriptional repressor, MeCP2…………………………………………………………….73 Figure 3-4. 24-hour treatment of hippocampal cultures with DNMT inhibitors reveals demethylation of genomic DNA……………………………………..74 Figure 3-5. Schematic of the CpG island in the BDNF gene…………………………….75 Figure 3-6. Treatment of hippocampal cultures with DNMT inhibitors reveals activity-dependent demethylation of DNA and concurrent alterations in synaptic transmission……………………………………………………...76 Figure 3-7. BDNF mRNA expression after chronic manipulation of DNA methylation in hippocampal cultures………………………………………...77 Figure 3-8. Model for activity-dependent demethylation of genomic DNA in post-mitotic neurons………………………………………………………….78 Figure 4-1. Treatment with HDAC inhibitors results in increased acetylation of H4 and defects in both spontaneous and evoked synaptic transmission…………90 Figure 4-2. Quantification of HDAC1 and 2 mRNA and protein expression levels one week after infection with Cre-recombinase lentivirus……………………….91 Figure 4-3. Loss of HDAC2, but not HDAC1, results in decreased frequency of spontaneous mEPSCs……………………………………………………..92 Figure 4-4. Miniature inhibitory synaptic currents from conditional HDAC1 and 2 xii KO neurons ………………………………………………………………….93 Figure 4-5. Increased evoked EPSC amplitudes and decreased paired pulse ratios in HDAC1, HDAC2, and MeCP2 KO neurons……………………………...94 Figure 4-6. Evoked inhibitory postsynaptic currents from HDAC1, HDAC2, and MeCP2 KO cultures………………………………………………………….95 Figure 5-1. List of possible presynaptic gene targets of MeCP2………………………...99 Figure 5-2. Quantitative Real-Time PCR results from MeCP2 KO cultures…………..100 xiii LIST OF ABBREVIATIONS 5azaC – 5-azacytidine Act D – Actinomycin D AMP – Adenosine monophosphate AP5 - 2-amino-5-phosphonovaleric acid BDNF – Brain-derived neurotrophic factor CaMK – Calcium/calmodulin-dependent protein kinase CBP – CREB binding protein CNQX - 6-cyano-7-nitroquinoxaline-2,3-dione CNS – central nervous system Cplx -Complexin CREB - Cyclic AMP response element-binding protein DG – dentate gyrus DIV – days in vitro DMSO – dimethyl sulfoxide DNA – deoxyribonucleic acid DNMT – DNA methyltransferase EPSC – excitatory postsynaptic current FMR – Fragile X mental retardation Gadd45α – Growth arrest and DNA damage 45α GFP – Green fluorescent protein HAT – Histone acetyltransferase HDAC – Histone deacetylase xiv ICF - Immunodeficiency, centromeric region instability, facial anomalies IPSC – inhibitory postsynaptic current KO - knockout LTD – long-term depression LTP – long-term potentiation MAP2 – Microtubule associated protein 2 MBD – methyl binding domain MeCP2 – Methyl-CpG binding protein 2 mEPSC – miniature EPSC mIPSC – miniature IPSC mRNA – messenger RNA MS-AFLP – Methylation-sensitive amplified fragment length polymorphism NBQX - 2,3-dihydroxy-6-nitro-7-sulfamoyl-benzo[f]quinoxaline-2,3-dione Nlgn - Neuroligin NMDA - N-methyl-D-aspartic acid Nrxn - Neurexin PCR – Polymerase chain reaction PP1 – Protein phosphatase 1 PSD-95 - postsynaptic density protein of 95 kDa PTX - Picrotoxin RE1 - repressor element 1 REST – RE1 silencing transcription factor RNA – ribonucleic acid xv RTS – Rubinstein-Taybi syndrome RTT – Rett syndrome SAM – S-adenosyl-L-methionine Synpor -Synaptoporin Syp1 – Synaptophysin 1 Syt7 – Synaptotagmin 7 TRD – transcriptional repression domain TSA – Trichostatin A TTX - Tetrodotoxin VPA – Valproic acid WT – wildtype Zeb – Zebularine xvi CHAPTER 1 INTRODUCTION Epigenetics is the study of stable, and potentially heritable, changes in gene expression without alteration of the actual DNA sequence. It is also believed to be a way that environment might control the phenotype of a cell. One epigenetic example is the ability of each cell in an organism, all genetically identical, to differentiate into a particular cell type to serve a specific purpose. Our kidney, skin, and blood cells, and even our neurons, all share the same genetic makeup, but all perform significantly different functions within our bodies due to the differential expression of cell-type specific genes. The expression of genes is partially controlled by how tightly DNA is bound around a core group of proteins called histones, and epigenetic phenomena are part of what regulates that association. The epigenetic control of gene expression is mediated by two main mechanisms: DNA methylation and histone modifications. DNA methylation is the covalent addition of methyl groups to the 5-position of cytosine residues within CpG dinucleotides and an important molecular tag for the repression of gene transcription. Certain proteins, such as the methyl-CpG-binding protein 2 (MeCP2), contain methyl-binding domains that recognize methylated DNA and repress transcription. There are a number of different histone modifications that are important for either the activation or repression of gene expression. Histone acetylation occurs on the amino side chain of specific lysine residues found on histone tails, which loosens the association between DNA and histones and allows for the activation of transcription. 17 Conversely, histone deacetylation refers to the removal of these acetyl groups, which causes the repression of gene expression by tightening the DNA-histone complex. My work has concentrated on both DNA methylation and histone deacetylation as means of repressing transcription due to their association with the repressor protein MeCP2. The study of epigenetics has focused mainly on its roles in cancer. Promoter hypermethylation of tumor suppressor genes and altered levels of individual histone modifications are found in many types of tumor cells. Outside of cancer, epigenetics has received less attention. Recent accumulating evidence is pointing towards a role for epigenetics in neurons. Some believe that these stable changes in gene expression might be the underlying basis for learning and memory and long lasting forms of synaptic plasticity in the brain. For example, extensive research has established multiple interdependent activities of the transcriptional activator cyclic-AMP-response element binding protein (CREB) and synaptic transmission. Expression levels of CREB have been shown to regulate certain types of late long-term synaptic potentiation (LTP) and longterm depression (LTD) (Ahn et al., 1999). Recent data suggests that mutations in its coactivator CREB binding protein (CBP), a histone acetyltransferase (HAT) enzyme that can influence CREB function, exert profound effects in human brain by causing a form of mental retardation, Rubinstein-Taybi syndrome (Petrij et al., 1995). While there is ample research connecting transcriptional activation and synaptic transmission, less attention has been focused on the role of transcriptional repression. The association of mutations in the methyl-binding protein MeCP2 gene and the human form of mental retardation called Rett Syndrome strongly suggests that transcriptional repression plays an important role in the CNS. My thesis work has been concerned with 18 studying the role of transcriptional repression in the control of basal synaptic function, with my focus centering on the repressor protein MeCP2. 19 MeCP2 The methyl-CpG-binding protein 2 (MeCP2) gene encodes a DNA binding protein that belongs to a family of proteins that bind methylated cytosines in the mammalian genome (Nan et al., 1996). The other members include methyl-binding domain (MBD) proteins 1-4. MeCP2 acts as a transcriptional repressor by binding to target gene promoters via its MBD and silences transcription via its transcriptional repression domain (TRD) by recruiting additional silencing proteins such as Sin3a and histone deacetylases (HDACs) 1 and 2 (Nan et al., 1998). MeCP2 protein is expressed at high levels in the brain, specifically in neurons, not in glia, and correlates with neuronal maturation (Akbarian et al., 2001; Shahbazian et al., 2002b). Recent evidence suggests that MeCP2 is phosphorylated in response to synaptic activity (Chen et al., 2003; Zhou et al., 2006). Under unstimulated conditions, MeCP2 associates with methylated CpG dinucleotides in the promoters of target genes and recruits Sin3a and HDAC1 or 2. In response to neuronal activity, the kinase CaMKII phosphorylates MeCP2 at serine 421, causing its release from methylated promoters and relieving its repression of target genes (Figure 1-1). Figure 1-1. Transcriptional repression by MeCP2 is relieved following synaptic activity. In the absence of activity, MeCP2 is bound to methylated CpG sequences in the promoters of certain genes. The expression of these genes is repressed due the inability of transcriptional activators to bind to promoter regions as well as to the deacetylation of histones by HDAC1 and 2 which are recruited to chromatin by MeCP2 and the corepressor Sin3a. Upon stimulation, CaMKII phosphorylates MeCP2 causing release of the repressor complex from CpG islands, allowing subsequent binding of transcriptional activators that can enhance gene expression. 20 A distinctive characteristic of MeCP2 is that, in humans, mutations in the MeCP2 gene result in a neurodevelopmental disorder called Rett Syndrome (RTT). RTT is a childhood neurological disorder that accounts for one of the leading causes of mental retardation and autistic behavior in females (Hagberg et al., 1983). In general, individuals affected with RTT experience normal development up to the age of 6-18 months at which time they fail to acquire new skills and enter a period of motor skill regression. With time, the RTT defects become more pronounced and include a wide range of neurological defects (mental retardation, autism-like behavior, seizures, disturbances of sleep, problems with gait, decelerated head growth, and stereotypical hand movements). In addition, most children afflicted with RTT show a loss of social and cognitive abilities. Recent work has demonstrated that RTT is an X-linked dominant disorder that results from mutations in the MeCP2 gene found on the X chromosome (Amir et al., 2000; Amir et al., 1999; Bienvenu et al., 2000; Huppke et al., 2000; Wan et al., 1999). Classical RTT is found primarily in females because they are heterozygous for MeCP2 mutations and the presence of a normal MeCP2 in approximately half of their cells allows them to survive past birth. The amount of X chromosome inactivation in these females accounts for the severity of an individual’s phenotypes (Shahbazian et al., 2002c; Wan et al., 1999). There are a few cases of males that present classical symptoms of RTT, but they are usually Klinefelter’s (XXY) or have greatly skewed somatic mosaicism (Armstrong et al., 2001; Clayton-Smith et al., 2000; Leonard et al., 2001; Schwartzman et al., 2001). 21 Figure 1-2. Common MeCP2 mutations in Rett Syndrome. Missense (below) and nonsense (above) mutations are found both in and around the functional domains of MeCP2. In RTT patients, several mutations in the MeCP2 gene have been identified (Figure 1-2). These mutations can be missense mutations that result in single amino acid changes, frameshift mutations that cause a shift in the reading frame of the gene, or nonsense mutations that result in a truncated protein. Most of the mutations affect either the MBD of MeCP2 that mediates its binding to DNA or the TRD that recruits co-repressor complexes after DNA binding. The disease causing mutations found in RTT patients are predicted to result in the loss of function of MeCP2 that then results in genes turned on in an inappropriate manner. However, only the expression of a few genes have been shown to be regulated by MeCP2, including brain-derived neurotrophic factor (BDNF) and the imprinting genes Dlx5 and Dlx6, although the direct relationship between these changes in gene expression and functional consequences in synaptic transmission have not been shown (Chen et al., 2003; Horike et al., 2005). Microarray analysis of RTT patients and mouse models of RTT have revealed little about possible genes whose expression levels are controlled by MeCP2 (Colantuoni et al., 2001; Tudor et al., 2002). One study tried to narrow down the possible list of MeCP2 target genes by suggesting that MeCP2 22 specifically binds to A/T rich sequences near methylated CpG dinucleotides, while another methylated DNA binding protein, MBD2, does not (Klose et al., 2005). Using a candidate gene approach, MeCP2 was found to control BDNF expression in mammalian cultured cortical neurons, in an activity-dependent manner (Chen et al., 2003; Martinowich et al., 2003). However, it is thought that there are a number of unidentified gene targets of MeCP2 whose expression levels are subtly increased in the absence of functional MeCP2 (Bienvenu et al., 2000). Therefore, how the loss of function of MeCP2 leads to the neurological phenotypes seen in RTT patients is of great interest. Attempts to model the disease by generating constitutive MeCP2 knockout (KO) mice results in the recapitulation of many of the neurological symptoms of RTT, although these mice die early in postnatal development (Chen et al., 2001; Guy et al., 2001). MeCP2-null mice develop normally until about 6 weeks of age and then begin to show a variety of defective neuronal phenotypes, such as ataxic gait, tremors, and hindlimb clasping (Guy et al., 2001). Studies examining the brains of RTT patients, as well as mouse models of the disease, have not found any major neuropathological abnormalities or neuronal loss but rather only subtle changes in neuronal morphology (Kaufmann and Moser, 2000; Shahbazian et al., 2002a). To more fully characterize the role of MeCP2 in the brain on behavioral phenotypes, our lab used conditional MeCP2 KO mice in which MeCP2 was selectively deleted in broad forebrain regions of the brain during early postnatal development (Gemelli et al., 2005). We found that these conditional MeCP2 KO mice have many of the behavioral abnormalities that are reminiscent of the symptoms seen in RTT patients, including, impaired motor coordination, increased anxiety, and abnormal social interaction with other mice. These data suggest that 23 expression of MeCP2 in postnatal neurons is crucial for normal development and that the loss of MeCP2 in postnatal neurons of broad forebrain areas is sufficient to recapitulate many features of RTT. Based on the CNS-specific phenotypes of RTT, and the necessity of neuronal expression of MeCP2, it is plausible that the mechanism underlying the disease progression of RTT involves defects in neurotransmission in the CNS. Indeed, there is evidence that individuals affected with RTT, as well as mouse models of the disorder, have alterations in dendritic and synaptic spine structure indicative of a malfunction of synaptic development and plasticity (Kaufmann and Moser, 2000). Recent work has demonstrated that alterations in dendritic structure can result from changes in synaptic activity (Zito and Svoboda, 2002). Therefore, these morphological changes in dendritic structure of RTT patients, and of mouse models of the disease, may suggest an underlying synaptic deficit. Recent data suggests that MeCP2 may serve as a link between synaptic activity and transcriptional regulation. Over the past couple of years, researchers have discovered a number of defects in synaptic function in different mouse models of Rett Syndrome. It appears that the loss of MeCP2 function can lead to changes in spontaneous synaptic transmission as well as in short- and long-term synaptic plasticity. Brain slices from mice overexpressing MeCP2 displayed an increase in paired pulse facilitation and long-term potentiation (LTP) (Collins et al., 2004), while MeCP2 null mice exhibited the converse (Asaka et al., 2006), suggesting that MeCP2 expression may contribute to functional alterations in synaptic transmission resulting in the disease phenotype. Two studies, one using an MeCP2-null mouse (Asaka et al., 2006) and another using a mouse expressing a 24 truncated form of MeCP2 (Moretti et al., 2006), found deficits in both LTP and long-term depression (LTD) in hippocampal slices from these mice compared to littermate controls. Interestingly, while the first study saw these changes only in older, symptomatic mice (Asaka et al., 2006), the second found them also in younger, asymptomatic mice, suggesting the possibility that these synaptic deficits may be occurring before the manifestation of RTT-like behaviors (Moretti et al., 2006). Patients with RTT are normal for the first 6-18 months of age, therefore much research is focusing on the role of MeCP2 in neurodevelopment in hopes that an early intervention might lessen some of the behavioral deficits. Since microarray studies have been inconclusive in identifying large-scale gene changes in RTT patients, and in mouse models of the disease, MeCP2’s role as a transcriptional repressor has come into question. There is new evidence that MeCP2 may also play a role in RNA splicing (Young et al., 2005). Another recent study found MeCP2 localized to the postsynaptic compartment, suggesting some additional role for MeCP2 outside of the nucleus (Aber et al., 2003). Despite this small amount of evidence to the contrary, research continues to focus mainly on MeCP2’s role as a transcriptional repressor, though no study has directly linked this function to the neurological deficits seen in RTT patients and mouse models. 25 DNA Methylation DNA methylation refers to the covalent addition of methyl groups to the C5position of cytosine residues within CpG dinucleotides. It is an important cellular instrument by which cells control the repression of gene expression. Methylated DNA can be used by the cell to exclude transcription factors important for activating gene expression (Hark et al., 2000) or to recruit transcriptional repressor proteins like MeCP2 (Nan et al., 1996). In addition, methylation of DNA plays roles in processes such as X chromosome inactivation, chromosome stability, and genomic imprinting. Figure 1-3. DNA methylation and demethylation. DNMTs 3a and 3b add de novo methyl groups to DNA. DNMT1 is responsible for copying these methyl groups onto newly synthesized DNA strands after replication. Without DNMT1, DNA becomes passively demethylated during DNA replication. Active demethylation is also believed to occur, though the enzyme responsible is still unknown. 26 DNA methyltransferases (DNMTs) are the enzymes responsible for adding methyl groups to CpG islands found in mammalian genomic DNA. There are three main DNMTs expressed in mammals: DNMT1, 3a and 3b. DNMT3a and DNMT3b are de novo methyltransferases that establish methylation patterns at specific sites within the genome (Okano et al., 1999). DNMT1 is responsible for the maintenance of these methylation patterns during DNA replication (Hermann et al., 2004) (Figure 1-3). The initiation signals for DNA methylation, and how DNMTs are targeted to specific gene promoters remains unclear. A few studies have found relationships between DNA methylation and histone modifications. DNA methylation has been linked with histone H3 lysine 9 methylation in silenced heterochromatin, though these modifications have been found to precede one another in reverse order in different species (Johnson et al., 2002; Lehnertz et al., 2003). Also, quite a few associations between DNMTs and HDACs have been discovered within transcriptional corepressor complexes (Geiman et al., 2004; Rountree et al., 2000). Nevertheless, there is still much to be discovered about what governs the control of DNA methylation to specific CpG sites. Much research has focused on DNA methylation changes during mammalian development. Both male and female primordial germ cells undergo methylation reprogramming via a putative active process of genome wide demethylation followed by de novo remethylation carried out by DNMT3a (Hajkova et al., 2002; Kaneda et al., 2004). In pre-implantation embryos, the paternal genome is reprogrammed by active demethylation while the maternal is done by a passive demethylation process that is dependent on DNA replication (Figure 1-3) (Haaf, 2006; Mayer et al., 2000). Once the 27 embryo reaches the blastocyst stage, genome-wide de novo methylation is performed by both DNMT3a and 3b (Okano et al., 1999). Following cellular differentiation, DNA methylation changes are less numerous and thought to control the tissue-specific gene expression required to maintain the identity of cells from one generation to the next. One example of this is seen with maspin, a human cancer gene that is unmethylated in tumor cells that express it and methylated in normal cells that don’t (Futscher et al., 2002). Evidence for DNA demethylation in differentiated cells suggests only a passive mechanism by which methylation patterns are lost during the DNA replication that occurs with each cellular division. The idea that an active mechanism of demethylation can occur in a replication-independent manner, or in non-dividing cells such as neurons, has garnered plenty of controversial attention. However, evidence for this type of demethylation does exist. For example, demethylation of transfected DNA into non-replicating cells has been shown to occur (Paroush et al., 1990). Many researchers have searched for a possible enzyme that can remove established methylation patterns and thereby turn on specific gene expression. One group discovered that the methyl-binding protein MBD2b can demethylate DNA by removing the methyl moiety on cytosine residues and replacing it with a hydrogen atom (Bhattacharya et al., 1999; Ramchandani et al., 1999), though this finding has been strongly contested by others (Ng et al., 1999; Wolffe et al., 1999). Instead, most demethylase supporters have focused on DNA damage and repair related-mechanisms. 5methylcytosine-DNA glycosylase can remove 5-methylcytosines in chick embryos, allowing them to be replaced by unmethylated cytosines via DNA repair enzymes (Jost et 28 al., 1995), though there are many caveats to this reaction. The same group also discovered that the protein MBD4 possesses this same demethylase activity (Zhu et al., 2000), while another group has suggested that the function of MBD4 is to remove G-T mismatched bases following 5-methylcytosine deamination (Hendrich et al., 1999). Most recently, the growth arrest and DNA damage protein Gadd45α was discovered to promote DNA repair and thereby erase methylation marks in non-dividing cells (Barreto et al., 2007). Interest in DNA methylation alterations in post-mitotic neurons has risen due its association with a number of neurodevelopmental disorders. As previously mentioned, mutations in the DNA methyl-binding protein MeCP2 result in Rett Syndrome, a form of mental retardation in humans. Two other neurodevelopmental disorders that manifest symptoms of mental retardation, Fragile X and ICF (Immunodeficiency, Centromeric region instability, Facial anomalies) syndromes, arise from malfunctions in the establishment of normal DNA methylation patterns. Fragile X mental retardation (FMR) is brought about by the expansion of CGG or CCG triplet repeats in the promoters of the FMR1 or FMR2 genes causing an increase in DNA methylation and therefore a decrease in the expression of these genes (Turner et al., 1996). ICF syndrome is caused by mutations in the DNMT3b gene resulting in decreased DNA methylation throughout the genome (Hansen et al., 1999). Defects in DNA methylation have also been suggested to play a role in schizophrenia, a serious cognitive disorder in humans. Evidence indicates that deficiencies in the protein reelin, an extracellular matrix protein shown to play a role in synaptic plasticity, may be responsible for the etiology of schizophrenia (Costa et al., 29 2002). These deficiencies in reelin expression are believed to result from DNA hypermethylation of the gene’s promoter region. DNMTs are known to be highly expressed in adult brain neurons along with high patterns of DNA methylation. DNMT mRNA expression and enzymatic activity levels are both high in mature neurons (Brooks et al., 1996; Goto et al., 1994), although specific DNMT isoforms are expressed at different levels in distinct areas of the brain. Both DNMT1 and DNMT3a are expressed in post-mitotic neurons found in the olfactory bulb (MacDonald et al., 2005). DNMT3a expression was also found in neurons of the cortex, hippocampus, striatum, and cerebellum (Feng et al., 2005). And although one study found DNMT1 expression in the adult cerebellum to be localized to the cytoplasmic compartment (Inano et al., 2000), research continues to focus on the functions DNMTs might have for controlling gene expression in the nuclei of mature, post-mitotic neurons. Currently, there is little understood about the possible functions of DNMTs and DNA methylation in mature, post-mitotic neurons. Some evidence points to them playing a role in the control of long-term synaptic plasticity and memory formation. Treatment of hippocampal slices with inhibitors of DNMT activity blocks both long-term potentiation and memory formation following contextual fear conditioning, a commonly used test for hippocampal-dependent associative learning and memory (Levenson et al., 2006; Miller and Sweatt, 2007). Interestingly, DNMT expression and promoter methylation levels of a specific learning and memory-suppressing gene, protein phosphatase 1 (PP1), are upregulated following fear conditioning, while methylation of the reelin promoter is decreased, suggesting that mechanisms controlling both DNA methylation and DNA demethylation are altered in correlation with hippocampal learning and synaptic plasticity 30 (Miller and Sweatt, 2007). These studies suggest that DNA methylation plays an important role in the control of synaptic transmission and indicate that changes can occur in the DNA methylation patterns found in post-mitotic neurons in response to synaptic activity. However, the exact mechanisms leading to these changes in DNA methylation and their effects on synaptic function remain unclear, and whether or not they are actually occurring in non-dividing neurons and not in the dividing glial cells surrounding the neurons remains unresolved. 31 Histone Deacetylation In the nucleus, DNA is wrapped around a core group of proteins called histones. These proteins contain histone tails whose amino-termini of specific residues can receive the covalent addition of a number of different post-translational modifications, such as phosphorylation, methylation, ubiquitination, SUMOylation, and acetylation. The addition of specific groups controls how tightly DNA is associated with histones, which in turn defines how readily accessible gene sequences are to the transcriptional machinery and other DNA-binding proteins. For example, acetylation of multiple lysine residues on the tail of histone H4, acetylation of two lysine residues (9 and 14) on the tail of histone H3, and dimethylation of lysine4 on H3 are all modifications commonly associated with active gene transcription, while dimethylation of lysine 9 on H3 is associated with transcriptionally silent chromatin (Turner, 2002). The combination of specific modifications and their resulting effects on gene expression are collectively referred to as the “histone code”. Many of the functions of these modifications are not completely understood, however the detailed role of histone acetylation and deacetylation is more apparent. Histone acetylation occurs on the amino side chain of specific lysine residues found within histone tails, thereby neutralizing their positive charge and interrupting their association with negatively-charged DNA, allowing for the activation of transcription (Figure 1-4). Histone acetyltransferases (HATs) are the enzymes responsible for utilizing the cofactor acetyl-CoA in this acetylation reaction (Varga-Weisz and Becker, 1998). Histone deacetylases (HDACs) repress transcription through removal of the acetyl group, 32 which then strengthens the histone-DNA interaction and blocks access of the transcriptional machinery to the DNA template (Fischle et al., 2003). Figure 1-4. Histone acetylation and deacetylation. Gene transcription is activated by histone acetylation via histone acetyltransferases (HATs). Removal of the acetyl groups by histone deacetylases (HDACs) results in repressed transcription. There are 3 distinct classes of HDACs in mammalian cells, referred to as Class I, II and III. Class I HDACs, which consist of HDACs 1, 2, and 3, are widely expressed. Their localization within cells is strictly nuclear, their simple structures contain only a catalytic domain, and they are frequently found to be associated with large corepressor complexes. Class II HDACs (4, 5, 7, and 9) are a bit more sophisticated in that, in addition to their catalytic domain, they contain an N-terminal extension that allows interaction with coactivator and corepressor proteins and also includes serine/threonine residues that can be phosphorylated by kinases in response to calcium. Most of the Class II HDACs can be shuttled into and out of the nucleus in response to phosphorylation or dephosphorylation (Fischle et al., 2001). The third class of HDACs is believed to be primarily responsible for the deacetylation of tubulin in microtubules, which gives them a very specific role in the modulation of cytoskeletal dynamics (Kovacs et al., 2004). 33 The association of Class I HDACs with corepressor protein complexes is widely corroborated. Both HDACs 1 and 2 are associated with the corepressor, Sin3a, a protein included in complexes with a number of different transcriptional repressors, such as Mad, REST, and MeCP2. HDACs 1 and 2’s involvement with Mad is important for the control of cellular proliferation by blocking cell cycle progression (Laherty et al., 1997). The REST, Sin3a, and HDAC1/2 complex is important in nonneuronal cells for repressing genes responsible for inducing a neuronal phenotype (Huang et al., 1999). The association of HDACs 1 and 2 with MeCP2 is clearly defined (Nan et al., 1998), though the functional consequences of transcriptional repression by these proteins are not entirely clear. Evidence is pointing to this complex playing a role in everything from cancer to neurodevelopment (Harikrishnan et al., 2005) (Amir et al., 1999). Therefore, it is becoming increasingly apparent that HDACs can serve as transcriptional repressors that modulate a variety of cellular functions. While most HDACs are expressed ubiquitously, interest in the role of histone acetylation and deacetylation in the brain has arisen due to a number of serendipitous findings. First, it was discovered that a widely-used drug to treat epilepsy and bipolar disorder, valproic acid (VPA), is also a potent inhibitor of HDACs (Phiel et al., 2001), suggesting that inhibitors of these proteins, and likely the proteins themselves, play an important role in the function of the CNS, perhaps in cellular excitability. Another link between histone acetylation and the brain came from the discovery that Rubinstein-Taybi syndrome, a form of mental retardation, is caused by mutations in the CREB-binding protein (CBP), a protein known to have intrinsic HAT activity (Kalkhoven et al., 2003). Finally, as was previously mentioned, mutations in the MeCP2 gene cause another form 34 of mental retardation called Rett syndrome, and it has been shown that part of MeCP2’s function as a transcriptional repressor is dependent on the recruitment of HDACs 1 and 2 (Nan et al., 1998). In neurons, histone acetylation has been shown to play roles in both neural development and synaptic plasticity. The association of HDACs with the transcriptional repressor REST has obvious implications towards having an effect on neuronal development. Overexpression of REST in neuronal precursor cells represses the expression of a voltage-gated Na channel, Nav1.2, and reduces neurite outgrowth, demonstrating its important function in repressing the induction of a neuronal phenotype in nonneuronal cells during development (Ballas et al., 2001). It has also been shown that HDAC inhibitors can induce neuronal differentiation, suppress glial differentiation and decrease proliferation in neural progenitor cells (Hsieh et al., 2004). A role for HATs and HDACs in learning and memory and long-term synaptic plasticity is quickly being established. To begin with, CBP heterozygous mice, models of the human mental retardation disorder Rubinstein-Taybi syndrome, display deficits in long-term memory after fear conditioning as well as in maintenance of the late phase of LTP, known to be dependent on gene transcription (Alarcon et al., 2004). These defects correlated with decreased acetylation of histone H2B, and the LTP deficit could be rescued by treatment with HDAC inhibitors. Treatment of Aplysia sensorimotor neurons with the HDAC inhibitor Trichostatin A (TSA) resulted in increased sensitivity for the induction of long-term facilitation (Guan et al., 2002), while treatment of rodent hippocampal slices caused an enhancement of LTP (Levenson et al., 2004). In addition, HDAC inhibitors have been shown to augment the locomotor and reward responses to 35 cocaine while overexpression of HDAC4 in the striatum had the opposite effect (Kumar et al., 2005), and HDAC5 overexpression in the hippocampus is able to block the antidepressant effects of imipramine following defeat stress (Tsankova et al., 2006). Though all of these studies point to a definite role for histone deacetylation in neuronal function, there is currently no information about the roles of specific HDACs in synaptic transmission. Therefore, there is still much to be discovered about the functional consequences of epigenetic alterations, in particular histone acetylation and DNA methylation, in neurons. 36 CHAPTER 2 MECP2-DEPENDENT TRANSCRIPTIONAL REPRESSION REGULATES EXCITATORY NEUROTRANSMISSION Introduction Mutations in the transcriptional repressor, methyl-CpG-binding protein 2 (MeCP2), result in a neurodevelopmental disorder called Rett Syndrome (RTT) (Amir et al., 2000; Amir et al., 1999; Bienvenu et al., 2000; Huppke et al., 2000; Wan et al., 1999). RTT is an Xlinked dominant disorder in which defects are predominantly expressed in the central nervous system, including stereotypical hand movements, mental retardation, autismrelated behavior, seizures, disturbances of sleep, and problems with gait (Hagberg et al., 1983). The MeCP2 gene encodes a DNA binding protein that binds to methylated cytosines in the mammalian genome. Normally, MeCP2 acts as a transcriptional repressor by binding to target gene promoters and silencing their transcription through the recruitment of additional silencing proteins such as Sin3a and histone deacetylases (HDACs) 1 and 2. The disease causing mutations found in RTT patients are predicted to result in the loss of function of MeCP2 that then results in genes turned on in an inappropriate manner (Ballestar et al., 2000; Yusufzai and Wolffe, 2000). It is intriguing how mutations in MeCP2, a gene present in many tissues, leads to the wide array of neurological phenotypes observed in RTT patients. Studies examining the brains of RTT patients, as well as recent mouse models of the disease, have not found any major neuropathological abnormalities or neuronal loss but rather only subtle changes in neuronal morphology (Kaufmann and Moser, 2000; Shahbazian and Zoghbi, 2002). 37 Attempts to model the disease by generating MeCP2 knockout (KO) mice results in the recapitulation of many of the neurological symptoms of RTT (Chen et al., 2001; Guy et al., 2001). There is evidence that individuals affected with RTT have alterations in dendritic and synaptic spine structure indicative of a malfunction of synaptic development and plasticity (Kaufmann and Moser, 2000). Recent work has demonstrated that alterations in dendritic structure can result from changes in synaptic activity (Zito and Svoboda, 2002). Therefore, it is possible that the MeCP2 mutations contribute to functional alterations in synaptic transmission resulting in the disease phenotype. Based on the neurological phenotypes observed in Rett patients, we examined the potential role of MeCP2 in synaptic function. We compared elementary properties of synaptic transmission between cultured hippocampal neurons from MeCP2 knockout and wild type littermate control mice and found a decrease in the frequency of spontaneous excitatory synaptic transmission (mEPSCs) in neurons lacking MeCP2. We also detected a significant increase in the rate of short-term synaptic depression. To explore whether these functional effects can be attributed to MeCP2’s role as a transcriptional silencer, we treated cultures with a drug that impairs histone deacetylation and examined spontaneous synaptic transmission. Treatment with this compound induced a similar decrease in mEPSC frequency in wild type control cultures but this decrease was occluded in MeCP2-deficient neurons. Interestingly, neither the loss of MeCP2, nor the drug treatment resulted in changes in mIPSC properties. Finally, using a lentivirus expressing Cre recombinase, we show that loss of MeCP2 function after neurodevelopment and synaptogenesis was sufficient to mimic the decrease in mEPSC frequency seen in 38 constitutive MeCP2 KO neurons. Taken together, these results suggest a role for MeCP2 in control of excitatory presynaptic function through regulation of gene expression. 39 Results To elucidate the role of MeCP2 in the regulation of synaptic transmission, we studied functional alterations of synapses in 11-14 days in vitro hippocampal cultures made from newly born MeCP2 KO mice. Recent electrophysiological measurements of synaptic plasticity in MeCP2-deficient mice were performed on both hippocampal and cortical slices (Asaka et al., 2006; Dani et al., 2005). Dissociated primary cultures allow examination of synaptic function independent of potential general alterations in brain homeostasis, thus enabling a distinction between cell autonomous defects and global systemic dysfunction. We quantified the frequency and amplitude of spontaneous miniature excitatory postsynaptic currents (mEPSCs) in MeCP2 KO and wild type (WT) littermate control cultures using whole-cell recordings performed in the presence of tetrodotoxin (TTX) to block action potential firing and picrotoxin to block inhibitory activity. We found a significant decrease in the frequency of mEPSCs in the KO neurons compared to WT controls (Figure 2-1A-C). The decrease was also observed in older cultures (>20 DIV) (Figure 2-2), suggesting that the loss of MeCP2 produces long-term alterations in excitatory synaptic transmission. This alteration in mEPSC frequency may implicate a presynaptic deficit in the MeCP2 KO neurons. In contrast, the frequency of spontaneous miniature inhibitory postsynaptic currents (mIPSCs) was unaffected, suggesting a specificity of MeCP2 function in excitatory neurotransmission (Figure 2-1EG). The amplitudes of individual synaptic events in both mEPSCs and mIPSCs were also unaffected by the loss of MeCP2, indicating no potential change in the number of postsynaptic receptors at either excitatory or inhibitory synapses (Figure 2-1D, H). 40 To better understand how the loss of MeCP2 may contribute to the alteration in mEPSC frequency, we examined the number of presynaptic terminals formed on pyramidal neuron dendrites in culture. Neurons were immunostained for microtubuleassociated protein (MAP2) and Synapsin, a synaptic vesicle protein, to identify presynaptic terminals on the dendrites and soma. This analysis revealed that the number of presynaptic terminals were unchanged in MeCP2 KO neurons compared with WT neurons, suggesting that the decrease in spontaneous synaptic events is not the result of a decreased number of presynaptic terminals (Figure 2-1I, J). We next examined whether there was a decrease in the size of the total recycling pool and the number of readily releasable vesicles. To probe the total pool size, we stimulated cultures with 47 mM K+ solution for 90 seconds in the presence of the styryl dye FM1-43 (Betz et al., 1996). This strong stimulation normally labels all recycling vesicles within a presynaptic terminal (Harata et al., 2001). After dye wash out (~10 minutes), we stimulated the cultures with 90 mM K+ solution applied four times, the first for 90s followed by three applications of 60s each (each separated by 60s intervals), to release all the dye trapped within presynaptic terminals. The kinetics of dye loss from synaptic terminals (Figure 2-3A), and the total amount of dye trapped in individual synapses was indistinguishable between KO and control neurons (Figure 2-3A insert), indicating that the sizes of the total vesicle pools were unchanged. To quantify differences in the number of readily releasable vesicles, we stimulated cultures with a brief hypertonic sucrose application, which selectively releases vesicles from the readily releasable pool (Rosenmund and Stevens, 1996). We found no difference between the MeCP2 KO and WT neurons, suggesting that the changes in spontaneous release 41 frequency in the KO cultures cannot be attributed to a reduction in the number of readily releasable vesicles (Figure 2-3B and C). However, we cannot fully exclude the possibility that the numbers of spontaneously recycling vesicles are reduced, but we consider this unlikely given that sizes of distinct vesicle pools are usually highly correlated in central synapses (Sara et al., 2005). We next examined the properties of evoked neurotransmission in response to action potential stimulation. Trains of action potentials applied at 10 Hz typically depress neurotransmission. This depression is, at least in part, elicited by a rapid decrease in the number of vesicles available for release within a synaptic terminal. When the kinetics of synaptic depression and recovery in the MeCP2 KO cultures were examined, we observed a more rapid depression during 10 Hz stimulation and a slower response recovery when the stimulation frequency was switched to 1 Hz at the end of the 10 Hz train, compared to WT cultures (Figure 2-4A, C, and D). The KO cultures also showed a slight increase in first response amplitudes and significantly smaller paired pulse ratios during high stimulation frequencies compared to controls (Figure 2-4B, C insert). These findings suggest that the loss of MeCP2 may contribute to an increase in neurotransmitter release probability leading to a faster depletion of releasable vesicles, as well as a delay in synaptic vesicle recycling after stimulation retarding the recovery of responses. Taken together with the decrease in spontaneous event frequency, these findings suggest a role for MeCP2 in presynaptic control of neurotransmitter release and vesicle recycling specifically in excitatory synapses. To examine whether the alterations in excitatory neurotransmission of neurons lacking MeCP2 are the result of impairments in gene silencing, we treated wild type 42 C57BL/6 hippocampal cultures chronically with drugs that inhibit either transcriptional activation or repression and then measured synaptic transmission. To suppress transcription, we treated cultures with the RNA polymerase inhibitor Actinomycin D (Act D). To suppress transcriptional repression, we used Trichostatin A (TSA), a histone deacetylase inhibitor. Cultures were treated with these individual drugs (and DMSO as a control) for 24 hours and then synaptic activity was measured. We preferred chronic rather than acute treatments because earlier studies did not reveal a significant change in baseline synaptic transmission after acute TSA application, although they reported a strong augmentation of long term synaptic plasticity (Levenson et al., 2004). We initially examined cell viability in the presence of these chronic treatments and found that it was not compromised (see Experimental Procedures). In these experiments, we focused on potential alterations in the frequency and amplitude of spontaneous miniature events, which is a more direct measurement of presynaptic machinery, since evoked transmission may be vulnerable to alterations in membrane excitability and Ca2+ signaling, two aspects of neuronal function that may also be targeted by transcriptional regulation. We found a significant decrease in mEPSC frequency in TSA treated cultures compared to DMSO treated cultures, while Actinomycin D did not have an effect (Figure 2-5A and B). The average amplitudes of individual events were not significantly affected by these treatments (data not shown). We also determined the number of presynaptic terminals in these cultures and found no changes between the control and TSA treatments (Figure 25C and D). Similarly to what was seen with the MeCP2 KO cultures, both the frequency and amplitudes of mIPSCs were unchanged between the DMSO and TSA treated neurons (Figure 2-5E, F and data not shown). To investigate whether newly transcribed genes are 43 involved in the suppression of synaptic function, we treated cultures with both Actinomycin D and TSA and found a reversal of the mEPSC deficits seen in the TSA treated neurons (Figure 2-5B). These findings indicate a selective impairment of excitatory presynaptic function after suppression of transcriptional repression but not transcriptional activation. To examine whether the TSA-mediated decrease in mEPSCs was related to MeCP2 function, we chronically treated MeCP2 KO cultures with TSA and did not detect a reduction in the frequency of spontaneous mEPSCs (Figure 2-5G and H). This result strongly suggests that the decrease in spontaneous miniature frequency we observed in the presence of this transcriptional activator was in significant part due to inhibition of MeCP2 function and thus was occluded in the absence of MeCP2. Interestingly, Actinomycin D did not rescue the reduction in mEPSC frequency seen in MeCP2 KOs back to control levels (Figure 2-5G and H). This may not be surprising since degradation of abnormally expressed presynaptic proteins may take longer than 24 hours. We also investigated whether an acute loss of MeCP2 function after neurodevelopment and synapse maturation would result in similar phenotypes found in the constitutive MeCP2 KO neurons. We made primary dissociated hippocampal cultures from newborn floxed MeCP2 mice and allowed them to age 7 days in vitro before infecting them with high or low titer lentivirus expressing the gene Cre recombinase, or GFP as a control (Figure 2-6). One week later, we found a significant decrease in the frequency of mEPSCs in high titer Cre-infected floxed MeCP2 neurons compared to GFP-infected neurons (Figure 2-7A and B). This significant frequency decrease was also seen when mEPSCs from Cre-infected floxed MeCP2 neurons were compared with Cre- 44 infected wild type littermate neurons, ruling out a nonspecific effect of Cre expression on mEPSCs (Figure 2-7A and B). These data suggest that MeCP2 acts as a regulator of synaptic transmission even in mature neurons and the loss of MeCP2 may have profound effects on synaptic function after neurodevelopment. We also recorded mEPSCs from uninfected floxed MeCP2 neurons receiving the majority of their inputs from Creinfected neurons and found the same decrease in event frequency, supporting the presynaptic origin of this observation (Figure 2-7A and B). In accordance with this premise, mEPSC frequency was not altered in floxed MeCP2 neurons infected with a low titer Cre-expressing lentivirus compared to GFP controls (Figure 2-7D and E). This data, as well as the fact that we saw no significant changes in mEPSC amplitudes among any of the lentiviral-infected cultures (Figure 2-7C and F), strongly suggests that MeCP2 plays a specific role in presynaptic function. 45 Discussion Taken together, these findings suggest that synaptic transmission, in particular presynaptic function, is under transcriptional control and that MeCP2-dependent transcriptional repression is a critical component of this regulation. In our experiments, we detected MeCP2 dependent alterations in spontaneous neurotransmission as well as in short-term synaptic depression. Spontaneous neurotransmission is important for a number of neuronal processes, which include maturation and stability of synaptic networks (McKinney et al., 1999; Zucker, 2005) and inhibition of local dendritic protein synthesis (Sutton et al., 2004). Therefore, the decrease in mEPSCs we describe here may well underlie some of the neuromorphological abnormalities seen in RTT patients as well as RTT mouse models (Chen et al., 2001; Guy et al., 2001; Kaufmann and Moser, 2000; Shahbazian and Zoghbi, 2002). However, our data also suggest genes involved in evoked transmission and short-term plasticity as potential transcriptional targets under MeCP2 control. Short-term synaptic depression is a fundamental synaptic mechanism, which underlies key brain functions such as sound localization and sensory adaptation (Abbott and Regehr, 2004; Chung et al., 2002; Cook et al., 2003). Therefore, changes in synaptic depression may have important implications for the synaptic basis of RTT, as well as other neurodevelopmental disorders. Recent evidence suggests that synaptic vesicles giving rise to evoked and spontaneous neurotransmission originate from different pools (Sara et al., 2005). Therefore it is possible to envision that simultaneous changes in expression levels of multiple synaptic proteins, which are under MeCP2 regulation, may have opposing effects (or a complex additive effect) on the two forms of neurotransmission. 46 Understanding the mechanisms underlying these synaptic alterations will require identification of synaptic molecules that are MeCP2-dependent in their transcription. Could the alterations in synaptic transmission produced by mutations in the MeCP2 gene underlie the behavioral phenotypes observed in RTT patients? Recent studies have shown that the loss of MeCP2 selectively in the brain is sufficient to recapitulate many features of RTT including impaired motor coordination, increased anxiety-related behavior and social deficits (Gemelli et al., 2005). In related studies, brain slices from mice overexpressing MeCP2 displayed an increase in paired pulse facilitation and long-term potentiation (LTP) (Collins et al., 2004), while MeCP2 null mice exhibited the converse (Asaka et al., 2006), suggesting MeCP2 expression may exert a profound role on synaptic plasticity. Our data indicates a specific deficit in excitatory synaptic transmission upon the loss of MeCP2 function. A recent study of cortical slices from 4-5 week old MeCP2 knockout mice also found changes in excitatory, as well as inhibitory, transmission (Dani et al., 2005). These additional inhibitory changes may be specific for cortical neurons, or they may just occur later in development. Nevertheless, it appears that an imbalance in excitatory and inhibitory input, as we see in hippocampal cultures, may underlie some of the neurological deficits in RTT, and possibly other related neurodevelopmental disorders. Indeed, other recent studies have shown that the neuroligin genes, a family of proteins implicated in autism spectrum disorders, are important for maintaining a balance of excitatory and inhibitory neurotransmission (Chih et al., 2004; Chih et al., 2005). It is worthwhile noting that we did not observe any morphological changes in neurons lacking MeCP2. This may well be due to the fact that our studies were done in 47 young cultures and may reflect a more immature neuronal population. This suggests that the functional deficits we observed in MeCP2 KO neurons may preclude the neuronal structural abnormalities observed in the disease state. These data suggest that transcriptional repression is important in regulating presynaptic function of hippocampal neurons, even after neurodevelopment. This provides insight into how the loss of function of the transcriptional repressor, MeCP2, may contribute to the disease state and may have profound implications, especially if additional neuronal populations are under MeCP2 control. This information is important in delineating the cellular and functional abnormalities that lead to the wide array of neurological deficits observed in RTT patients. 48 Figure 2-1. Spontaneous miniature synaptic currents in cultured hippocampal neurons from MeCP2 knockout and control mice (A-D) Spontaneous excitatory synaptic currents. Representative recordings of miniature excitatory events in control (A) and MeCP2 knockout (B) neurons recorded in 1 μM TTX and 50 μM picrotoxin. (C) Bar graph showing a decrease in the frequency of spontaneous excitatory events in knockout neurons compared to controls (*; p<0.05). (D) Cumulative histogram of mEPSC amplitudes. (E-H) Spontaneous inhibitory synaptic currents. Representative recordings of miniature inhibitory events in control (E) and MeCP2 knockout (F) neurons recorded in 1 μM TTX and 10 μM NBQX. (G) Bar graph showing similar frequencies of spontaneous inhibitory events in control and knockout neurons. (H) Cumulative histogram of mIPSC amplitudes. (I and J) Immunostaining of cultured neurons. (I) Dissociated neurons from control and knockout mice were labeled with primary antibodies to MAP2 (green) and Synapsin (red). (J) Bar graph depicts the number of presynaptic terminals found in control and mutant neurons. There was no significant difference in the number of presynaptic terminals between control and MeCP2 knockout neurons (p>0.2). 49 Figure 2-2. Miniature EPSC frequency is reduced in older MeCP2 KO neurons (A) Representative recordings of miniature excitatory events in control and MeCP2 knockout neurons >20 DIV. (B) Bar graph showing a decrease in the frequency of spontaneous excitatory events in older knockout neurons compared to controls (*; p<0.05). 50 Figure 2-3. Total pool and readily releasable pool of synaptic vesicles from MeCP2 knockout and control neurons (A) Fluorescence destaining of MeCP2 knockout and control synapses in response to high K+ stimulation. Synapses were loaded with FM1-43 by 47 mM K+ induced depolarization and destained using 90 mM K+. Insert, bar graph depicts the total vesicle pool sizes in control and mutant synapses measured by the total change in fluorescence during 90 mM K+ destaining. (B and C) Synaptic responses to hypertonic sucrose stimulation. (B) Representative traces from control and knockout neurons showing synaptic responses to 500 mM sucrose. (C) Bar graph depicting the average amplitudes of the peak currents during hypertonic sucrose stimulation. 51 Figure 2-4. Evoked synaptic responses in MeCP2 knockout and control neurons during 10 Hz field stimulation immediately followed by 1Hz recovery stimulation (A) Representative whole-cell recordings of the first 20 responses. (B) Paired pulse ratios of the first two responses during various stimulation frequencies were measured from control and knockout neurons. The paired pulse ratios recorded from MeCP2 knockout neurons were significantly less than control at both 30 Hz and 10 Hz frequencies (*, p<0.05, **, p<0.01). (C) Average response amplitudes of control and mutant neurons measured during the first two seconds of 10 Hz stimulation. Recorded responses from field stimulated knockout neurons depressed significantly faster than control responses (*, p<0.05, **, p<0.01). Insert is a bar graph depicting similar average first peak amplitudes of these responses. (D) Average response amplitudes measured during 1 Hz stimulation following the 10 Hz depression. Knockout response amplitudes did not recover as quickly as those recorded from control neurons (*, p<0.05). 52 Figure 2-5. Spontaneous miniature synaptic responses from wild type C57BL/6 and MeCP2 knockout hippocampal cultures after a 24-hour treatment with inhibitors of transcriptional repression and activation (A and B) Spontaneous excitatory synaptic currents from C57BL/6 neurons. (A) Representative miniature excitatory synaptic currents from drug treated neurons recorded in 1 μM TTX and 50 μM picrotoxin. (B) Bar graph of the significant decrease in mEPSC frequencies in neurons treated for 24 hours with the inhibitor of transcriptional repression, TSA (*; p<0.05). This decrease was reversed when treatment included both TSA and the inhibitor of transcriptional activation, Act D. (C and D) Immunostaining of C57BL/6 cultured neurons. (C) Control and TSA treated dissociated neurons were labeled with primary antibodies to MAP2 (green) and Synapsin (red). (D) Bar graph depicting a similar number of presynaptic terminals found in control and TSA treated neurons. (E and F) Spontaneous inhibitory synaptic currents from C57BL/6 neurons. (E) Representative recordings of miniature inhibitory events from control and TSA treated neurons recorded in 1 μM TTX and 10 μM NBQX. (F) Bar graph showing the frequencies of spontaneous miniature inhibitory events. (G and H) Spontaneous excitatory synaptic currents from MeCP2 knockout neurons. (G) Representative traces from drug treated knockout neurons recorded in the presence of 1 μM TTX and 50 μM picrotoxin. (H) Bar graph showing that mEPSC frequencies in knockout neurons were not significantly affected by 24-hour treatment with either Act D or TSA. 53 Figure 2-6. High and low titer lentiviral infection of dissociated hippocampal cultures (Top) Light and fluorescent microscopic images and an overlay of the two demonstrate the low titer (<20% of neurons infected) Cre lentivirus used to knockdown the expression of MeCP2 in floxed MeCP2 neurons. (Bottom) Light, fluorescent and overlaid images of neurons infected with the high titer (>80% infected) Cre lentivirus. 54 Figure 2-7. Spontaneous miniature excitatory synaptic currents in floxed MeCP2 neurons infected with a lentivirus expressing Cre recombinase (A-C) Miniature excitatory events from floxed neurons infected with high titer lentivirus. (A) Representative traces from infected neurons recorded in the presence of 1 μM TTX and 50 μM picrotoxin. (B) Bar graph revealing a significant decrease in mEPSC frequencies from floxed MeCP2 neurons infected after synapse formation with high titer lentiviral Cre recombinase (*; p<0.05). This decrease was seen in recordings from both Cre infected and uninfected postsynaptic floxed neurons and is compared to both floxed neurons infected with GFP and wildtype neurons infected with Cre. (C) Cumulative histogram showing no significant changes in mEPSC amplitudes. (D-F) Miniature excitatory currents from floxed neurons infected with low titer lentivirus. (D) Representative traces of mEPSCs recorded from infected neurons. (E) Bar graph revealing similar mEPSC frequencies from floxed MeCP2 neurons infected after synapse formation with low titer lentiviral Cre recombinase (*; p<0.05). (F) Cumulative histogram showing no significant changes in mEPSC amplitudes. 55 CHAPTER 3 ACTIVITY-DEPENDENT SUPRESSION OF EXCITATORY MINIATURE NEUROTRANSMISSION THROUGH THE REGULATION OF DNA METHYLATION Introduction DNA methylation is a key cellular mechanism used to repress gene expression and promote genome stability in various species. In mammals, methylation of DNA plays roles in many processes, such as X chromosome inactivation, genomic imprinting and the control of tissue-specific gene expression. DNA methyltransferases (DNMTs) are the enzymes responsible for adding methyl groups at the 5-position of cytosine residues within CpG dinucleotides. During development, widespread methylation changes occur in primordial germ cells and pre-implantation embryos (Jaenisch and Bird, 2003; Reik et al., 2001). After differentiation, alterations in DNA methylation are less abundant and primarily control local gene expression to preserve cellular identity. These methylation changes are commonly believed to occur only in actively dividing cells, where specific methylation sites on genomic DNA are established and maintained by DNMTs during replication. More recently however, the Gadd45a protein was discovered to play a role in active demethylation of DNA in proliferating as well as non-dividing cells (Barreto et al., 2007). The role of DNA methylation in neurons has recently become of interest due to alterations in the methylation status of genes associated with a number of mental retardation syndromes including, Rett, ICF and Fragile X (Robertson and Wolffe, 2000). 56 DNMTs are highly expressed in neurons in the adult brain suggesting they may have a functional role in postmitotic neurons (Brooks et al., 1996; Feng et al., 2005; Goto et al., 1994; Inano et al., 2000). Methylcytosine analogs, including 5-azacytidine, can inhibit DNA methylation in many cell types (Robertson and Jones, 2000). Recent studies suggest that inhibiting DNA methylation in hippocampal slices blocks long-term synaptic plasticity (Levenson et al., 2006). In other studies, prolonged depolarization of cultured cortical neurons has been reported to result in a decrease in methylation in the promoter region of BDNF, a neurotrophin important for synaptic plasticity (Martinowich et al., 2003). These studies suggest that there may be a relationship between synaptic activity and DNA methylation in mature neurons. However, the mechanisms behind these changes in DNA methylation and synaptic activity are unknown. We are interested in looking more closely at the role of DNA methylation in synaptic function, therefore we used an array of pharmacological approaches to impair DNMT activity in hippocampal neurons and tested basic properties of neurotransmission using electrophysiological and imaging techniques. 57 Results To examine whether alterations in DNA methylation influence synaptic transmission, hippocampal neurons were cultured from newborn C57BL/6 mice, matured (13-21 DIV) (Mozhayeva et al., 2002), and then treated for 24 hours with two different DNMT inhibitors, the methylcytosine analogs 5-azacytidine (5azaC, 2.5 μM) and Zebularine (Zeb, 50 μM) (Tawa et al., 1990). Whole-cell voltage clamp recordings were performed on cultured hippocampal neurons immediately following 24-hour chronic treatments. Spontaneous miniature excitatory postsynaptic currents (mEPSCs) were measured in neurons treated with 5azaC, Zeb or vehicle (DMSO). These recordings were done in the presence of TTX to block action potential firing and picrotoxin to block inhibitory activity. We found a significant decrease in the frequency of mEPSCs in both the 5azaC and Zeb treated neurons compared to controls, but no change in the amplitudes of these events (Fig. 3-1A-C). The changes in mEPSC frequency, but not amplitudes, point to a possible presynaptic deficit following DNMT inhibition. To test whether this effect on spontaneous activity is specific for excitatory synapses, miniature inhibitory postsynaptic currents (mIPSCs) were recorded in neurons in the presence of TTX and the AMPA-type glutamate receptor blocker, NBQX. No changes were observed in the frequency or amplitudes of mIPSCs in neurons with either 5azaC or Zeb treatments compared to controls, suggesting that DNMT inhibition is affecting only excitatory synaptic activity (Fig. 3-1D-F). The fact that both DNMT inhibitors caused similar deficits in excitatory synaptic transmission suggests that inhibiting DNA methylation produces specific alterations in synapse function and not generalized, global nonspecific effects. 58 The deficits in mEPSC frequency produced by DNMT inhibition could be caused by decreases in excitatory synapse number and/or release probability from excitatory presynaptic terminals. Therefore, the number of functional excitatory synapses formed onto cultured hippocampal neurons was assessed following 24 hours of DNMT inhibition. Neurons were immunostained for Synapsin, a presynaptic vesicle protein, and PSD-95, a postsynaptic scaffolding protein found specifically at excitatory synapses. The analysis of colocalized Synapsin and PSD-95 revealed that the number of excitatory synapses was unaffected by treatment with either 5azaC or Zeb, indicating that the decreases in spontaneous mEPSCs are not the result of a decreased number of excitatory inputs (Fig. 3-1G, H). We also examined the properties of evoked excitatory neurotransmission in response to action potential stimulation to determine if there were deficits in release probability after inhibition of DNMT activity. After treatment with 5azaC or Zeb for 24 hours, there were no changes in excitatory postsynaptic response depression during a 10 Hz field stimulation (Fig. 3-2A, B) or in the paired pulse ratios of these responses recorded at various stimulation frequencies (Fig. 3-2C). These observations are consistent with a previous experiment which did not find effects of these drugs on basal synaptic transmission or short term synaptic plasticity in hippocampal slices (Levenson et al., 2006). These findings suggest that inhibition of DNA methyltransferases by methylcytosine analogs specifically affects the release probability of spontaneous miniature excitatory events. To further explore the presynaptic properties of these neurons, we used FM dye imaging to measure aspects of both evoked and spontaneous synaptic vesicle recycling. 59 To label all recycling vesicles within presynaptic terminals, cultures were stimulated with 47 mM K+ solution for 90 seconds in the presence of the styryl dye FM1-43 (Betz et al., 1996; Harata et al., 2001). After a brief wash, the kinetics of release of these vesicles was measured by destaining synapses with 90 mM K+ solution for 90 seconds. The rates of dye loss from total vesicle pools in control and DNMT inhibitor treated synapses was similar (Fig. 3-2D), as were the total amounts of dye trapped within individual synapses (Fig. 3-2E), indicating that the sizes of the total vesicle pools were unchanged. We then assessed the properties of spontaneously recycling vesicles from neurons treated with inhibitors of DNMT activity. Vesicles were loaded for 15 minutes in the presence of TTX to block activity, washed, and then the destaining of individual synapses was measured for 20 minutes, again in the presence of TTX to block synaptic activity. Treatment with 5azaC resulted in slower destaining kinetics compared to controls (Fig. 3-2F), suggesting a specific deficit in spontaneous vesicle fusion following DNMT inhibition. The total amount of dye released during destaining was similar between control and 5azaC treated synapses (Fig. 3-2G), implying that the number of vesicles that recycle spontaneously was unaffected. Together, these results support a specific role for DNA methylation in the control of spontaneous vesicle release from presynaptic terminals. The molecular substrate for the DNMT methylation reaction is S-adenosyl-Lmethionine (SAM). The interaction of methylcytosine analogs with DNA methyltransferases interferes with the enzymes’ abilities to transfer methyl groups from this endogenous substrate to cytosines in CpG dinucleotides. However, in the presence of excess SAM, DNMTs can actually methylate 5azaC-containing DNA (Gabbara and Bhagwat, 1995). Therefore, we surmised that the addition of excess SAM may reverse 60 the DNMT inhibitor-induced deficits in spontaneous synaptic transmission. The previously seen decrease in mEPSC frequency after 24-hour 5azaC and Zeb treatments was no longer seen after the addition of SAM (Fig. 3-3A). The proposed functions of 5azaC and Zeb are to inhibit DNMT-dependent methylation of appropriate CpG dinucleotides thus resulting in the improper transcription of specific genes. Therefore, the effects of these two drugs should be dependent on active gene expression. To investigate whether the deficit in mEPSC frequency caused by inhibition of DNMT activity is dependent on transcription, we treated cultures with the RNA polymerase inhibitor, Actinomycin D (Act D), in combination with either 5azaC or Zeb. Act D treatment was able to block the decrease in mEPSC frequency seen in neurons treated with DNMT inhibitors (Fig. 3-3B). These results demonstrate that the defects in miniature excitatory event frequency seen after blocking the ability of DNMTs to add methyl groups to DNA is, in fact, a result of increased gene transcription and not some nonspecific effect of the drugs. In mammalian cells, one primary role of DNA methylation is to promote the binding of transcriptional repressor proteins to the promoters of specific genes. One such DNA methyl-binding protein is methyl-CpG binding protein 2 (MeCP2). Mutations in the MeCP2 gene are associated with the neurological disorder, Rett syndrome. We previously reported a defect in excitatory neurotransmission in cultured hippocampal neurons from MeCP2-deficient mice, of which included a specific decrease in the frequency of mEPSCs (Nelson et al., 2006). We were intrigued by the similar decrease in mEPSC frequency seen after DNMT inhibition, suggesting a putative shared mechanism for the control of excitatory spontaneous transmission by DNA methylation and MeCP2. 61 To investigate this possibility, primary hippocampal cultures were made from newborn MeCP2 knockout mice and mature cultures were treated with DNMT inhibitors. No further reduction in the frequency of mEPSCs from these neurons was detected after the addition of either 5azaC or Zeb (Fig. 3-3C, D), effectively denoting that the loss of MeCP2 function, either in the knockout or by eliminating DNA methylation, is the primary cause of this deficit. These results also suggest that the effects of DNMT inhibition occur in post-mitotic neurons since MeCP2 is not expressed in glial cells (Shahbazian et al., 2002b). Next, we examined the possibility that the methyl donor, SAM, may also alleviate the synaptic defects seen in MeCP2-deficient neurons. A decrease in mEPSC frequency was still seen after a 24-hour treatment with the DNMT substrate, however, after 48 hours, SAM was able to rescue the defect in spontaneous event frequency seen in MeCP2 knockout neurons (Fig. 3-3C, D). The need for a longer exposure to S-adenosyl-Lmethionine is not surprising since previous work has shown that an increase in DNA methylation does not occur until after >36 hours treatment with SAM (Noh et al., 2005). The transcriptional repressor, MeCP2, is the only methyl-binding protein that can bind to singly methylated CpG dinucleotides (Lewis et al., 1992), while other proteins containing a methyl-binding domain (MBDs) require additional sites (Hendrich and Bird, 1998). It is possible that the addition of excess SAM leads to an increase in the number of methylated CpGs on specific promoters, allowing for other MBDs to bind in the absence of MeCP2. We saw no effect of SAM treatment, for either 24 or 48 hours, on wildtype neurons (Fig. 3-3A, C, D), suggesting that MeCP2’s binding to sufficiently methylated promoter regions has already repressed the expression of genes responsible for 62 suppressing synaptic function. The ability of SAM to rescue the synaptic deficits in the MeCP2 knockout is intriguing and future studies will be important to determine whether SAM can rescue the behavioral deficits in mouse models of Rett Syndrome. Our data, thus far, support a role for DNA methylation in synaptic function, which overlaps with MeCP2’s role as a transcriptional repressor. To ensure that the DNMT inhibitors were indeed effective at blocking methylation, we employed the use of methylation-sensitive restriction endonucleases on genomic DNA extracted from 24-hour treated hippocampal cultures. Using the technique, NotI-MseI methylation-sensitive amplified fragment length polymorphism (MS-AFLP) we looked for decreases in the methylation patterns of total genomic DNA compared to controls (Yamamoto et al., 2001). NotI is a restriction enzyme that cleaves unmethylated DNA in CpG islands, or areas rich in G+C content; NotI is unable to cleave these sites when methylated. With NotI-MseI MS-AFLP, hypomethylation of DNA is depicted by an increase in band intensity from a fluorescent DNA fingerprint covering >95% of the entire genome. A comparison of the methylation patterns from 5azaC and Zeb treated cells revealed that ~1% of the increased band intensities overlapped (Fig. 3-4A, B and data not shown). Though these changes are small, they demonstrate the successful demethylation of genomic DNA by DNMT inhibitors. The modest size of this decrease in methylation is consistent with the extensive stability of overall DNA methylation patterns in postmitotic neurons in the adult brain. Recently, MeCP2’s function was shown to be susceptible to activity-dependent regulation via phosphorylation in response to Ca2+ (Chen et al., 2003; Zhou et al., 2006). There is recent evidence that DNA methylation can also be regulated by neuronal 63 activity. A commonly used learning and memory paradigm, contextual fear conditioning, increases the levels of DNMT enzymes in the brain and alters promoter methylation patterns in two genes associated with synaptic plasticity (Miller and Sweatt, 2007), however the molecular signaling mechanisms that drive these changes in DNA methylation are still not understood. To examine whether DNA methylation indeed forms an additional target for activity-dependent regulation of gene expression, we focused on the BDNF gene, due to its earlier association of high potassium depolarization mediated demethylation in its promoter region (Martinowich et al., 2003). After exposing dissociated hippocampal cultures to a chronic, 24-hour treatment with the DNMT inhibitor, 5azaC, we isolated genomic DNA and processed it for bisulfite modification. Using quantitative Real-Time PCR with primers specific for a CpG island located in the promoter region for exon 1 of the BDNF gene (Fig. 3-5), we found a significant increase in unmethylated DNA after 5azaC treatment compared to vehicle controls (Fig. 3-6A, B), suggesting that demethylation of this BDNF promoter occurs in mature, hippocampal cultures. We hypothesized that the spontaneous network activity present in these mature cultures (Virmani et al., 2006) may exert control over BDNF expression by regulating methylation at the BDNF promoter I. Therefore, we measured the methylation status of the CpG island after a 24-hour co-treatment with 5azaC and either tetrodotoxin (TTX) or 2-amino-5-phosphonopentanoic acid (AP5). The increase in demethylation after 5azaC treatment was prevented when either evoked synaptic activity was blocked with TTX or when NMDA receptor-activity was blocked using AP5 (Fig. 3-6A, B). We also measured the amount of unmethylated BDNF in hippocampal cultures treated with SAM in combination with 5azaC. The addition of SAM was able to reverse the effects of 5azaC 64 on DNA methylation (Fig. 3-6A, B), indicating a rescuing effect of the substrate in the presence of DNMT inhibition. These results demonstrate that spontaneous network activity present in these cultures is sufficient to render the BDNF promoter susceptible to demethylation and alter BDNF expression. The mechanism by which neuronal activity regulates the methylation status of genes, such as BDNF, may be that activity makes DNA susceptible to demethylation by causing damage/repair or perhaps facilitates putative demethylation activity. To determine whether the deficit in synaptic transmission caused by DNMT inhibitors is dependent on activity, mEPSCs were recorded from cultures silenced with TTX during the entire 24 hours they were exposed to either 5azaC or Zeb. In the absence of action potential firing, treatment with these DNMT inhibitors no longer resulted in decreased frequency of spontaneous miniature excitatory events (Fig. 3-6C). Thus, the ability of DNA methylation to influence synaptic transmission requires neuronal activity, suggesting that the methylation status of genes that play a role in controlling synapse function may be regulated by synaptic activity. A well-known mechanism by which synaptic activity can drive changes in gene expression is by calcium influx through postsynaptic N-methyl-D-aspartate-type (NMDA) glutamate receptors (Bito et al., 1997). Since we observed a requirement of synaptic activity for DNA methylation-induced alterations in synaptic function, we tested the importance of NMDA receptor-dependent calcium signaling in this regulation. We exposed our cultures to the NMDA receptor antagonist, AP5, and measured the effects of DNMT inhibition on mEPSCs. The previously seen decrease in mEPSC frequency after treatments with either 5azaC or Zeb was blocked in the presence of AP5 (Fig. 3-6D), 65 suggesting that calcium influx through NMDA receptors is, at least in part, responsible for the effects DNMT inhibition has on excitatory synaptic transmission. Taken together, these data indicate that the regulation of DNA methylation in mature neurons is dependent on neuronal activity and NMDA receptor-dependent calcium signaling, and that these activity-dependent changes in DNA methylation play a fundamental role in controlling basal synaptic function. Finally, we assayed whether the changes in methylation of the BDNF promoter I resulted in changes in BDNF mRNA expression. Using quantitative Real-Time PCR with primers specific to BDNF exon 5, the mRNA coding exon of the gene, we determined the levels of BDNF expression in our cultures following treatment with the DNMT inhibitor, 5azaC. Interestingly, there was no change in the level of BDNF expression following 24 hour treatment with 5azaC (Figure 3-7), as would not have been expected given the increase in BDNF promoter demethylation seen previously. However, 5azaC treatment for 48 hours did lead to a significant increase in BDNF mRNA levels compared to controls. This treatment also resulted in demethylation of BDNF promoter I (data not shown). One caveat of this experiment is that BDNF mRNA expression is controlled by four different promoters, I-IV, in mice. We may not have been able to detect changes in expression due to differential regulation of these three promoters, at least not until longer exposure to 5azaC possibly leads to additional decreases in promoter methylation. Future analyses of methylated cytosines within the different BDNF promoters following DNMT inhibition should help clarify these discrepancies. Next, we further tested the role of synaptic activity in controlling changes in BDNF expression in response to alterations in DNA methylation by combining the 5azaC treatments with either TTX or AP5. Both the 66 blocker of action potentials and the antagonist of NMDA receptors significantly decreased the expression of BDNF (Figure 3-7). These results were expected given that there is ample evidence indicating an activity-dependent regulation of BDNF gene expression through the regulation of promoters III or IV (Tao et al., 1998). Again, our assay cannot differentiate between specific promoters driving changes in BDNF expression. In conclusion, our studies suggest a novel mechanism by which synaptic activity can drive an increase in BDNF transcription through the demethylation of BDNF promoters. 67 Discussion Collectively, our findings suggest that spontaneous synaptic transmission between postmitotic neurons is regulated by alterations in DNA methylation that occur in response to synaptic activity. Spontaneous synaptic currents are known to be important for controlling synapse maturation and the stability of neuronal networks (McKinney et al., 1999; Zucker, 2005). In addition, spontaneous neurotransmission can be an important read-out for homeostatic mechanisms such as synaptic scaling (Kilman et al., 2002; Turrigiano et al., 1998). In response to 24-hour DNMT inhibition, we found a significant decrease in the frequency of mEPSCs, which correlates with a decrease in BDNF promoter I methylation. Interestingly, an increase in BDNF mRNA expression was seen only after longer exposure to 5azaC treatment. Previous work has established a role for BDNF in the induction of homeostatic plasticity. A chronic decrease in endogenous BDNF signaling in cortical cultures increases the intrinsic excitability of pyramidal neurons, a similar effect to what occurs following chronic activity blockade (Desai et al., 1999). In our hands, the deficit in neurotransmission in response to DNMT inhibition is seen prior to the increase in BDNF expression. This suggests that the regulation of homeostatic plasticity can act in two ways: neurons can respond to decreases in the expression of normal activators of synaptic plasticity, like BDNF, and consequently increase excitatory synaptic function, or they can respond to decreases in neurotransmission by increasing the expression of genes that will enhance neuronal excitability. We also demonstrate that both effects on synaptic transmission and BDNF promoter methylation are dependent on the spontaneous neuronal activity occurring in 68 our cultures. This activity-dependent regulation is likely via increased postsynaptic calcium influx through NMDA receptors and presumably leads to the demethylation of specific gene promoters. Alas, the presence of an active demethylating enzyme in neurons has yet to be discovered which leads to the possible conclusion that activity is driving demethylation through a process utilizing DNA damage and repair machinery (Brooks et al., 1996). Nevertheless, there is additional support for activity-driven changes in DNA methylation. Both increases and decreases in DNA methylation patterns of two genes involved in synaptic plasticity were found in the hippocampus following fear conditioning (Miller and Sweatt, 2007). While this study does not explore a molecular mechanism controlling activity-dependent alterations in DNA methylation, the association of learning and memory behaviors with postsynaptic signaling cascades activated by synaptic inputs implicates potential pathways that may be able to drive these changes. Furthermore, our studies demonstrate an intimate relationship between DNA methylation in neurons and the transcriptional repressor MeCP2. The deficit seen in spontaneous synaptic transmission following 5azaC and Zeb treatments was occluded in the absence of MeCP2, the loss of which causes a similar defect in synapse function (Nelson et al., 2006). In addition, treatment of MeCP2 KO neurons with the methyl donor SAM was able to reverse this decrease in miniature neurotransmission. An interesting future avenue to pursue is the possible affects of SAM treatment on many of the behavioral deficits seen in MeCP2-deficient mice. These findings suggest that MeCP2 is the methyl-binding protein responsible for mediating the effects of DNA methylation on 69 neuronal function. Our results also have implications towards treatment of RTT patients with SAM in an attempt to ameliorate some of their neurological symptoms. In conclusion, our data indicate a role for DNA methylation in the control of synaptic function, which shares a common pathway with the methyl-binding protein, MeCP2. Furthermore, our data suggest that neuronal activity can drive the transcription of genes important for controlling spontaneous neurotransmitter release by regulating the methylation status of these genes (Fig. 3-8). Previous work has demonstrated that activity can also induce the phosphorylation of MeCP2, causing its dissociation with target genes and relieving its repression of transcription (Chen et al., 2003). Activity-dependent loss of DNA methylation may be an additional mechanism by which MeCP2 is released from the promoters of target genes. Our results concerning DNA methylation and its impact on neurotransmission may suggest a homeostatic mechanism by which neuronal nuclei can monitor alterations in activity levels and adjust neurotransmitter output via altering gene expression and thus impact network excitability. 70 Figure 3-1. Inhibiting DNMT activity in neurons causes a deficit in excitatory synaptic transmission. (A) Sample traces of miniature EPSCs recorded from mature hippocampal cultures treated 24 hours with DNMT inhibitors, 5azaC and Zeb. (B) Bar graph showing a decrease in the frequencies of mEPSCs recorded from 5azaC and Zeb treated neurons compared to controls (**p<0.01; *p<0.05). (C) Histograms of mEPSC amplitudes show no differences among control, 5azaC, or Zeb treatments. (D) Sample recordings of miniature IPSCs from cultures treated with inhibitors of DNMT activity. (E) Bar graph depicts no change in mIPSC frequencies from 5azaC or Zeb treated neurons compared with controls. (F) Cumulative histograms of mIPSC amplitudes show no differences between control and DNMT inhibitor treated neurons. (G) Dissociated hippocampal cultures were immunostained with antibodies to Synapsin (red) and PSD-95 (green) to determine the number of excitatory synapses. (H) Bar graph shows no alterations in excitatory synapse number among control, 5azaC, or Zeb treated cultures. 71 Figure 3-2. DNMT inhibition for 24 hours specifically affects spontaneous presynaptic function. (A) Normalized sample traces of the first 20 evoked EPSCs in response to 10 Hz field stimulation from neurons treated with inhibitors of DNMTs. (B) Average normalized EPSC amplitudes from treated neurons measured during 20 sec of 10 Hz stimulation show no alterations in response depression after treatment with DNMT inhibitors compared to controls (DMSO n=15, 5azaC n=14, Zeb n=12). (C) Paired pulse ratios of the first two evoked EPSCs in response to various stimulation frequencies were not significantly different in DNMT inhibitor treated neurons compared with controls. (D) Total synaptic vesicle pools were loaded with FM1-43 by 47 mM K+ induced depolarization and destained using 90 mM K+. The kinetics of destaining were not different between control and 5azaC treated neurons. (E) Bar graph depicts no change in total recycling synaptic vesicles from control and 5azaC treated synapses measured by the total change in fluorescence during 90 mM K+ destaining (DMSO n=8 coverslips, 5azaC n=8). (F) Spontaneously recycling synaptic vesicles were loaded for fifteen minutes in the presence of TTX and destained for twenty minutes in the same manner. Destaining of spontaneous vesicles was slower in 5azaC treated synapses compared to controls (**p<0.01). (G) Bar graph indicates no difference in the numbers of spontaneously recycling synaptic vesicles between control and DNMT inhibitor treated neurons (DMSO n=6, 5azaC n=5). 72 Figure 3-3. Changes in spontaneous excitatory neurotransmission after DNMT inhibition are mediated by the loss of function of the transcriptional repressor, MeCP2. (A) Bar graph demonstrating that cotreatments with DNMT inhibitors and the methyl donor, SAM, or treatment with SAM alone, results in no alterations in mEPSC frequency compared with controls. (B) Bar graph revealing no changes in mEPSC frequencies when neurons were treated with DNMT inhibitors in combination with the inhibitor of transcriptional activation, Act D, suggesting that gene transcription is required for the deficit seen with DNMT inhibitor treatment alone (black bar +/- gray S.E.M.). (C) Representative traces of mEPSCs recorded from MeCP2 deficient neurons. (D) Bar graph showing a decrease in mEPSC frequency in MeCP2 knockout neurons that is not significantly affected by 24-hour treatments with 5azaC, Zeb or SAM. However, a 48hour application of SAM onto MeCP2 deficient neurons was able to reverse this frequency deficit (**p<0.01; *p<0.05). 73 Figure 3-4. 24-hour treatment of hippocampal cultures with DNMT inhibitors reveals demethylation of genomic DNA. (A) Example NotI-MseI methylation-sensitive amplified fragment length polymorphism (MS-AFLP) fingerprints of genomic DNA samples from control and DNMT inhibitor treated neurons. (B) Plot profiles of average band intensities from MS-AFLP example in (A) (n=2 for each treatment). Blue arrows indicate increases in band intensities with both 5azaC and Zeb treatments compared with controls. Red arrowheads indicate examples where band intensities increased with 5azaC treatment, but not Zeb. 74 Figure 3-5. Schematic of the CpG island in the BDNF gene. After bisulfite modification of genomic DNA, primers specific for a CpG island located in the promoter region for exon I were used with quantitative Real-time PCR to measure the amount of unmethylated DNA. 75 Figure 3-6. Treatment of hippocampal cultures with DNMT inhibitors reveals activity-dependent demethylation of DNA and concurrent alterations in synaptic transmission. (A) Bisulfite modification of genomic DNA followed by Quantitative PCR to measure levels of unmethylated BNDF promoter I. Representative gel electrophoresis of PCR products following control and 5azaC treatments ± TTX, AP5, or SAM. (B) Bar graph shows a 4-fold increase in the level of unmethylated BDNF promoter I following 5azaC treatment compared to controls (black bar ± gray S.E.M.), but no changes in the level of demethylation when 5azaC treatments were in combination with TTX, AP5, or SAM (*p<0.05). (C) Bar graph shows no changes in mEPSC frequency when cultures were treated with DNMT inhibitors in the presence of TTX, suggesting that neuronal activity is required for the decrease in frequency seen with DNMT inhibitor treatment alone (black bar +/- gray S.E.M.). (D) Bar graph indicates no difference in the frequencies of mEPSCs when 5azaC and Zeb treatments included the NDMA antagonist, AP5. 76 Figure 3-7. BDNF mRNA expression after chronic manipulation of DNA methylation in hippocampal cultures. Expression of BDNF was unchanged in cultures treated 24 hours with the DNMT inhibitor, 5azaC, compared to controls. However, after 48 hr exposure to 5azaC, BDNF mRNA levels were significantly increased. Blocking activity with TTX, or calcium influx through NDMA receptors using AP5, caused a significant decrease in the amount of BDNF mRNA compared to controls (*, p<0.05; **, p<0.01; ***, p<0.001). 77 Figure 3-8. Model for activitydependent demethylation of genomic DNA in post-mitotic neurons. (A) In the absence of activity, MeCP2 is able to bind to methylated CpG dinucleotides and repress the transcription of target genes. (B) In the presence of activity, demethylation occurs (possibly through DNA damage/repair) and MeCP2 can no longer bind to the promoter unless remethylation occurs via DNMT activity. (C) In the presence of activity and 5azaC, DNMTs become covalently bound to the methylcytosine analog and can no longer methylate the promoter. (D) In the presence of excess SAM, 5azaC can be methylated by the covalently bound DNMT enzyme, thereby allowing MeCP2 to bind and repress transcription. (E) In MeCP2 deficient neurons, the addition of excess SAM results in multiple methylated sites at the promoter that allows the binding of other MBD transcriptional repressors. 78 CHAPTER 4 LOSS OF HDAC1 AND HDAC2 IN HIPPOCAMPAL NEURONS RESULTS IN SPECIFIC ALTERATIONS IN EXCITATORY SYNAPTIC TRANSMISSION Introduction Post-translational modifications of chromatin, the complex of DNA wound around a core group of proteins called histones, are fundamental in controlling gene expression in all eukaryotes. These modifications can occur on cytosine nucleotides within the DNA itself or on numerous amino acid residues found in histone tails. The addition of different combinations of these modifications leads to either the activation or repression of gene transcription, a system that is commonly referred to as the “histone code” (Turner, 2002). Acetylation of histones, via histone acetyltransferase (HAT) activity, allows for the activation of gene expression by interfering with the electrophilic interaction between histones and DNA that then permits binding of the transcriptional machinery to specific gene promoters (Varga-Weisz and Becker, 1998). Conversely, the removal of acetyl groups by histone deacetylases (HDACs) results in the tighter compaction of DNA within chromatin, which therefore leads to the repression of gene transcription. There are 3 distinct classes of HDACs in mammals. Class I HDACs, consisting of HDACs 1, 2, and 3, are known to interact with multiple corepressor complexes, like those containing Mad, REST, and MeCP2, via their direct binding to Sin3a (Huang et al., 1999; Laherty et al., 1997; Nan et al., 1998). The presence of particular corepressor proteins within these complexes dictates the repression of specific target genes. Class II HDACs 79 (4, 5, 7, and 9) are unique in that they are shuttled back and forth from the nucleus to the cytoplasm in response to activity-driven phosphorylation or dephosphorylation (Fischle et al., 2001). Finally, the Class III HDACs are responsible for the deacetylation of tubulin, among other proteins, and are thereby believed to regulate the cytoskeletal dynamics of cells (Kovacs et al., 2004). Most HDACs are expressed ubiquitously within an organism, and histone acetylation and deacetylation have been shown to play important roles in a number of biological disorders, from multiple forms of cancer to neurodegenerative diseases such as Huntington’s. It is increasingly becoming apparent that HAT and HDAC activities are fundamental for normal brain function. In humans, mutations in the histone acetyltransferase CREB-binding protein (CBP) cause Rubinstein-Taybi syndrome (RTS), a disorder associated with mental retardation (Kalkhoven et al., 2003). Mouse models of RTS, in particular mice heterozygous for CBP, show increased acetylation of histone H2B that correlates with decreases in both long-term memory and synaptic plasticity (Alarcon et al., 2004). Interactions of HDACs1 and 2 with the corepressor complex containing MeCP2 (Nan et al., 1998), a gene in which mutations lead to the neurodevelopmental disorder Rett syndrome, suggest an important role for histone deacetylases in the brain. MeCP2 KO mice have defects in excitatory spontaneous neurotransmission and short and long-term synaptic plasticity (Dani et al., 2005; Moretti et al., 2006; Nelson et al., 2006). They also display deficits in behavior tests of learning and memory, as well as anxiety and social interaction (Gemelli et al., 2005; Moretti et al., 2006). 80 Recent studies suggest that small molecule inhibitors of HDAC activity can have profound effects on neuronal function. In humans, valproic acid (VPA) is commonly utilized for patients in order to treat both epilepsy and bipolar disorder (Phiel et al., 2001). Treatment of rodent hippocampal slices with Trichostatin A (TSA) results in enhanced long-term potentiation (LTP) (Levenson et al., 2004), while TSA treatment of dissociated hippocampal cultures causes a decrease in excitatory spontaneous synaptic currents, an effect that was occluded in MeCP2 KO neurons (Nelson et al., 2006). Since most research regarding HDAC activity in the brain has been done using these broadscale inhibitors, evidence for the functions of specific HDAC proteins in neurons is considerably lacking. Using mice, two studies were able to demonstrate that overexpressing HDAC4 in the striatum can have effects on cocaine reward (Kumar et al., 2005), while HDAC5 overexpression in the hippocampus can alter the effects of antidepressants on stress (Tsankova et al., 2006). Unfortunately, many questions are left unanswered about the exact functions these HDACs are carrying out in those particular areas of the brain. Are they somehow controlling cellular excitability via the regulation of genes involved in synaptic transmission or membrane polarity? If so, how is their deacetylase activity being regulated? In an attempt to delve further into the functional roles for histone deacetylation in neurons, we have measured various aspects of synaptic function in hippocampal neurons lacking specific HDAC proteins. Given our previous interest in MeCP2 and the association of HDACs 1 and 2 with the MeCP2 transcriptional repressor complex, we have focused on these two HDACs and their potential roles in the regulation of synapse function. 81 Results We previously discovered a role for HDAC function in the control of spontaneous excitatory synaptic transmission using the HDAC inhibitor TSA (Nelson et al., 2006). To further support this finding, we treated dissociated hippocampal cultures for 24 hours with an additional inhibitor of HDAC activity, Valproic acid (VPA), and then used whole-cell voltage clamp electrophysiology to measure miniature excitatory postsynaptic currents (mEPSCs). Both TSA and VPA treatments resulted in a specific decrease in the frequency of mEPSCs, but had no effects on event amplitudes (Figure 4-1A, B). To determine if HDAC inhibition had any additional effects on synapse function, we measured the short-term synaptic plasticity of TSA treated neurons in response to 10 Hz field stimulation. Chronic treatment with TSA caused a significantly faster depression of evoked EPSCs, indicating an increase in the release probability of evoked synaptic vesicles following HDAC inhibition (Figure 4-1C). To be certain that TSA and VPA were indeed inhibiting HDAC activity, we measured the amount of histone acetylation following 24-hour treatment with these drugs. Both HDAC inhibitors caused a significant increase in the amount of acetylated histone H4 (AcH4) immunoreactivity (Figure 4-1D, E), indicating that the effects of these drugs on synaptic transmission are being mediated by increases in histone acetylation. Following this initial investigation of synaptic properties in response to treatment with broad scale HDAC inhibitors, we went on to explore the roles of specific HDACs in synapse function. Due to our previous discovery of a relationship between HDAC inhibition and the loss of MeCP2’s effects on synaptic transmission, we decided to focus our study on HDACs 1 and 2, the two HDACs known to interact with MeCP2 in a 82 transcriptional repressor complex (Nan et al., 1998). Since constitutive HDAC1 and HDAC2 knockout mice die during embryonic development, we decided to use a viralmediated approach to knockout these individual HDACs, which would also allow us to study the importance of these proteins after neurodevelopment. To investigate whether an acute loss of HDAC function would result in alterations in synaptic transmission, we made primary dissociated hippocampal cultures from newborn floxed HDAC1 or HDAC2 mice and allowed them to age 7 days in vitro before infecting them with a lentivirus expressing the gene Cre recombinase, or GFP as a control. One week later, we assayed the amount of HDAC1 and 2 mRNA levels in both knockout cultures. In Creinfected floxed HDAC1 (KO) neurons, we found a significant decrease in HDAC1 mRNA levels, indicating successful knockout of the gene (Figure 4-2A). Furthermore, the expression level of HDAC2 mRNA was unchanged, arguing against any compensation for the loss of HDAC1 (Figure 4-2B). In floxed HDAC2 (KO) neurons infected with Cre-lentivirus, we successfully induced a significant decrease in HDAC2 mRNA and saw no difference in the level of HDAC1 mRNA compared to controls (Figure 4-2A, B). We also looked at protein levels in our cultures using western blotting techniques. We found a significant decrease of HDAC1 in HDAC1 KO cultures and HDAC2 protein was significantly decreased in the HDAC2 KO cultures (Figure 4-2C, D). After establishing that we had induced substantial knockdowns of HDAC1 and 2, we went on to measure various aspects of synaptic transmission. We found a significant decrease in the frequency of spontaneous mEPSCs in HDAC2 KO neurons compared to GFP-infected neurons, but not in HDAC1 KO neurons. (Figure 4-3A, B). The amplitudes 83 of these events were unchanged in both HDAC1 and 2 KO cultures (Figure 4-3C). The alteration in mEPSC frequency may implicate a presynaptic deficit in the HDAC2 KO neurons and suggests that HDAC2 may play more of a role than HDAC1 in the control of excitatory spontaneous transmission in hippocampal neurons. A recent report has demonstrated higher HDAC2 expression, compared to HDAC1 levels, in the hippocampus (DG), suggesting that HDAC2 may be the predominant histone deacetylase in hippocampal neurons (Cassel et al., 2006). In addition, both control and HDAC1 and 2 KO cultures were treated for 24 hours with TSA to determine the effect of this broadscale HDAC inhibitor on mEPSC properties within each genetic background. Similarly to the previous TSA results, treatment with this HDAC inhibitor caused a significant decrease in mEPSC frequency in all three cultures compared to untreated controls (Figure 4-3A, B). The fact that we saw a small, but more severe, decrease in the HDAC2 KO after TSA treatment suggests that there may be additional HDACs causing this deficit, or perhaps that the knockdown of HDAC2 was not complete enough to cause the same level of decrease in event frequency. To better understand how the loss of HDAC2 may contribute to the alteration in mEPSC frequency, we examined the number of functional excitatory synapses formed onto cultured hippocampal neurons after acute knockdown of HDAC2. Neurons were immunostained for Synapsin, a presynaptic vesicle protein, and PSD-95, a postsynaptic scaffolding protein found specifically at excitatory synapses. The analysis of colocalized Synapsin and PSD-95 revealed that the number of excitatory synapses was unaffected by the loss of HDAC2, indicating that the decreases in spontaneous mEPSCs are not the result of a decreased number of excitatory inputs (Fig. 4-3D, E). 84 To determine whether the loss of either HDAC1 or HDAC2 resulted in deficits in inhibitory synaptic transmission, we measured miniature inhibitory postsynaptic currents (mIPSCs) from these neurons. The frequencies of mIPSCs were unaffected by the loss of either HDAC1 or HDAC2, suggesting a specificity for HDAC2’s function in excitatory spontaneous neurotransmission (Figure 4-4A, B). The amplitudes of individual synaptic events were also unaffected by the loss of HDAC1 and HDAC2, indicating no potential change in the number of postsynaptic receptors at inhibitory synapses (Figure 4-4C). We next examined the properties of evoked neurotransmission in response to action potential stimulation. We analyzed the amplitudes and paired pulse ratios of EPSCs evoked with a number of different stimulation frequencies from the conditional HDAC1 and HDAC2 KO cultures, as well as from constitutive MeCP2 KO neurons for comparison. Both HDAC1 and 2 KO cultures showed a significant increase in first response amplitudes and significantly smaller paired pulse ratios at the 20Hz stimulation frequency compared to controls (Figure 4-5A-C). Interestingly, these results are very similar to those seen in the constitutive MeCP2 KO neurons. MeCP2 deficient neurons showed a significantly larger first EPSC amplitude and significantly smaller paired pulse ratios at 20, 10 and 5 Hz stimulation frequencies (Figure 4-5A-C) It is not surprising that the viral-mediated knockdowns of HDAC1 and HDAC2 did not cause the same severity of deficits as the constitutive loss of MeCP2 since they were knocked down after neurodevelopment. Taken together with the decrease in spontaneous event frequency, these findings suggest a role for both HDAC1 and 2 in the presynaptic control of neurotransmitter release at excitatory synapses. 85 To be certain of this specific effect on excitatory neurotransmission, we analyzed the amplitudes and paired pulse ratios of evoked IPSCs from the HDAC1, HDAC2, and MeCP2 KO cultures. No differences in IPSC amplitudes or paired pulse ratios were seen as a result of the loss of any of these components of the transcriptional repressor complex (Figure 4-6A-C). Therefore, it appears that the loss of either HDAC1 or HDAC2 results in specific defects in excitatory synaptic function, very similar to those seen after the constitutive KO of MeCP2. These data suggest that the transcriptional repression complex containing MeCP2 and HDACs 1 or 2 acts to regulate excitatory synaptic transmission in mature neurons and that the loss of these proteins may have profound effects on synaptic function after neurodevelopment. 86 Discussion New studies are beginning to reveal a role for histone deacetylation in the central nervous system. The availability of HDAC inhibitors has allowed for simple but important discoveries concerning histone deacetylation and its implications in brain function. Treatment with these compounds can enhance long-term memory and LTP (Fischer et al., 2007; Levenson et al., 2004) and decrease spontaneous excitatory synaptic transmission in mice (Nelson et al., 2006). We have further extended these findings with the discovery that TSA can induce a faster synaptic depression of EPSCs in response to train stimulation, indicating that decreases in HDAC activity, and therefore increases in histone acetylation, can also effect basal synaptic transmission. Major concerns of these studies arise from the fact that these are nonspecific HDAC inhibitors, and they give no clear indication as to the molecular mechanisms behind the effects on synaptic function. While there has been a small amount of work done looking at the roles of Class II HDACs in animal behavior (Kumar et al., 2005; Tsankova et al., 2006), no studies have yet to explore the regulation of synaptic transmission by specific HDACs. We decided to focus our research on the Class I HDACs 1 and 2 due to their associations with the corepressor protein MeCP2 (Nan et al., 1998). MeCP2’s function in the brain is clearly significant given that mutations in the gene lead to a form of mental retardation, Rett syndrome, and that MeCP2 KO mice show deficits in spontaneous neurotransmission and short- and long-term synaptic plasticity (Dani et al., 2005; Moretti et al., 2006; Nelson et al., 2006). Interestingly, the loss of MeCP2 causes a decrease in LTP while HDAC inhibition results in enhanced LTP (Levenson et al., 2004; Moretti et al., 2006), but both are believed to result in the increased expression of certain genes important for 87 controlling synapse function. Again, these differences could be due to the nonspecificity of the HDAC inhibitors used that would result in increased transcription of a number of genes, many of which may not be regulated by MeCP2 function. To reconcile these differences, we measured spontaneous and evoked synaptic transmission in neurons lacking either HDAC1 or 2, the two HDACs known to bind MeCP2. We used a viral-mediated KO approach for these studies, which allowed us to determine the roles of these HDACs in mature synapse function, separate from any neurodevelopmental defects that might be caused by the loss of these proteins at an earlier time point. With respect to spontaneous synaptic activity, the loss of HDAC2, but not HDAC1, resulted in a decrease in the frequency of mEPSCs. This specific alteration in mEPSC frequency in the HDAC2 KO neurons suggests that HDAC2 may play more of a role than HDAC1 in the control of excitatory spontaneous transmission in hippocampal neurons. In fact, HDAC2 mRNA expression is more prevalent in the hippocampus than HDAC1 (Cassel et al., 2006). However, evoked synaptic transmission was effected by the loss of both HDACs 1 and 2, resulting in increased EPSC amplitudes and decreased paired pulse ratios at high stimulation frequencies. All of these defects were specific for excitatory synaptic activity as both miniature and evoked IPSCs were unaltered in HDAC1 or 2 KO neurons compared to controls. These results are very similar to those seen in constitutive MeCP2 KO neurons which show defects in mEPSC frequency (Nelson et al., 2006) and evoked excitatory synaptic transmission but no alterations in inhibitory synapse function. In conclusion, our findings support a role for both HDACs 1 and 2 in the control of excitatory synaptic transmission. It appears that HDAC2 may play a part in controlling 88 both spontaneous and evoked synaptic activity, while HDAC1 is more specific in only controlling evoked neurotransmission. The fact that these results occur in response to acute knockdown of HDAC expression after neurodevelopment highlights the function of these proteins in mature neurons. In addition, the loss of both HDACs closely mimicked the defects seen in MeCP2 KO neurons indicating a shared mechanism for controlling excitatory synapse function. More work needs to be done to explore this relationship and to determine if the loss of these proteins results in the increased expression of similar genes important for regulating synaptic transmission. 89 Figure 4-1. Treatment with HDAC inhibitors results in increased acetylation of H4 and defects in both spontaneous and evoked synaptic transmission. (A and B) Spontaneous miniature EPSCs recorded from neurons treated 24 hours with inhibitors of HDAC activity. (A) Both TSA and VPA treatments caused a significant decrease in the frequency of mEPSCs compared to controls (*, p<0.05). (B) No alterations in mEPSC amplitudes were seen with these drugs. (C) TSA treatment caused a significantly faster depression of evoked postsynaptic currents in response to 10 Hz field stimulation (**, p<0.01). (D and E) Immunostaining for histone H4 acetylation. (D) Images depicting an increase in fluorescence intensity after TSA and VPA treatments compared to controls. (E) HDAC inhibitor treatments resulted in significant increases of acetylated H4. 90 Figure 4-2. Quantification of HDAC1 and 2 mRNA and protein expression levels one week after infection with Cre-recombinase lentivirus. (A and B) HDAC1 and 2 mRNA measured by quantitative Real-Time PCR. (A) HDAC1 mRNA was significantly knocked down after lentiviral Cre infection in the floxed HDAC1 neurons, while the levels of HDAC1 were unchanged in HDAC2 KO cultures (***, p<0.001). (B) HDAC2 mRNA levels were significantly decreased in floxed HDAC2, but not floxed HDAC1, neurons after lentiviral infection (**, p<0.01). (C and D) Western blot analysis of HDAC1 and 2 protein expression. (C) Infection with Crerecombinase resulted in a significant decrease in HDAC1 protein in the floxed HDAC1 neurons (*, p<0.05). (D) Floxed HDAC2 neurons showed a significant knock down of HDAC2 protein levels after infection (**, p<0.01). 91 Figure 4-3. Loss of HDAC2, but not HDAC1, results in decreased frequency of spontaneous mEPSCs. (A-C) Miniature excitatory events from floxed HDAC1 or 2 neurons infected with lentivirus expressing Cre-recombinase. (A) Representative traces from infected neurons recorded in the presence of 1 μM TTX and 50 μM picrotoxin. (B) Bar graph revealing a significant decrease in mEPSC frequency from floxed HDAC2 neurons, but not floxed HDAC1 neurons, after infection with lentiviral Cre recombinase. The HDAC inhibitor, TSA, reduced mEPSC frequencies in all neurons compared to untreated controls (*, p<0.05; **, p<0.01). (C) Cumulative histogram showing no significant changes in mEPSC amplitudes. (D and E) Immunostaining of cultured neurons. (I) Dissociated neurons from control and HDAC2 knockout mice were labeled with primary antibodies to PSD-95 (blue) and Synapsin (red). (J) Bar graph depicts the number of excitatory synapses found in control and HDAC2 neurons. 92 Figure 4-4. Miniature inhibitory synaptic currents from conditional HDAC1 and 2 KO neurons. (A) Representative recordings of miniature inhibitory events from control, HDAC1, and HDAC2 knockout neurons recorded in 1 μM TTX and 10 μM NBQX. (G) Bar graph showing similar frequencies of spontaneous inhibitory events in control and floxed HDAC1 and 2 neurons after Cre infection. (H) Cumulative histogram of mIPSC amplitudes reveals no differences between either HDAC1 or 2 neurons and controls. 93 Figure 4-5. Increased evoked EPSC amplitudes and decreased paired pulse ratios in HDAC1, HDAC2, and MeCP2 KO neurons. (A) Representative traces of evoked excitatory currents recorded in 50 μM PTX at 1 Hz and 20 Hz stimulation frequencies. (B) First peak amplitudes were significantly increased in all three KO neurons compared to controls (*, p<0.05). (C) MeCP2 KO neurons showed decreased paired pulse ratios at 5, 10 and 20 Hz stimulation frequencies compared to controls, while both HDAC1 and HDAC2 neurons had reduced paired pulse ratios at 20 Hz (*, p<0.05; **, p<0.01). 94 Figure 4-6. Evoked inhibitory postsynaptic currents from HDAC1, HDAC2, and MeCP2 KO cultures. (A) Representative traces of IPSCs recorded in 10 μM NBQX. (B) First peak amplitudes of inhibitory currents were the same in all KO neurons compared with controls. (C) Paired pulse ratios recorded at numerous stimulation frequencies were unaffected by the loss of HDAC1, HDAC2, or MeCP2. 95 CHAPTER 5 CONCLUSIONS AND FUTURE DIRECTIONS An obvious role for transcriptional repression in the control of neuronal function is quickly emerging. Upon the discovery of associations between a number of neurological disorders and repressor proteins, like MeCP2 and DNMT3b, much research has begun to focus on these different components of the transcriptional repression machinery and their functions in the brain. One particular transcriptional repressor of great recent interest is MeCP2. Mutations in this gene lead to a neurodevelopmental disorder in humans called Rett syndrome (RTT), therefore much research has begun to focus on MeCP2’s role in the brain. My thesis work was concerned with delineating MeCP2’s function in the regulation of synaptic transmission. In addition, we looked at two mechanisms important for directing and mediating the transcriptional repression activity of MeCP2, DNA methylation and histone deacetylation. In order to study the effects of these repressor proteins on synapse function, we made dissociated hippocampal cultures from a number of different mouse strains and recorded postsynaptic currents using whole-cell voltage clamp electrophysiology. Overall, our findings agree significantly with what is known about the molecular interaction between MeCP2, DNA methylation, and HDACs 1 and 2. MeCP2 knockout neurons displayed deficits specifically in excitatory synaptic transmission, while showing no alterations in inhibitory activity. Interestingly, there are a number of studies suggesting an abnormal ratio of excitation/inhibition in the brains of autistic patients (Purcell et al., 2001; Rubenstein and Merzenich, 2003; Serajee et al., 2003), and since 96 RTT is considered an autism-spectrum disorder it is not unreasonable to hypothesize that something similar may be occurring in the brains of RTT patients. This specificity for excitatory synaptic transmission was also seen after manipulation of DNA methylation as well as after knockdown of HDAC1 and 2 in post-mitotic neurons. Alterations in excitatory synapse function included both defects in spontaneous neurotransmission and deficits in short-term synaptic plasticity. Interestingly, the loss of MeCP2, DNA methylation, or HDAC2 caused defects in spontaneous synaptic currents, while the loss of MeCP2 and both HDACs resulted in alterations in evoked neurotransmission. This suggests that genes important for controlling spontaneous versus evoked synaptic activity are differentially expressed depending on the components of the transcriptional repressor complex. Evidence suggests a separation of presynaptic vesicles that is dependent on whether they are released spontaneously or in response to activity (Sara et al., 2005). Perhaps understanding the molecular mechanisms behind this distinction would help determine possible genes regulated by the MeCP2 complex. The identification of MeCP2 target genes has proved to be a challenging task. Microarray analysis of RTT brains and MeCP2 KO mice revealed essentially nothing about genes whose expression levels were increased after the loss of MeCP2-dependent transcriptional repression (Colantuoni et al., 2001; Tudor et al., 2002). This has lead some to believe that MeCP2 plays alternate functional roles in neurons, such as in the regulation of RNA splicing (Young et al., 2005). The work presented in this thesis demonstrates that MeCP2’s transcriptional repression activity is responsible for the defects seen in synapse function. Future work will be directed towards identifying the MeCP2 target genes responsible for these deficits. 97 For wide-scale analysis of MeCP2 target genes, a chromatin immunoprecipitation (CHIP) assay will be utilized, followed by the identification of gene promoters using microarray or sequence specific primers. A relatively new method by which to pull down targets of MeCP2 is the use of the methyl-binding domain of the protein in CHIP experiments (Ballestar et al., 2003). To expand the list of genes to include those also affected by DNMT or HDAC inhibition, we can pull down using antibodies to methylated cytosines or particular acetylated histones. Our hope is that by making use of the CHIP technique, we can filter out many of the genes not regulated by MeCP2 and hopefully resolve the question of MeCP2’s function as a transcriptional repressor. We have also undertaken a more specified approach to identifying MeCP2 target genes. Using viral-mediated knockdown of MeCP2, we were able to attribute some of the synaptic deficits to the loss of MeCP2’s regulation of presynaptic function. Therefore, we will attempt to identify presynaptic target genes using quantitative Real-Time PCR with mRNA isolated from MeCP2-deficient neurons. We have comprised a list of possible presynaptic genes based on their known localization and function at the synaptic terminal (Figure 5-1). Real-Time PCR has been carried out on a number of these genes. In order to narrow down the list for preliminary studies, we searched for DNA sequences thought to be important for the specificity of MeCP2 to certain target genes. Previous work has implicated an association of MeCP2 with the corepressor coREST, a protein that recognizes RE1 sequences in the promoters of its target genes (Lunyak et al., 2002). We narrowed down the list by looking for RE1 sequences in the promoters of these genes. We then looked for A/T rich sequences located near CpG islands, since one study 98 suggests a need for A/T rich DNA in order to recruit MeCP2 to methylated promoters (Klose et al., 2005). Figure 5-1. List of possible presynaptic gene targets of MeCP2. Genes with RE1 sequences are highlighted as well as those containing A/T rich sequences near CpG islands. The purple * indicates genes whose expression levels were previously reported to be unaffected by the loss of MeCP2 (Asaka et al., 2006). Our Real-Time PCR results showed the significant upregulation of a couple of possible MeCP2 targets, Complexin 2 (Cplx2) and Synaptoporin (Synpor) (Figure 5-2). Not surprisingly, there were no dramatic alterations in the expression levels of any of the genes, which may help explain the inability to use microarray analysis to identify these targets. Cplx2 is an interesting candidate since Complexins have been shown to inhibit fast neurotransmitter release in response to synaptic activity and have no effect on spontaneous synaptic transmission (Reim et al., 2001; Tang et al., 2006). The fact that Cplx2 differentially regulates evoked versus spontaneous activity makes it an intriguing candidate. In addition, Cplx2 is specific for excitatory synapses, while Cplx1 is found at inhibitory synapses, and the fact that we did not see changes in Cplx1 expression further supports the specificity of MeCP2 for excitatory synapse function. Synpor, also called Synaptophysin 2, is a little more difficult to understand because its function in the brain is unknown, but we will continue to pursue it as a possible MeCP2 target gene. 99 Figure 5-2. Quantitative Real-Time PCR results from MeCP2 KO cultures. Only the mRNA expression levels of Cplx2 and Synpor were significantly increased after the loss of MeCP2. Nrxns 1 and 3 appear to decrease in the MeCP2 KO. We also looked at the expression of Neuroligins (Nlgn) and Neurexins (Nrxn) in our MeCP2 KO cultures. Although Nlgns are postsynaptic scaffolding proteins, Nlgn3 mutations have been found in some cases of autism (Jamain et al., 2003). Nlgn1 is the isoform specific for excitatory synapses and has been shown to modulate presynaptic neurotransmitter release in a retrograde manner (Futai et al., 2007). While both of these genes are promising candidates with respect to their functions and effects on the brain, we found no changes in Nlgn1 or 3 mRNA expression in MeCP2 KO neurons (Figure 52). Nrxns are the presynaptic binding partners of Nlgns. Any regulation of synaptic transmission by Nrxns would presumably be similar to that for Nlgns (Craig and Kang, 2007). Interestingly, we saw a decreasing trend in Nrxn1 and Nrxn3 expression (Figure 5-2), a change that may become significant with repeated experiments. Though this argues against Nrxns being direct targets of MeCP2 repression, they may be indirectly regulated in response to the loss of MeCP2 and may also play a role in the neurophysiological defects seen in the KO. 100 Another exciting discovery from this work is the activity-dependent demethylation of genomic DNA in mature, post-mitotic neurons. The practice of active DNA demethylation in nondividing cells is quite controversial, mainly due to the lack of convincing identification of a demethylating enzyme in mammals. Our data demonstrate that DNA demethylation indeed occurs in post-mitotic neurons and that this process can be used to regulate spontaneous synaptic transmission. Furthermore, our findings demonstrate that these DNA methylation changes are regulated by synaptic activity. This finding is intriguing because it adds to the many ways in which gene transcription can be altered in response to synaptic input. Activity-dependent gene regulation is critical for the brain to convert synaptic inputs into long-term alterations in synapse structure and function. A recent study has suggested that learning can actually drive both DNA demethylation and methylation, presumably through some activity-dependent postsynaptic signaling cascade (Miller and Sweatt, 2007). Our work directly implicates synaptic activity-driven changes in DNA methylation, possibly as a result of calcium influx through postsynaptic NMDA receptors. Finally, two of our most significant findings relate back to MeCP2 and RTT. Our viral-mediated deletions of MeCP2, as well as HDAC1 and 2, after early maturation demonstrate the importance of these proteins in the regulation of synapse function in mature neurons. These proteins were present throughout neurodevelopment but the loss of them after synapse formation resulted in synaptic deficits similar to those seen in the constitutive MeCP2 KO. These data imply that MeCP2-, along with HDAC1- and 2-, dependent transcriptional repression regulates excitatory synaptic transmission after neurodevelopment. The data also suggest that RTT is not strictly a neurodevelopmental 101 disorder and indicate the possibility that late therapeutic intervention might reverse the neurological symptoms seen in RTT patients. Along this idea, a recent study garnered a great deal of attention by demonstrating a significant reversal of the behavioral phenotypes and synaptic plasticity deficits in MeCP2 KO mice by returning MeCP2 expression to control levels (Guy et al., 2007). The authors generated a conditional knockin mouse that did not express MeCP2 until the introduction of Cre-recombinase caused the excision of a stop codon within the gene that then allowed for MeCP2 expression. Unfortunately, this study was problematical due to the method by which they manipulated MeCP2 expression, but it supports the idea that RTT is not strictly a neurodevelopmental disorder. In humans, using gene therapy to introduce MeCP2 to RTT patients, who are mosaics of normal and mutant MeCP2-containing cells, would most likely result in many of their cells expressing too much MeCP2. Both humans and mice with MeCP2 overexpression display many of the same neurological deficits as RTT patients (Collins et al., 2004; Luikenhuis et al., 2004; Van Esch et al., 2005), signifying the importance for proper control of MeCP2 expression levels. The discovery that RTT symptoms are not necessarily a result of neurodevelopmental defects puts forward the idea that treatments intended to reverse these phenotypic deficits may be successful later in life. While gene therapy to increase MeCP2 expression levels may not be the answer, it may be possible to modulate the expression levels of putative target genes of MeCP2 and ameliorate some of the symptoms. One study attempted to do this by crossing mice overexpressing BDNF with MeCP2 KO mice. The increased expression in BDNF was able to rescue some of the deficits in the KO, however they provide no data on the effects of BDNF overexpression 102 in normal wildtype mice (Chang et al., 2006). Our results showing a reversal of the mEPSC deficit seen in MeCP2 null mice using treatment with SAM could be another therapeutic avenue. The next step would be to treat MeCP2 KO mice with SAM in vivo and see if there is a rescue of any of their behavioral deficits. Interestingly, there has already been a study in human RTT patients who received folinic acid treatments. Folinic acid is the molecular precursor to methionine, among other reaction products. Folinic acid is used to convert homocysteine to methionine, which is then activated by methionine adenosyltransferase to make S-adenosylmethionine (SAM), the major methyl donor for cellular methyltransferase reactions, including those involving DNMTs. RTT patients treated with folinic acid showed improvements in social behavior and presented less stereotypical “hand-washing” movements and fewer seizures (Ormazabal et al., 2005), signifying folinic acid as a viable treatment for patients with Rett syndrome. In conclusion, these studies implicate a role for the MeCP2 transcriptional repressor complex in controlling excitatory synaptic transmission. Future work will be directed towards the identification of genes whose expression is regulated by any or all of the components of this complex. In addition, there will be continued investigation into the association of MeCP2, DNA methylation, and HDACs 1 and 2 and their combined effects on synapse function. Could the deficits caused by the loss of one protein, perhaps MeCP2, be rescued by manipulating the amounts of another component like DNA methylation or HDAC activity? Our findings support this idea and we look forward to future work aimed at answering some of these questions. 103 MATERIALS AND METHODS Cell Culture Dissociated hippocamal cultures were prepared from the brains of MeCP2 null knockout mice, (Jackson Laboratories) floxed MeCP2 mice, floxed HDAC1 mice, floxed HDAC2 mice, or C57BL/6 mice, according to previously published protocols (Kavalali et al., 1999). Briefly, whole hippocampi were dissected from the floxed mice or C57BL/6 mice on postnatal days 0-3, or MeCP2 knockout mice on postnatal day 0. Tissue was trypsinized for 10 min at 37ºC, mechanically dissociated using siliconized glass pipettes, and then plated onto matrigel-coated coverslips. For DNA methylation, RNA and protein level measurements, neurons were plated directly onto matrigel-coated 6-well plates. All experiments were done on cultures 11-22 days in vitro (DIV). Drug treatments and cell viability 24 or 48-hour treatments of hippocampal cultures were done with the following drugs: dimethyl sulfoxide (DMSO) (1:1000), Trichostatin A (TSA) (1 μM), 5-azacytidine (5azaC) (2.5 μM), Zebularine (Zeb) (50 μM), S-Adenosyl-L-methionine (SAM) (100 ug/ml), Actinomycin D (Act D) (2.5 μM), Tetrodotoxin (TTX) (1 μM), Picrotoxin (PTX) (50 μM), Vaproic Acid (VPA) (0.8mM), or 2-amino-5-phosphonopentanoic acid (AP5) (50 μM). SAM (50 ug/ml) treatment was also done for 48 hours where indicated. After drug treatments, cell viability was checked using Trypan Blue exclusion (Sigma). For the first study, the percentages of dead cells were not significantly different between control cultures (6.8±1.8% S.E.M., DMSO treated) and cultures treated with drugs (6.8±1.1% TSA and 3.5±1.8% Act-D). For the second study, the percentages of dead cells were not 104 significantly different between control cultures (7.4±1.5% S.E.M., DMSO treated) and cultures treated with DNMT inhibitors (8.1±2.4% 5azaC and 7.4±2.1% Zeb). Immunocytochemistry For the first study, dissociated hippocampal neurons were fixed for 30 min with 4% paraformaldehyde, rinsed twice with 1X PBS/Glycine, then blocked in 2% goat serum for 1 hour. The cells were then incubated with primary antibodies, anti-MAP2 monoclonal (1:200, Chemicon), and anti-synapsin polyclonal (1:1000, Synaptic Systems) overnight at 4oC. The next day the cells were washed then incubated with fluorescent secondary antibodies, goat-anti-rabbit (1:200, Molecular Probes), and goat-anti-mouse (1:200, Molecular Probes). For the second and third studies, dissociated hippocampal neurons were fixed for 2 min at room temperature in PBS with 2% formaldehyde and 2% sucrose followed by treatment with cold methanol for 10 min at -20 oC. Then, the neurons were blocked in 2% goat serum for 1 hr at room temperature. The cells were then incubated with primary antibodies, anti-PSD-95 monoclonal (1:200, Affinity Bioreagents), antiAcH4 polyclonal (1:500, Upstate), or anti-Synapsin polyclonal (1:1000, Synaptic Systems) added to 0.02% gelatin and 0.5% Triton X-100 in PBS overnight at 4oC. The next day, neurons were washed with PBS and then incubated with fluorescent secondary antibodies, goat-anti-rabbit (1:200, Molecular Probes), and goat-anti-mouse (1:200, Molecular Probes). Coverslips were mounted with Vectashield (Vector Laboratories) and neurons were visualized on a Zeiss Confocal microscope. 105 Electrophysiology Synaptic activity was recorded from hippocampal pyramidal cells using a whole-cell voltage clamp technique. Data were acquired using an Axopatch 200B amplifier and Clampex 9.0 software (Axon Instruments). Recordings were filtered at 2 kHz and sampled at 200 μsec. A modified Tyrode solution containing (in mM): 150 NaCl, 4 KCl, 2 MgCl2, 2 CaCl2, 10 Glucose, 10 HEPES, pH 7.4) was used as external bath solution for all experiments unless otherwise noted. A hypertonic Tyrode solution was made by adding 500 mM sucrose. The pipette internal solution contained (in mM): 115 CsMeSO3, 10 CsCl, 5 NaCl, 10 HEPES, 0.6 EGTA, 20 TEA-Cl, 4 Mg-ATP, 0.3 Na3GTP, pH 7.35 (300 mOsm). The pipette solution for field stimulation also contained 10 mM QX-314. Field stimulation was applied through parallel platinum electrodes immersed in the perfusion chamber delivering 20 mA pulses. Fluorescence Imaging For high potassium stimulation, synaptic boutons were loaded with FM1-43 during a 90 s incubation in Tyrode solution containing 47 mM K+. After washing with a dye-free Tyrode solution for 10 min, synaptic terminals were destained using a 90 mM K+ Tyrode solution for 90 s followed by 3 applications of 60 s (each separated by 60 s intervals). For spontaneous recycling pool experiments, boutons were loaded with FM2-10 during a 15 min incubation in Tyrode solution containing tetrodotoxin (TTX), washed for 10 min in dye-free Tyrode, then destained with Tyrode solution containing TTX for 20 min. This was followed by 3 applications of 90 mM K+ Tyrode solution for 60 s (each separated by 60 s) to release all dye from synapses. All staining and washing solutions contained 10 106 μM CNQX and 50 μM AP-5 to prevent recurrent activity. Isolated boutons were selected during the wash and fluorescence changes were measured during destaining. Images were obtained by a cooled, intensified digital CCD camera (Roper Scientific) during illumination (1 Hz and 40 ms) at 480 nm via an optical switch (Sutter Instruments). Images were acquired and analyzed using Axon Imaging software (Axon Instruments). Lentivirus Production HEK 293 cells were transfected using the Fugene 6 transfection system (Roche Molecular Biochemicals) with the expression plasmid, pFUGW or pFUGW-Cre and two helper plasmids, delta 8.9 and vesicular stomatitis virus G protein, at 3 µg of each DNA per 75 cm2 flask (Dittgen et al., 2004). After 48 hours, lentivirus containing culture medium was harvested, filtered at a 0.45-µm pore size, and immediately used for infection. Hippocampal cultures were infected at 7 DIV by adding 300 μl of viral suspension to each well and recordings were done 13-17 DIV. Titer was determined by counting the number of infected neurons per coverslip (high: >80%; low: <20%). Methylation-Sensitive Amplified Fragment Length Polymorphism Genomic DNA was extracted from hippocampal cultures (DNeasy tissue kit; Qiagen, Valencia, CA) and then 1 μg DNA was processed for fluorescent NotI-MseI MS-AFLP according to previous protocols (Yamamoto et al., 2001). DNA was digested for 2 hr at 37ºC with 10 units each of NotI and MseI. NotI adaptor sequences (5’CTCGTAGACTGCGTACC-3’ and 5’-GGCCGGTACGCAGTCTAC-3’) and MseI adaptor sequences (5’-GACGATGAGTCCTGAG-3’ and 5’-TACTCAGGACTCAT-3’) 107 were duplexed together by adding RNase-Free Duplex Buffer (IDT) and incubating at 94º C for 2 min. Then, 1.5 μL each of 50 μM NotI and 50 μM MseI adaptors were ligated to the digest product by adding 2 μL T4 DNA ligase, 4 μL 10x buffer, 11 μL H2O and incubating overnight at 16ºC. Two PCR reactions were then run. For PCR1, 5 μL 1:2.5 dilution of ligation product was added to 0.5 μL Taq Polymerase (Invitrogen), 5 μL 10x PCR buffer, 3 μL MgCl2 (25 mM), 4 μL dNTPs (2.5 mM), 1 μL formamide, 30 μL H2O and 0.75 μL of both NotI and MseI PCR1 primers (20 μM). PCR1 primer sequences: Not1 5’-GACTGCGTACCGGCCGC-3’ and Mse1 5’-GATGAGTCCTGAGTAA-3’. PCR1 program: 72ºC for 1 min, 94ºC for 2 min, 35 cycles of 94ºC for 30s, 52ºC for 1 min, and 72ºC for 2 min, then 72ºC for 7 min and 10ºC to end. For PCR2, 5 μL 1:15 dilution of PCR1 product was added to 0.25 μL Taq Polymerase (Invitrogen), 2.5 μL 10x PCR buffer, 1.5 μL MgCl2 (25 mM), 3 μL dNTPs (2.5 mM), 0.5 μL formamide, 11 μL H2O, 0.25 μL fluorescently labeled NotI PCR2 primer (20 μM), and 1 μL MseI PCR2 primer (20 μM). PCR2 primer sequences were the same for PCR1+N (A/C/T/G) added to the 3’ ends of both Not1 and MseI primers and 56-FAM custom synthesized (IDT) to the 5’ ends of each Not1 primer, giving a total of 4 Mse1+N primers and 4 labeled Not1+N primers. A total of 16 PCR2 reactions were run with different combinations of Mse1+N and Not1+N primers. PCR2 program: 72ºC for 1 min, 94ºC for 2 min, 12 cycles of 94ºC for 30s, touchdown 64ºC to 52ºC for 1 min, and 72ºC for 2 min, the 23 cycles of 94ºC for 30s, 52ºC for 1 min, and 72ºC for 2 min followed by 72ºC for 7 min and 10ºC to end. PCR products were purified by centrifugation through Sephadex G-75 beads (Sigma) 108 then 10 μL were loaded onto an ABI 377 DNA sequencer and electrophoresed according to manufacturer’s protocols. Measurement of Unmethylated DNA Genomic DNA was extracted from hippocampal cultures (DNeasy tissue kit; Qiagen, Valencia, CA) and then bisulfite modification of 0.4 μg DNA was performed (CpGenome DNA modification kit; Chemicon, Temecula, CA). Quantitative Real-Time PCR was used to determine the amount of unmethylated CpG island present in the BDNF promoter I according to previously published work (Levenson et al., 2006). Briefly, 2 μL DNA was added to 10 μL iQ SYBR Green Supermix (Bio-rad, Hercules, CA), 7 μL DEPC H2O and 1 μL of each primer. The following primers were used at 18 uM concentration: forward (5’-GGGTAGTGATTTTGGGGAGGAAGTAT-3’) and reverse (5’CAACCTCTATACACAACTAAATCCACC-3’). GAPDH primers were used as controls: forward (5’-AGGTCGGTGTGAACGGATTTG-3’) and reverse (5’TGTAGACCATGTAGTTGAGGTCA-3’). Each sample was run in triplicate. Reactions were run on an Mx3000P real-time PCR machine (Stratagene, La Jolla, CA) with the following cycling program: 95°C for 3 min, 40 cycles of 95°C for 15 s, 60°C for 1 min, and 74°C for 15 s. Detection of fluorescent products was at the end of the last step. For each sample, a ΔCt value was determined (Ct BDNF – Ct GAPDH) followed by a ΔΔCt value relative to DMSO controls (ΔCt Experimental treatment - ΔCt Control treatment). Fold changes were determined by taking 2 to the power of ΔΔCt values. PCR products were run out on an agarose gel and visualized using ethidium bromide. 109 RNA Isolation and Reverse Transcription Hippocampal cultures were washed once with PBS. Neurons were scraped from 6-well plate in 500 μL RNA STAT-60 reagent (Tel-Test, Inc., Friendswood, TX) and transferred to 1.5 mL eppendorf tubes. Tubes were incubated on ice for 5 min, then 100 μL chloroform was added, mixed thoroughly and incubated on ice for 2 min. Tubes were spun for 15 min at 12,000xg at 4°C. Upper, aqueous layer was transferred to new tubes, then 250 μL isopropanol and 4 μL linear acrylamide (Ambion, Austin, TX) was added, mixed and incubated at -80°C for 1 hour. Tubes were spun for 15 min at 12,000xg at 4°C. Pellets were washed with 1 mL 70% ethanol and then resuspended in 20 μL DEPCtreated H20. 0.8 μg RNA was brought up to a total volume of 17 μL with DEPC H20, treated with 1 μL TURBO DNase (Ambion, Austin, TX) in 2 μL DNase buffer, mixed and then incubated at 37°C for 25 min. 5 μL DNase Inactivation Reagent was mixed in for 2 min at 23°C, then pelleted by spinning at 10,000xg for 1 min and supernatant was transferred to new tubes. 2 μL each of Random Hexamers (50 ng/uL), dNTPs (10 μM), and DEPC H20 was mixed in with the samples and then incubated at 65°C for 5 min. Tubes were put on ice for 1 min, then 8 μL 5x 1st Strand Buffer (Invitrogen) and 2 μL each of DTT (0.1M) (Invitrogen), RNase OUT (Invitrogen), and Superscript III Reverse Transcriptase (Invitrogen) was mixed in and incubated for 5 min at 25°C, 60 min at 50°C, then 15 min at 70°C. Quantitative RT-PCR 1 μL cDNA was added to 10.5 μL iQ SYBR Green Supermix (Bio-rad, Hercules, CA), 7.5 μL H2O and 1 μL of each primer. The following primers were used at 10 μM 110 concentration: HDAC1 forward (5’-TCTACCGCCCTCACAAAGC-3’) and reverse (5’ACAGAACTCAAACAAGCCATCA-3’), HDAC2 forward (5’-GCGTACAGTCAAGGAGGCGG-3’) and reverse (5’-GCTTCATGGGATGACCCTGGC-3’), Syt7 forward (5’-ACGGCCACTACCCTTGAGT-3’) and reverse (5’-AAGGATTTCATGTCCAAGCCTC-3’), Cplx1 forward (5’-AGTTCGTGATGAAACAAGCCC-3’) and reverse (5’-TCTTCCTCCTTCTTAGCAGCA-3’), Cplx2 forward (5’-AAGAGCGCAAGGCGAAAC-3’) and reverse (5’-TGGCAGATATTTGAGCACTGT-3’), Syp1 forward (5’AGTGCCCTCAACATCGAAGTC-3’) and reverse (5’-CGAGGAGGAGTAGTCACCAAC-3’), Synpor forward (5’-GGCACCTTTCGGGCATTGA-3’) and reverse (5’CCTCCTGAATAGCCACCACA-3’), Rab3a forward (5’-ACCACAGAATATTACCGAGG-3’) and reverse (5’-GCATTGTCCCACGAGTAAGTTTT-3’), Nlgn1 forward (5’CTTGGGGTACTTGAGAAAGAGAC-3’) and reverse (5’-CTTGTTTGGGTATAAAGCCTCCA-3’), Nlgn3 forward (5’-TCGCCACTTATATCCAGGAGC-3’) and reverse (5’ATCCCCGTCATTATCCGCTAA-3’), Nrxn1 forward (5’-AATCTGCGTCAGGTGACAATAC-3’) and reverse (5’-GCCACCACACCGTGAATCTT-3’), Nrxn3 forward (5’AGACCCCAGAGGCTTACATCA-3’) and reverse (5’-CGTGAGTGAAGAGAATCAGGC-3’), and BDNF forward (5'-CCTGCATCTGTTGGGGAGAC-3') and reverse (5'-GCCTTGTCCGTGGACGTTTA-3'). GAPDH primers were used as controls: forward (5’-AGGTCGGTGTGAACGGATTTG-3’) and reverse (5’- TGTAGACCATGTAGTTGAGGTCA-3’). Each sample was run in triplicate. Reactions were run on an Mx3000P real-time PCR machine (Stratagene, La Jolla, CA) with the following cycling program: 95°C for 10 min, 40 cycles of 95°C for 20 s, 59°C for 30 s, and 72°C for 20 s. Detection of fluorescent products was at the end of the second step. 111 Protein Extraction and Immunoblotting Hippocampal cultures were washed once with PBS. Neurons were scraped from 6-well plate in 500 μL PBS and transferred to 1.5 mL eppendorf tubes. Tubes were spun for 5 min at 1,000xg and pellets were washed in cold PBS. Pellets were resuspended in 30 μL Lysis buffer (25 mM HEPES pH 7.9, 150 mM NaCl, 2 mM EDTA, 1mM DTT, 0.1% NP-40, 1 mM PMSF, 1 μM aprotinin, 1 μM leupeptin, 20 mM NaF, 1X protease inhibitor) and tubes were put on ice for 15 min. Incubated tubes for 1 hour at -20°C. Tissue was homogenized using a 25 gauge needle and syringe. Tubes were spun for 5 min at 15,000xg at 4°C, then supernatant was kept for protein. 10 μg protein was mixed with 5x loading dye (0.4 M Tris pH 6.8, 10% SDS, 60% glycerol, 2g bromophenol blue) and loaded onto 10% 19:1 acrylamide stacking gels (1.5 M Tris pH 8.8, 10% SDS, 10% ammonium persulfate, 0.04% TEMED). Gels were run at 100V for 1.5 hours, then protein was transferred to nitrocellulose membranes at 4°C at 100V for 1.5 hours. Membranes were blocked in 3% milk for 1 hour, then incubated in primary antibodies made in 3% milk overnight at 4°C: rabbit anti-HDAC1 (1:2000, Sigma) and rabbit antiHDAC2 (1:2000, Sigma). Membranes were washed in TBS-10% Tween for 1 hour, then incubated in secondary antibody, anti-rabbit (1:2000) in 3% milk, for 1.5 hours. Membranes were washed in TBS-10% Tween for 1 hour, then incubated in ECL-Plus reagent (Amersham Biosciences) for 5 min and exposed to film. 112 Statistical Analysis All error bars represent the standard error of the mean (S.E.M) and all data was tested for statistical significance by means of a two-tailed Student’s t test. 113 REFERENCES Abbott, L. F., and Regehr, W. G. (2004). Synaptic computation. Nature 431, 796-803. Aber, K. M., Nori, P., MacDonald, S. M., Bibat, G., Jarrar, M. H., and Kaufmann, W. E. (2003). Methyl-CpG-binding protein 2 is localized in the postsynaptic compartment: an immunochemical study of subcellular fractions. Neuroscience 116, 77-80. Ahn, S., Ginty, D. D., and Linden, D. J. (1999). A late phase of cerebellar long-term depression requires activation of CaMKIV and CREB. Neuron 23, 559-568. Akbarian, S., Chen, R. Z., Gribnau, J., Rasmussen, T. P., Fong, H., Jaenisch, R., and Jones, E. G. (2001). Expression pattern of the Rett syndrome gene MeCP2 in primate prefrontal cortex. Neurobiol Dis 8, 784-791. Alarcon, J. M., Malleret, G., Touzani, K., Vronskaya, S., Ishii, S., Kandel, E. R., and Barco, A. (2004). Chromatin acetylation, memory, and LTP are impaired in CBP+/mice: a model for the cognitive deficit in Rubinstein-Taybi syndrome and its amelioration. Neuron 42, 947-959. Amir, R., Dahle, E. J., Toriolo, D., and Zoghbi, H. Y. (2000). Candidate gene analysis in Rett syndrome and the identification of 21 SNPs in Xq. Am J Med Genet 90, 69-71. Amir, R. E., Van den Veyver, I. B., Wan, M., Tran, C. Q., Francke, U., and Zoghbi, H. Y. (1999). Rett syndrome is caused by mutations in X-linked MECP2, encoding methylCpG-binding protein 2. Nat Genet 23, 185-188. Armstrong, J., Pineda, M., Aibar, E., Gean, E., and Monros, E. (2001). Classic Rett syndrome in a boy as a result of somatic mosaicism for a MECP2 mutation. Ann Neurol 50, 692. Asaka, Y., Jugloff, D. G., Zhang, L., Eubanks, J. H., and Fitzsimonds, R. M. (2006). Hippocampal synaptic plasticity is impaired in the Mecp2-null mouse model of Rett syndrome. Neurobiol Dis 21, 217-227. Ballas, N., Battaglioli, E., Atouf, F., Andres, M. E., Chenoweth, J., Anderson, M. E., Burger, C., Moniwa, M., Davie, J. R., Bowers, W. J., et al. (2001). Regulation of neuronal traits by a novel transcriptional complex. Neuron 31, 353-365. Ballestar, E., Paz, M. F., Valle, L., Wei, S., Fraga, M. F., Espada, J., Cigudosa, J. C., Huang, T. H., and Esteller, M. (2003). Methyl-CpG binding proteins identify novel sites of epigenetic inactivation in human cancer. Embo J 22, 6335-6345. Ballestar, E., Yusufzai, T. M., and Wolffe, A. P. (2000). Effects of Rett syndrome mutations of the methyl-CpG binding domain of the transcriptional repressor MeCP2 on selectivity for association with methylated DNA. Biochemistry 39, 7100-7106. 114 Barreto, G., Schafer, A., Marhold, J., Stach, D., Swaminathan, S. K., Handa, V., Doderlein, G., Maltry, N., Wu, W., Lyko, F., and Niehrs, C. (2007). Gadd45a promotes epigenetic gene activation by repair-mediated DNA demethylation. Nature 445, 671-675. Betz, W. J., Mao, F., and Smith, C. B. (1996). Imaging exocytosis and endocytosis. Curr Opin Neurobiol 6, 365-371. Bhattacharya, S. K., Ramchandani, S., Cervoni, N., and Szyf, M. (1999). A mammalian protein with specific demethylase activity for mCpG DNA. Nature 397, 579-583. Bienvenu, T., Carrie, A., de Roux, N., Vinet, M. C., Jonveaux, P., Couvert, P., Villard, L., Arzimanoglou, A., Beldjord, C., Fontes, M., et al. (2000). MECP2 mutations account for most cases of typical forms of Rett syndrome. Hum Mol Genet 9, 1377-1384. Bito, H., Deisseroth, K., and Tsien, R. W. (1997). Ca2+-dependent regulation in neuronal gene expression. Curr Opin Neurobiol 7, 419-429. Brooks, P. J., Marietta, C., and Goldman, D. (1996). DNA mismatch repair and DNA methylation in adult brain neurons. J Neurosci 16, 939-945. Cassel, S., Carouge, D., Gensburger, C., Anglard, P., Burgun, C., Dietrich, J. B., Aunis, D., and Zwiller, J. (2006). Fluoxetine and cocaine induce the epigenetic factors MeCP2 and MBD1 in adult rat brain. Mol Pharmacol 70, 487-492. Chang, Q., Khare, G., Dani, V., Nelson, S., and Jaenisch, R. (2006). The disease progression of Mecp2 mutant mice is affected by the level of BDNF expression. Neuron 49, 341-348. Chen, R. Z., Akbarian, S., Tudor, M., and Jaenisch, R. (2001). Deficiency of methyl-CpG binding protein-2 in CNS neurons results in a Rett-like phenotype in mice. Nat Genet 27, 327-331. Chen, W. G., Chang, Q., Lin, Y., Meissner, A., West, A. E., Griffith, E. C., Jaenisch, R., and Greenberg, M. E. (2003). Derepression of BDNF transcription involves calciumdependent phosphorylation of MeCP2. Science 302, 885-889. Chih, B., Afridi, S. K., Clark, L., and Scheiffele, P. (2004). Disorder-associated mutations lead to functional inactivation of neuroligins. Hum Mol Genet 13, 1471-1477. Chih, B., Engelman, H., and Scheiffele, P. (2005). Control of excitatory and inhibitory synapse formation by neuroligins. Science 307, 1324-1328. Chung, S., Li, X., and Nelson, S. B. (2002). Short-term depression at thalamocortical synapses contributes to rapid adaptation of cortical sensory responses in vivo. Neuron 34, 437-446. 115 Clayton-Smith, J., Watson, P., Ramsden, S., and Black, G. C. (2000). Somatic mutation in MECP2 as a non-fatal neurodevelopmental disorder in males. Lancet 356, 830-832. Colantuoni, C., Jeon, O. H., Hyder, K., Chenchik, A., Khimani, A. H., Narayanan, V., Hoffman, E. P., Kaufmann, W. E., Naidu, S., and Pevsner, J. (2001). Gene expression profiling in postmortem Rett Syndrome brain: differential gene expression and patient classification. Neurobiol Dis 8, 847-865. Collins, A. L., Levenson, J. M., Vilaythong, A. P., Richman, R., Armstrong, D. L., Noebels, J. L., David Sweatt, J., and Zoghbi, H. Y. (2004). Mild overexpression of MeCP2 causes a progressive neurological disorder in mice. Hum Mol Genet 13, 26792689. Cook, D. L., Schwindt, P. C., Grande, L. A., and Spain, W. J. (2003). Synaptic depression in the localization of sound. Nature 421, 66-70. Costa, E., Chen, Y., Davis, J., Dong, E., Noh, J. S., Tremolizzo, L., Veldic, M., Grayson, D. R., and Guidotti, A. (2002). REELIN and Schizophrenia:: A Disease at the Interface of the Genome and the Epigenome. Mol Interv 2, 47-57. Craig, A. M., and Kang, Y. (2007). Neurexin-neuroligin signaling in synapse development. Curr Opin Neurobiol 17, 43-52. Dani, V. S., Chang, Q., Maffei, A., Turrigiano, G. G., Jaenisch, R., and Nelson, S. B. (2005). Reduced cortical activity due to a shift in the balance between excitation and inhibition in a mouse model of Rett syndrome. Proc Natl Acad Sci U S A 102, 1256012565. Desai, N. S., Rutherford, L. C., and Turrigiano, G. G. (1999). BDNF regulates the intrinsic excitability of cortical neurons. Learn Mem 6, 284-291. Dittgen, T., Nimmerjahn, A., Komai, S., Licznerski, P., Waters, J., Margrie, T. W., Helmchen, F., Denk, W., Brecht, M., and Osten, P. (2004). Lentivirus-based genetic manipulations of cortical neurons and their optical and electrophysiological monitoring in vivo. Proc Natl Acad Sci U S A 101, 18206-18211. Feng, J., Chang, H., Li, E., and Fan, G. (2005). Dynamic expression of de novo DNA methyltransferases Dnmt3a and Dnmt3b in the central nervous system. J Neurosci Res 79, 734-746. Fischer, A., Sananbenesi, F., Wang, X., Dobbin, M., and Tsai, L. H. (2007). Recovery of learning and memory is associated with chromatin remodelling. Nature 447, 178-182. Fischle, W., Kiermer, V., Dequiedt, F., and Verdin, E. (2001). The emerging role of class II histone deacetylases. Biochem Cell Biol 79, 337-348. 116 Fischle, W., Wang, Y., and Allis, C. D. (2003). Histone and chromatin cross-talk. Curr Opin Cell Biol 15, 172-183. Futai, K., Kim, M. J., Hashikawa, T., Scheiffele, P., Sheng, M., and Hayashi, Y. (2007). Retrograde modulation of presynaptic release probability through signaling mediated by PSD-95-neuroligin. Nat Neurosci 10, 186-195. Futscher, B. W., Oshiro, M. M., Wozniak, R. J., Holtan, N., Hanigan, C. L., Duan, H., and Domann, F. E. (2002). Role for DNA methylation in the control of cell type specific maspin expression. Nat Genet 31, 175-179. Gabbara, S., and Bhagwat, A. S. (1995). The mechanism of inhibition of DNA (cytosine5-)-methyltransferases by 5-azacytosine is likely to involve methyl transfer to the inhibitor. Biochem J 307 ( Pt 1), 87-92. Geiman, T. M., Sankpal, U. T., Robertson, A. K., Zhao, Y., Zhao, Y., and Robertson, K. D. (2004). DNMT3B interacts with hSNF2H chromatin remodeling enzyme, HDACs 1 and 2, and components of the histone methylation system. Biochem Biophys Res Commun 318, 544-555. Gemelli, T., Berton, O., Nelson, E. D., Perrotti, L. I., Jaenisch, R., and Monteggia, L. M. (2005). Postnatal loss of MeCP2 in the forebrain is sufficient to mediate behavioral aspects of Rett Syndrome in mice. Biol Psychiatry In press. Goto, K., Numata, M., Komura, J. I., Ono, T., Bestor, T. H., and Kondo, H. (1994). Expression of DNA methyltransferase gene in mature and immature neurons as well as proliferating cells in mice. Differentiation 56, 39-44. Guan, Z., Giustetto, M., Lomvardas, S., Kim, J. H., Miniaci, M. C., Schwartz, J. H., Thanos, D., and Kandel, E. R. (2002). Integration of long-term-memory-related synaptic plasticity involves bidirectional regulation of gene expression and chromatin structure. Cell 111, 483-493. Guy, J., Gan, J., Selfridge, J., Cobb, S., and Bird, A. (2007). Reversal of neurological defects in a mouse model of Rett syndrome. Science 315, 1143-1147. Guy, J., Hendrich, B., Holmes, M., Martin, J. E., and Bird, A. (2001). A mouse Mecp2null mutation causes neurological symptoms that mimic Rett syndrome. Nat Genet 27, 322-326. Haaf, T. (2006). Methylation dynamics in the early mammalian embryo: implications of genome reprogramming defects for development. Curr Top Microbiol Immunol 310, 1322. Hagberg, B., Aicardi, J., Dias, K., and Ramos, O. (1983). A progressive syndrome of autism, dementia, ataxia, and loss of purposeful hand use in girls: Rett's syndrome: report of 35 cases. Ann Neurol 14, 471-479. 117 Hajkova, P., Erhardt, S., Lane, N., Haaf, T., El-Maarri, O., Reik, W., Walter, J., and Surani, M. A. (2002). Epigenetic reprogramming in mouse primordial germ cells. Mech Dev 117, 15-23. Hansen, R. S., Wijmenga, C., Luo, P., Stanek, A. M., Canfield, T. K., Weemaes, C. M., and Gartler, S. M. (1999). The DNMT3B DNA methyltransferase gene is mutated in the ICF immunodeficiency syndrome. Proc Natl Acad Sci U S A 96, 14412-14417. Harata, N., Pyle, J. L., Aravanis, A. M., Mozhayeva, M., Kavalali, E. T., and Tsien, R. W. (2001). Limited numbers of recycling vesicles in small CNS nerve terminals: implications for neural signaling and vesicular cycling. Trends Neurosci 24, 637-643. Harikrishnan, K. N., Chow, M. Z., Baker, E. K., Pal, S., Bassal, S., Brasacchio, D., Wang, L., Craig, J. M., Jones, P. L., Sif, S., and El-Osta, A. (2005). Brahma links the SWI/SNF chromatin-remodeling complex with MeCP2-dependent transcriptional silencing. Nat Genet 37, 254-264. Hark, A. T., Schoenherr, C. J., Katz, D. J., Ingram, R. S., Levorse, J. M., and Tilghman, S. M. (2000). CTCF mediates methylation-sensitive enhancer-blocking activity at the H19/Igf2 locus. Nature 405, 486-489. Hendrich, B., and Bird, A. (1998). Identification and characterization of a family of mammalian methyl-CpG binding proteins. Mol Cell Biol 18, 6538-6547. Hendrich, B., Hardeland, U., Ng, H. H., Jiricny, J., and Bird, A. (1999). The thymine glycosylase MBD4 can bind to the product of deamination at methylated CpG sites. Nature 401, 301-304. Hermann, A., Goyal, R., and Jeltsch, A. (2004). The Dnmt1 DNA-(cytosine-C5)methyltransferase methylates DNA processively with high preference for hemimethylated target sites. J Biol Chem 279, 48350-48359. Horike, S., Cai, S., Miyano, M., Cheng, J. F., and Kohwi-Shigematsu, T. (2005). Loss of silent-chromatin looping and impaired imprinting of DLX5 in Rett syndrome. Nat Genet 37, 31-40. Hsieh, J., Nakashima, K., Kuwabara, T., Mejia, E., and Gage, F. H. (2004). Histone deacetylase inhibition-mediated neuronal differentiation of multipotent adult neural progenitor cells. Proc Natl Acad Sci U S A 101, 16659-16664. Huang, Y., Myers, S. J., and Dingledine, R. (1999). Transcriptional repression by REST: recruitment of Sin3A and histone deacetylase to neuronal genes. Nat Neurosci 2, 867872. Huppke, P., Laccone, F., Kramer, N., Engel, W., and Hanefeld, F. (2000). Rett syndrome: analysis of MECP2 and clinical characterization of 31 patients. Hum Mol Genet 9, 13691375. 118 Inano, K., Suetake, I., Ueda, T., Miyake, Y., Nakamura, M., Okada, M., and Tajima, S. (2000). Maintenance-type DNA methyltransferase is highly expressed in post-mitotic neurons and localized in the cytoplasmic compartment. J Biochem (Tokyo) 128, 315-321. Jaenisch, R., and Bird, A. (2003). Epigenetic regulation of gene expression: how the genome integrates intrinsic and environmental signals. Nat Genet 33 Suppl, 245-254. Jamain, S., Quach, H., Betancur, C., Rastam, M., Colineaux, C., Gillberg, I. C., Soderstrom, H., Giros, B., Leboyer, M., Gillberg, C., and Bourgeron, T. (2003). Mutations of the X-linked genes encoding neuroligins NLGN3 and NLGN4 are associated with autism. Nat Genet 34, 27-29. Johnson, L., Cao, X., and Jacobsen, S. (2002). Interplay between two epigenetic marks. DNA methylation and histone H3 lysine 9 methylation. Curr Biol 12, 1360-1367. Jost, J. P., Siegmann, M., Sun, L., and Leung, R. (1995). Mechanisms of DNA demethylation in chicken embryos. Purification and properties of a 5-methylcytosineDNA glycosylase. J Biol Chem 270, 9734-9739. Kalkhoven, E., Roelfsema, J. H., Teunissen, H., den Boer, A., Ariyurek, Y., Zantema, A., Breuning, M. H., Hennekam, R. C., and Peters, D. J. (2003). Loss of CBP acetyltransferase activity by PHD finger mutations in Rubinstein-Taybi syndrome. Hum Mol Genet 12, 441-450. Kaneda, M., Okano, M., Hata, K., Sado, T., Tsujimoto, N., Li, E., and Sasaki, H. (2004). Essential role for de novo DNA methyltransferase Dnmt3a in paternal and maternal imprinting. Nature 429, 900-903. Kaufmann, W. E., and Moser, H. W. (2000). Dendritic anomalies in disorders associated with mental retardation. Cereb Cortex 10, 981-991. Kavalali, E. T., Klingauf, J., and Tsien, R. W. (1999). Activity-dependent regulation of synaptic clustering in a hippocampal culture system. Proc Natl Acad Sci U S A 96, 12893-12900. Kilman, V., van Rossum, M. C., and Turrigiano, G. G. (2002). Activity deprivation reduces miniature IPSC amplitude by decreasing the number of postsynaptic GABA(A) receptors clustered at neocortical synapses. J Neurosci 22, 1328-1337. Klose, R. J., Sarraf, S. A., Schmiedeberg, L., McDermott, S. M., Stancheva, I., and Bird, A. P. (2005). DNA binding selectivity of MeCP2 due to a requirement for A/T sequences adjacent to methyl-CpG. Mol Cell 19, 667-678. Kovacs, J. J., Hubbert, C., and Yao, T. P. (2004). The HDAC complex and cytoskeleton. Novartis Found Symp 259, 170-177; discussion 178-181, 223-175. 119 Kumar, A., Choi, K. H., Renthal, W., Tsankova, N. M., Theobald, D. E., Truong, H. T., Russo, S. J., Laplant, Q., Sasaki, T. S., Whistler, K. N., et al. (2005). Chromatin remodeling is a key mechanism underlying cocaine-induced plasticity in striatum. Neuron 48, 303-314. Laherty, C. D., Yang, W. M., Sun, J. M., Davie, J. R., Seto, E., and Eisenman, R. N. (1997). Histone deacetylases associated with the mSin3 corepressor mediate mad transcriptional repression. Cell 89, 349-356. Lehnertz, B., Ueda, Y., Derijck, A. A., Braunschweig, U., Perez-Burgos, L., Kubicek, S., Chen, T., Li, E., Jenuwein, T., and Peters, A. H. (2003). Suv39h-mediated histone H3 lysine 9 methylation directs DNA methylation to major satellite repeats at pericentric heterochromatin. Curr Biol 13, 1192-1200. Leonard, H., Silberstein, J., Falk, R., Houwink-Manville, I., Ellaway, C., Raffaele, L. S., Engerstrom, I. W., and Schanen, C. (2001). Occurrence of Rett syndrome in boys. J Child Neurol 16, 333-338. Levenson, J. M., O'Riordan, K. J., Brown, K. D., Trinh, M. A., Molfese, D. L., and Sweatt, J. D. (2004). Regulation of histone acetylation during memory formation in the hippocampus. J Biol Chem 279, 40545-40559. Levenson, J. M., Roth, T. L., Lubin, F. D., Miller, C. A., Huang, I. C., Desai, P., Malone, L. M., and Sweatt, J. D. (2006). Evidence that DNA (cytosine-5) methyltransferase regulates synaptic plasticity in the hippocampus. J Biol Chem 281, 15763-15773. Lewis, J. D., Meehan, R. R., Henzel, W. J., Maurer-Fogy, I., Jeppesen, P., Klein, F., and Bird, A. (1992). Purification, sequence, and cellular localization of a novel chromosomal protein that binds to methylated DNA. Cell 69, 905-914. Luikenhuis, S., Giacometti, E., Beard, C. F., and Jaenisch, R. (2004). Expression of MeCP2 in postmitotic neurons rescues Rett syndrome in mice. Proc Natl Acad Sci U S A 101, 6033-6038. Lunyak, V. V., Burgess, R., Prefontaine, G. G., Nelson, C., Sze, S. H., Chenoweth, J., Schwartz, P., Pevzner, P. A., Glass, C., Mandel, G., and Rosenfeld, M. G. (2002). Corepressor-dependent silencing of chromosomal regions encoding neuronal genes. Science 298, 1747-1752. MacDonald, J. L., Gin, C. S., and Roskams, A. J. (2005). Stage-specific induction of DNA methyltransferases in olfactory receptor neuron development. Dev Biol 288, 461473. Martinowich, K., Hattori, D., Wu, H., Fouse, S., He, F., Hu, Y., Fan, G., and Sun, Y. E. (2003). DNA methylation-related chromatin remodeling in activity-dependent BDNF gene regulation. Science 302, 890-893. 120 Mayer, W., Niveleau, A., Walter, J., Fundele, R., and Haaf, T. (2000). Demethylation of the zygotic paternal genome. Nature 403, 501-502. McKinney, R. A., Capogna, M., Durr, R., Gahwiler, B. H., and Thompson, S. M. (1999). Miniature synaptic events maintain dendritic spines via AMPA receptor activation. Nat Neurosci 2, 44-49. Miller, C. A., and Sweatt, J. D. (2007). Covalent modification of DNA regulates memory formation. Neuron 53, 857-869. Moretti, P., Levenson, J. M., Battaglia, F., Atkinson, R., Teague, R., Antalffy, B., Armstrong, D., Arancio, O., Sweatt, J. D., and Zoghbi, H. Y. (2006). Learning and memory and synaptic plasticity are impaired in a mouse model of Rett syndrome. J Neurosci 26, 319-327. Mozhayeva, M. G., Sara, Y., Liu, X., and Kavalali, E. T. (2002). Development of vesicle pools during maturation of hippocampal synapses. J Neurosci 22, 654-665. Nan, X., Ng, H. H., Johnson, C. A., Laherty, C. D., Turner, B. M., Eisenman, R. N., and Bird, A. (1998). Transcriptional repression by the methyl-CpG-binding protein MeCP2 involves a histone deacetylase complex. Nature 393, 386-389. Nan, X., Tate, P., Li, E., and Bird, A. (1996). DNA methylation specifies chromosomal localization of MeCP2. Mol Cell Biol 16, 414-421. Nelson, E. D., Kavalali, E. T., and Monteggia, L. M. (2006). MeCP2-dependent transcriptional repression regulates excitatory neurotransmission. Curr Biol 16, 710-716. Ng, H. H., Zhang, Y., Hendrich, B., Johnson, C. A., Turner, B. M., Erdjument-Bromage, H., Tempst, P., Reinberg, D., and Bird, A. (1999). MBD2 is a transcriptional repressor belonging to the MeCP1 histone deacetylase complex. Nat Genet 23, 58-61. Noh, J. S., Sharma, R. P., Veldic, M., Salvacion, A. A., Jia, X., Chen, Y., Costa, E., Guidotti, A., and Grayson, D. R. (2005). DNA methyltransferase 1 regulates reelin mRNA expression in mouse primary cortical cultures. Proc Natl Acad Sci U S A 102, 1749-1754. Okano, M., Bell, D. W., Haber, D. A., and Li, E. (1999). DNA methyltransferases Dnmt3a and Dnmt3b are essential for de novo methylation and mammalian development. Cell 99, 247-257. Ormazabal, A., Artuch, R., Vilaseca, M. A., Aracil, A., and Pineda, M. (2005). Cerebrospinal fluid concentrations of folate, biogenic amines and pterins in Rett syndrome: treatment with folinic acid. Neuropediatrics 36, 380-385. Paroush, Z., Keshet, I., Yisraeli, J., and Cedar, H. (1990). Dynamics of demethylation and activation of the alpha-actin gene in myoblasts. Cell 63, 1229-1237. 121 Petrij, F., Giles, R. H., Dauwerse, H. G., Saris, J. J., Hennekam, R. C., Masuno, M., Tommerup, N., van Ommen, G. J., Goodman, R. H., Peters, D. J., and et al. (1995). Rubinstein-Taybi syndrome caused by mutations in the transcriptional co-activator CBP. Nature 376, 348-351. Phiel, C. J., Zhang, F., Huang, E. Y., Guenther, M. G., Lazar, M. A., and Klein, P. S. (2001). Histone deacetylase is a direct target of valproic acid, a potent anticonvulsant, mood stabilizer, and teratogen. J Biol Chem 276, 36734-36741. Purcell, A. E., Jeon, O. H., Zimmerman, A. W., Blue, M. E., and Pevsner, J. (2001). Postmortem brain abnormalities of the glutamate neurotransmitter system in autism. Neurology 57, 1618-1628. Ramchandani, S., Bhattacharya, S. K., Cervoni, N., and Szyf, M. (1999). DNA methylation is a reversible biological signal. Proc Natl Acad Sci U S A 96, 6107-6112. Reik, W., Dean, W., and Walter, J. (2001). Epigenetic reprogramming in mammalian development. Science 293, 1089-1093. Reim, K., Mansour, M., Varoqueaux, F., McMahon, H. T., Sudhof, T. C., Brose, N., and Rosenmund, C. (2001). Complexins regulate a late step in Ca2+-dependent neurotransmitter release. Cell 104, 71-81. Robertson, K. D., and Jones, P. A. (2000). DNA methylation: past, present and future directions. Carcinogenesis 21, 461-467. Robertson, K. D., and Wolffe, A. P. (2000). DNA methylation in health and disease. Nat Rev Genet 1, 11-19. Rosenmund, C., and Stevens, C. F. (1996). Definition of the readily releasable pool of vesicles at hippocampal synapses. Neuron 16, 1197-1207. Rountree, M. R., Bachman, K. E., and Baylin, S. B. (2000). DNMT1 binds HDAC2 and a new co-repressor, DMAP1, to form a complex at replication foci. Nat Genet 25, 269-277. Rubenstein, J. L., and Merzenich, M. M. (2003). Model of autism: increased ratio of excitation/inhibition in key neural systems. Genes Brain Behav 2, 255-267. Sara, Y., Virmani, T., Deak, F., Liu, X., and Kavalali, E. T. (2005). An isolated pool of vesicles recycles at rest and drives spontaneous neurotransmission. Neuron 45, 563-573. Schwartzman, J. S., Bernardino, A., Nishimura, A., Gomes, R. R., and Zatz, M. (2001). Rett syndrome in a boy with a 47,XXY karyotype confirmed by a rare mutation in the MECP2 gene. Neuropediatrics 32, 162-164. 122 Serajee, F. J., Zhong, H., Nabi, R., and Huq, A. H. (2003). The metabotropic glutamate receptor 8 gene at 7q31: partial duplication and possible association with autism. J Med Genet 40, e42. Shahbazian, M., Young, J., Yuva-Paylor, L., Spencer, C., Antalffy, B., Noebels, J., Armstrong, D., Paylor, R., and Zoghbi, H. (2002a). Mice with truncated MeCP2 recapitulate many Rett syndrome features and display hyperacetylation of histone H3. Neuron 35, 243-254. Shahbazian, M. D., Antalffy, B., Armstrong, D. L., and Zoghbi, H. Y. (2002b). Insight into Rett syndrome: MeCP2 levels display tissue- and cell-specific differences and correlate with neuronal maturation. Hum Mol Genet 11, 115-124. Shahbazian, M. D., Sun, Y., and Zoghbi, H. Y. (2002c). Balanced X chromosome inactivation patterns in the Rett syndrome brain. Am J Med Genet 111, 164-168. Shahbazian, M. D., and Zoghbi, H. Y. (2002). Rett syndrome and MeCP2: linking epigenetics and neuronal function. Am J Hum Genet 71, 1259-1272. Sutton, M. A., Wall, N. R., Aakalu, G. N., and Schuman, E. M. (2004). Regulation of dendritic protein synthesis by miniature synaptic events. Science 304, 1979-1983. Tang, J., Maximov, A., Shin, O. H., Dai, H., Rizo, J., and Sudhof, T. C. (2006). A complexin/synaptotagmin 1 switch controls fast synaptic vesicle exocytosis. Cell 126, 1175-1187. Tao, X., Finkbeiner, S., Arnold, D. B., Shaywitz, A. J., and Greenberg, M. E. (1998). Ca2+ influx regulates BDNF transcription by a CREB family transcription factordependent mechanism. Neuron 20, 709-726. Tawa, R., Ono, T., Kurishita, A., Okada, S., and Hirose, S. (1990). Changes of DNA methylation level during pre- and postnatal periods in mice. Differentiation 45, 44-48. Tsankova, N. M., Berton, O., Renthal, W., Kumar, A., Neve, R. L., and Nestler, E. J. (2006). Sustained hippocampal chromatin regulation in a mouse model of depression and antidepressant action. Nat Neurosci 9, 519-525. Tudor, M., Akbarian, S., Chen, R. Z., and Jaenisch, R. (2002). Transcriptional profiling of a mouse model for Rett syndrome reveals subtle transcriptional changes in the brain. Proc Natl Acad Sci U S A 99, 15536-15541. Turner, B. M. (2002). Cellular memory and the histone code. Cell 111, 285-291. Turner, G., Webb, T., Wake, S., and Robinson, H. (1996). Prevalence of fragile X syndrome. Am J Med Genet 64, 196-197. 123 Turrigiano, G. G., Leslie, K. R., Desai, N. S., Rutherford, L. C., and Nelson, S. B. (1998). Activity-dependent scaling of quantal amplitude in neocortical neurons. Nature 391, 892896. Van Esch, H., Bauters, M., Ignatius, J., Jansen, M., Raynaud, M., Hollanders, K., Lugtenberg, D., Bienvenu, T., Jensen, L. R., Gecz, J., et al. (2005). Duplication of the MECP2 region is a frequent cause of severe mental retardation and progressive neurological symptoms in males. Am J Hum Genet 77, 442-453. Varga-Weisz, P. D., and Becker, P. B. (1998). Chromatin-remodeling factors: machines that regulate? Curr Opin Cell Biol 10, 346-353. Virmani, T., Atasoy, D., and Kavalali, E. T. (2006). Synaptic vesicle recycling adapts to chronic changes in activity. J Neurosci 26, 2197-2206. Wan, M., Lee, S. S., Zhang, X., Houwink-Manville, I., Song, H. R., Amir, R. E., Budden, S., Naidu, S., Pereira, J. L., Lo, I. F., et al. (1999). Rett syndrome and beyond: recurrent spontaneous and familial MECP2 mutations at CpG hotspots. Am J Hum Genet 65, 15201529. Wolffe, A. P., Jones, P. L., and Wade, P. A. (1999). DNA demethylation. Proc Natl Acad Sci U S A 96, 5894-5896. Yamamoto, F., Yamamoto, M., Soto, J. L., Kojima, E., Wang, E. N., Perucho, M., Sekiya, T., and Yamanaka, H. (2001). Notl-Msell methylation-sensitive amplied fragment length polymorhism for DNA methylation analysis of human cancers. Electrophoresis 22, 1946-1956. Young, J. I., Hong, E. P., Castle, J. C., Crespo-Barreto, J., Bowman, A. B., Rose, M. F., Kang, D., Richman, R., Johnson, J. M., Berget, S., and Zoghbi, H. Y. (2005). Regulation of RNA splicing by the methylation-dependent transcriptional repressor methyl-CpG binding protein 2. Proc Natl Acad Sci U S A 102, 17551-17558. Yusufzai, T. M., and Wolffe, A. P. (2000). Functional consequences of Rett syndrome mutations on human MeCP2. Nucleic Acids Res 28, 4172-4179. Zhou, Z., Hong, E. J., Cohen, S., Zhao, W. N., Ho, H. Y., Schmidt, L., Chen, W. G., Lin, Y., Savner, E., Griffith, E. C., et al. (2006). Brain-specific phosphorylation of MeCP2 regulates activity-dependent Bdnf transcription, dendritic growth, and spine maturation. Neuron 52, 255-269. Zhu, B., Zheng, Y., Angliker, H., Schwarz, S., Thiry, S., Siegmann, M., and Jost, J. P. (2000). 5-Methylcytosine DNA glycosylase activity is also present in the human MBD4 (G/T mismatch glycosylase) and in a related avian sequence. Nucleic Acids Res 28, 4157-4165. 124 Zito, K., and Svoboda, K. (2002). Activity-dependent synaptogenesis in the adult Mammalian cortex. Neuron 35, 1015-1017. Zucker, R. S. (2005). Minis: whence and wherefore? Neuron 45, 482-484. 125 VITAE Erika Dawn Nelson was born in Kansas City, MO on July 28, 1980 to the parents of Ann Cunningham and Jon Nelson. In 1998, she graduated in the top ten percent of her class at Olathe South High School in Olathe, KS. Following graduation, she attended Southern Methodist University in Dallas, TX for two years and then transferred to the University of Kansas in Lawrence, KS in the fall of 2000. In May of 2002, she graduated from the University of Kansas with honors and distinction and was awarded a Bachelor of Science in Biology with an emphasis in Genetics. In the fall of 2002, she came back to Dallas, TX where she entered graduate school at the University of Texas Southwestern Medical Center in Dallas, TX. She did her graduate research in the lab of Dr. Lisa Monteggia, funded by the University Cell and Molecular Biology training grant. She was awarded her Doctorate of Philosophy in Neuroscience in June, 2007. Permanent address: 2502 Live Oak St. #308 Dallas, TX 75204