* Your assessment is very important for improving the work of artificial intelligence, which forms the content of this project
Download Cell migration: mechanisms of rear detachment and the formation of
Cytoplasmic streaming wikipedia , lookup
Biochemical switches in the cell cycle wikipedia , lookup
Cell encapsulation wikipedia , lookup
Cell membrane wikipedia , lookup
Programmed cell death wikipedia , lookup
Cell culture wikipedia , lookup
Endomembrane system wikipedia , lookup
Cellular differentiation wikipedia , lookup
Organ-on-a-chip wikipedia , lookup
Cell growth wikipedia , lookup
Signal transduction wikipedia , lookup
Hyaluronic acid wikipedia , lookup
Cytokinesis wikipedia , lookup
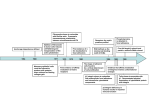
Eur. J. Cell Biol. 83 (2004); 717 ± 724 http: // www.elsevier.de/ejcb Cell migration: mechanisms of rear detachment and the formation of migration tracks Gregor Kirfel1), Alexander Rigort, Bodo Borm, Volker Herzog Institute of Cell Biology, University of Bonn, Bonn, Germany Migration tracks; Keratinocytes; Integrins; Cytoskeleton; Extracellular matrix Cell migration is central to many biological and pathological processes, including embryogenesis, tissue repair and regeneration as well as cancer and the inflammatory response. In general, cell migration can be usefully conceptualized as a cyclic process. The initial response of a cell to a migration-promoting agent is to polarize and extend protrusions in the direction of migration. These protrusions can be large, broad lamellipodia or spike-like filopodia, are usually driven by actin polymerization, and are stabilized by adhering to the extracellular matrix (ECM) via transmembrane receptors of the integrin family linked to the actin cytoskeleton. These adhesions serve as traction sites for migration as the cell moves forward over them, and they must be disassembled at the cell rear, allowing it to detach. The mechanisms of rear detachment and the regulatory processes involved are not well understood. The disassembly of adhesions that is required for detachment depends on a coordinated interaction of actin and actin-binding proteins, signaling molecules and effector enzymes including proteases, kinases and phosphatases. Originally, the biochemically regulated processes leading to rear detachment of migrating cells were thought not to be necessarily accompanied by any loss of cell material. However, it has been shown that during rear detachment long tubular extensions, the retracting fibers, are formed and that ™membrane ripping∫ occurs at the cell rear. By this process, a major fraction of integrin-containing cellular material is left behind forming characteristic migration tracks that exactly mark the way a cell has taken. 1) Corresponding author: Dr. Gregor Kirfel, Institute of Cell Biology, University of Bonn, Ulrich-Haberlandstr. 61a, D-53121 Bonn, Germany, e-mail: [email protected], Fax: 49 228 735 302. Integrins and the formation of cellsubstrate adhesions Cell migration is a complex process that leads to translocation through the co-ordinated sequence of distinct functions such as actin assembly-driven lamellipodia protrusion (Fig. 1), formation of cell-substrate adhesion at the tips of lamellipodia, actomyosin-powered contraction of the cell body, and detachment of the cell rear (Stossel, 1993; Sheetz, 1994; Lauffenburger and Horwitz, 1996; Mitchison and Cramer, 1996). Cell adhesion and attachment to extracellular matrix (ECM) is critical for cell migration and mediated by low-affinity transmembrane glycoprotein adhesion receptors including members of the heterodimeric integrin family (Buck and Horwitz, 1987; Ruoshlahti and Pierschbacher, 1987; Hynes, 1992; Sonnenberg, 1993). The integrins are heterodimers of two type I membrane proteins, the a and b chains, with large ligand-binding extracellular domains and short cytoplasmic domains. Cell-substrate adhesions are discrete transmembrane regions in which the extracellular domains of integrins are bound to ECM ligands such as fibronectin and the cytoplasmic domains are linked by bridging molecules to cytoskeletal components, either microfilaments or intermediate filaments. Microfilament-associated adhesion sites, also referred to as focal complexes and focal adhesions (Kaverina et al., 2002), are highly dynamic structures, whereas intermediate filament-associated adhesion sites, also referred to as hemidesmosomes (Yamada et al., 1996), function in the more stable anchorage of epithelial cells to their substrate (Jones et al., 1998). During migration, cell-substrate adhesions become only transiently detectable (Dunlevy and Couchman, 1993; Matsumoto et al., 1994) and they must be continuously formed at the cell front and disrupted at the cell rear for a cell to move. It has been shown that the dynamic behavior of integrins in cell-substrate adhesion structures at the rear of the cell is important in governing cell migration and that, at least in some cell types, the rate of this rear release determines the migration velocity (Chen, 1981). Thus, the rate of locomotion of Dictyostelium cells is limited by their ability to detach at the rear (Jay et al., 1995), and for Chinese hamster ovary (CHO) 0171-9335/04/083/11-12-717 $30.00/0 EJCB 718 G. Kirfel et al. ECM ligands (Fig. 2b) and their cytosolic adaptor proteins as well as proteases cleaving the adhesion complex including the cytosolic cysteine protease calpain (Fig. 2b), matrix proteases (Fig. 2c) and sheddases of the ADAM family (Fig. 2d). Contractility-promoted release Fig. 1. Scanning electron micrograph of an epidermal keratinocyte showing the characteristic polarized morphology of cells migrating on fibronectin-coated surfaces after stimulation with a motogenic mediator such as the epidermal growth factor (EGF). At the cell front numerous filopodia (white arrows) and lamellipodia (white asterisks) are formed by membrane protrusion. These membrane protrusions must adhere to the substrate for allowing transformation of cellular contraction forces into cell body translocation. At the cell rear, where the cell must detach during migration, long tubular extensions, the retracting fibers (white arrowheads), are formed. N nucleus. Black arrow direction of migration. Bar, 20 mm. cells transfected with aIIb3 integrins the rate of translocation on fibronectin-coated surfaces is approximately equal to the rate of cell rear detachment (Palecek et al., 1998). If the cytoskeletal contractility exceeds the adhesive strength of the focal contacts, contractile forces might directly promote detachment from the substrate during migration (Fig. 2a). In permeabilized cells ATP-induced contractility that usually is provided by actin-myosin interactions appears to promote the disassembly of focal adhesions (Crowley and Horwitz, 1995). Recent evidence suggests that in fibroblast cells a myosin transport might pull filaments centripetally inward (Cramer et al., 1997; Galbraith and Sheetz, 1997). In ultra-structural studies, it was found that there is a graded polarity of actin filaments, with barbed ends orientated outward at the cell front and rear, and a mixed polarity in the cell center (Cramer et al., 1997). Measurements of traction forces have shown that the direction of the forces exerted against the substrate agree with the structural information: forces are directed inward at the front and the rear of the cell and they oscillate towards the cell center. Together, these studies (Cramer et al., 1997; Galbraith and Sheetz, 1997) indicate that cytoskeletal contraction orientated centripetally generates forces that contribute to the detachment of cell adhesion sites at the cell rear. For migration to occur, cytoskeletal contractility needs to be regulated between the front and the rear of the cell. Evidence for a spatially regulated contraction comes from studies that indicate that myosin II may help the cell rear to detach from the substrate and to retract (Jay et al., 1995). Furthermore, traction forces exerted against the substrate are much higher in the rear of the cell when a thickened tail forms (Galbraith and Sheetz, 1997). This suggests that spatiotemporarily regulated contraction contributes to the process of rear detachment during cell migration. Mechanisms of rear detachment Regulation of rear detachment by transient changes of Ca2 levels Breakage of cell-substratum attachment needed to allow locomotion can, in principle, occur either by intracellular disruption of the cytoskeleton-integrin linkage (Fig. 2b) or by extracellular release of the integrin-matrix linkage (Fig. 2a, c, d). The kinetic model for integrin-mediated adhesion release during migration predicts two distinct detachment phenomena integrating the biochemical and biophysical interactions between integrins, the cytoskeleton and the ECM that affect rear retraction and linkage dissociation mechanisms (Palecek et al., 1998). In the first, detachment is extremely rapid, dominated by integrin-ECM dissociation and it occurs when high forces are generated or when adhesiveness is low. In the second, detachment is much slower, dominated by integrin-cytoskeleton dissociation occurring at low forces and high adhesiveness (DiMilla et al., 1991; Huttenlocher et al., 1995; Sheetz, 1994). Dissociation of adhesion sites depends on highly regulated events that are mediated by mechanical contraction forces (Fig. 2a) but also by a variety of tyrosine kinases and phosphatases that modulate the affinity of integrins for their Intracellular Ca2 regulates many of the molecular processes that are essential for cell movement including the production of actomyosin-based contractile forces, the regulation of the structure and the dynamics of the actin cytoskeleton, and the formation and disassembly of cell-substratum adhesions (Sjaastad and Nelson, 1997). Waves of increased intracellular free Ca2 levels have been implicated in the release of migrating neutrophils where the Ca2/calmodulin-dependent phosphatase calcineurin has been shown to be responsible for the detachment of integrin-mediated cell adhesion and the recycling of integrins to the cell front (Maxfield, 1993; Hendey et al., 1992). Besides calcineurin, the calpain proteases (see below) might also represent a direct link between the mechanism of rear detachment and cellular Ca2 levels. However, in most cases, it is still unknown how the transient and highly localized changes of Ca2 levels (Ca2 transients) are generated. In fish keratocytes Ca2 transients have been reported to arise from the activation of stretch-activated Ca2 channels, which triggers an influx of extracellular Ca2 (Lee et al., 1999). EJCB Fig. 2. Schematic illustration summarizing the different strategies of rear detachment. Mechanical forces generated by actomyosin-driven contraction are thought to contribute to the dissociation of substrate adhesions at both, cytosolic and extracellular sites (a). The cytosolic dissociation of cell-substrate adhesions can be performed by the calpain cysteine proteases, by phosphorylation/dephosphorylation of cytosolic Rear detachment by modulation of integrin affinity for their ECM ligands The binding of ECM ligands such as fibronectin to the extracellular portion of integrins leads to conformational changes in the receptors and to integrin clustering which is a prerequisite for integrin activation (Miyamoto et al., 1995). Although integrins themselves do not have any catalytic activity, signals are transmitted outside-in by activated integrins through direct and indirect interactions with many partners of integrins including direct and indirect integrin-actin crosslinkers such as talin and vinculin, tyrosine kinases such as FAK and Src family members, phosphatases and modulators of small GTPases (Schwartz and Ginsberg, 2002; Giancotti, 2000). Thus, integrin clustering can initiate intracellular signals such as tyrosine phosphorylation and the activation of small GTPases that regulate the formation and strengthening of adhesion sites Mechanisms of rear detachment during cell migration 719 adapter proteins and by posttranslational modification of integrins or adapter proteins (b). Extracellular dissociation of cell-substrate adhesions can be achieved by proteolytic cleavage of matrix constituents mediated by matrix proteases (c) or by shedding of matrix receptors such as integrins by specific sheddases leaving parts of the receptors on the substrate (d). and the organization and the dynamics of the cytoskeleton (DeMali et al., 2003; Sieg et al., 2000). Activated integrins preferentially localize to the leading edge, where new adhesions form (Kiosses et al., 2001). Integrin affinity is modulated in large part by interactions at the integrin cytoplasmic tail that trigger alterations in the conformation of the extracellular domains (Fig. 2b). The cytoskeletal linker protein talin promotes integrin activation by binding to a subset of integrin bsubunit tails and disrupting a-b-subunit tail interactions (Cram and Schwarzbauer, 2004). The binding capacity of integrins can also be modified by posttranslational modifications of the cytoplasmic domains (Fig. 2b). For example, integrin a4 phosphorylation on serine blocks the binding of paxillin, a adaptor protein involved in signaling (Liu et al., 2002). The phosphorylation of a4 integrin at the leading edge and the consequent release of bound paxillin are required to maintain stable lamellipodia of T cells migrating on ligands for integrin 720 G. Kirfel et al. a4b1 (Han et al., 2003). For neutrophils migrating on vitronectin the release of avb3 integrins and their recycling to the leading edge has been reported to depend on the activity of calcineurin, a calcium-calmodulin activated protein phosphatase type 2B (Lawson and Maxfield, 1995). Blocking of calcineurin has been shown to result in reduced neutrophil migration on vitronectin by reducing the efficiency of detachment (Hendey et al., 1992). Rear detachment by modulation of affinity between integrins and the cytosolic adaptors Besides the proteolytic cleavage of cytosolic adhesion components, their phosphorylation/dephosphorylation by kinases and phosphatases has been implicated in the mechanisms involved in rear release (Fig. 2b) (Larsen et al., 2003). In neutrophils the plasma membrane-actin cross-linker moesin has been shown to be modulated by phosphorylation/dephosphorylation in a spatiotemporal fashion (Yoshinaga-Ohara et al., 2002). Video microscopy showed that dephosphorylation of moesin preceded rear release. Calyculin A, an inhibitor of type 1 and type 2A serine/threonine phosphatases suppressed moesin dephosphorylation and impaired rear release in a dose-dependent manner (Yoshinaga-Ohara et al., 2002). Phosphorylation of moesin is thought to be regulated downstream of the small GTPase Rho since its inhibition by C3 exoenzyme impairs phosphorylation and inhibits rear release. It has also been shown that gradual loss of tyrosine phosphorylation of FAK coincided with disruption of focal adhesions and conversion to a motile phenotype (Matsumoto et al., 1994). Src and FAK tyrosine kinases are also thought to be involved in focal adhesion turnover associated with cell migration (Carragher and Frame, 2004). The selective inhibition of Src kinase has been shown to decrease the rate constants for focal adhesion disassembly (Webb et al., 2004). The role of Src in focal adhesion turnover is also shown in studies on the activation of oncogenic v-Src. Many components of focal adhesions, including b1 integrin, tensin, paxillin and FAK become highly activated upon activation of v-Src resulting in focal adhesion disassembly and cell-substrate detachment (Frame et al., 2002). In permeabilized fibroblasts, however, a rapid breakdown of focal adhesions has been observed upon the addition of ATP leading to tyrosine phosphorylation of cytoskeletal components (Crowley and Horwitz, 1995). Rear detachment by calpains ± cytosolic cysteine proteases The calpains represent a well conserved family of intracellular calcium-dependent cysteine proteases (Beckerle et al., 1987). The two ubiquitous calpains, m-calpain and M-calpain, named according to their relative requirement for Ca2 in vitro, with mcalpain requiring micromolar and M-calpain requiring millimolar levels, have been implicated in adhesion formation and rear detachment (Fig. 2b) (Bhatt et al., 2002; Huttenlocher et al., 1997). Due to this dual function calpain must be regulated differentially in a front versus rear fashion. Calpain binding to phospholipids has been shown to target calpain to the membrane and decreases the Ca2 requirement thus facilitating EJCB its activation. Adhesion-relevant targets of calpain are the adhesion complex components talin, paxillin, a-actinin, ezrin, and FAK as well as the cytoplasmic domains of b1 and b3 integrin (Du et al., 1995; Cooray et al., 1996; Perrin and Huttenlocher, 2002). Inhibition of calpain has been shown to lead to a stabilization of peripheral FA, increased adhesiveness, decreased detachment and inhibited b1 and b3 integrindependent migration. Reduced expression of calpain has been reported to be correlated with reduced migration of CHO cells. Treatment of migrating cells with pharmacological inhibitors of calpain activity and knock-out studies with calpain / fibroblasts result in impaired retraction of the cell rear, increased tail length and suppression of cell migration (Dourdin et al., 2001; Palecek et al., 1998). Calpain-mediated proteolysis has therefore been proposed as a mechanism for promoting disassembly of focal adhesion structures, leading to the turnover of integrin-dependent cell-matrix adhesion that is needed for cell locomotion (Glading et al., 2002). Rear detachment by pericellular proteolysis Recruitment of cell surface proteases such as matrix-metalloproteinases (MMP) and cathepsins, to ECM contacts sites and localized proteolysis is a key event during invasive migration of tumor cells. This pericellular proteolysis is also a crucial process during epidermal wound healing, when keratinocytes from the wound margin must move along dermal ECM at the interface between the fibrin clot and the dermal matrix, i.e., the leading keratinocytes have to proteolytically dissolve and to remodel the fibrin barrier ahead of them to create a path (Murphy and Gavrilovic, 1999). The main enzyme for fibrinolysis is plasmin derived from plasminogen within the clot. Plasmin can be activated either by tissue-type specific plasminogen activator (tPA) or urokinase-type plasminogen activator (uPA) (Ossowski and Aguirre-Ghiso, 2000). In migrating keratinocytes both activators and the receptor for uPA are up-regulated. In addition, various members of the MMP family (McCawley and Matrisian, 2001; Seiki, 2002), each of which cleaves a specific subset of matrix proteins, are also up-regulated by wound-edge keratinocytes (Grondahl-Hansen et al., 1988, Romer et al., 1994). MMP-9 can cleave collagens type IV and VII in the basement membrane and is thought to be responsible for detaching keratinocytes from their substrate (Salo et al., 1994). In addition, certain MMPs can bind to integrins, thereby providing a mechanism for localized matrix degradation (Chapman et al., 1999). For example the collagen-cleaving MMP-1 was shown to bind to a2 integrins and to be co-localized with the collagen-binding a2b1 integrin at the leading edge of migrating keratinocytes. Since MMP-1 cleavage of collagen results in the exposure of integrin-binding sites, there might be a co-operation between matrix receptors and proteinases to perform an efficient migration on collagens. More recently, the uPA receptor (Blasi, 1999, Mondino et al., 1999), a glycosyl phosphatidylinositol-anchored membrane protein (Chapman et al., 1999), was recognized as a multifunctional protein that interacts with integrins and, thereby, regulates integrin function and initiates signaling events that alter cell adhesion, migration and proliferation (Wei et al., 1996; Ossowski and AguirreGhiso, 2000; Preissner et al., 2000; Simon et al., 2000). Comparable processes of highly localized pericellular proteolysis might EJCB also contribute to the continuous detachment of the cell rear during cell migration by weakening the interaction between integrins and the ECM (Fig. 2c). Protein shedding and rear detachment Ectodomain shedding, the proteolytic release of membraneanchored cell-surface proteins, is essential for physiological and developmental events (Peschon et al., 1998; Yamamoto et al., 1999). Shedding is mediated by membrane-resident sheddases of the ADAM (a disintegrin and metalloprotease) protein family (Werb, 1998; Wolfsberg et al., 1995) and has been described for a variety of growth factors including TNF-a, TGF-a and the Alzheimer amyloid precursor protein (APP) (Black et al., 1997; Blobel, 1997; Buxbaum et al., 1998; Arribas et al., 1996). The recent finding that collagen XVII/BP 180, an epithelial adhesion molecule belonging to the group of collageneous transmembrane proteins (Banyard et al., 2003), is shed by a member of the ADAM family (Franzke et al., 2002, 2004) points to the involvement of sheddases in the process of rear detachment (Fig. 2d). Further evidence for sheddasemediated detachment strategies comes from observations showing that CD44 (Lesley et al., 1993; Lesley and Hyman, 1998), a widely expressed integral membrane glycoprotein that serves as specific adhesion receptor for the ECM glycosaminoglycan hyaluronan (Aruffo et al., 1990) is shed from the surface of fibroblasts and monocytes by members of the ADAM family (Shi et al., 2001). Shedding of CD44 has also been reported to be critical for the induction of tumor cell migration (Okamoto et al., 1999). Recently, the shedding of a 55-kDa fragment of b1 integrin has been shown for cardiac fibroblasts and myoblasts both in vitro and in vivo (Goldsmith et al., 2003). Although integrins have been shown to associate with specific members of the ADAM protein family through the disintegrin domain (Chen et al., 1999), no evidence for cleavage of an integrin by ADAM proteins has yet been reported. More recently four ADAMs have been shown to cleave the ECM proteins fibronectin and collagen IV in vitro (Alfandari et al., 2001). ADAM-mediated cleavage of ECM proteins could foster rear detachment during cell migration but it might also release growth factors, previously bound to ECM, for downstream signaling. Integrin loss and migration tracks Originally, these biochemically regulated processes were thought to facilitate rear detachment of migrating cells by a process which does not necessarily induce any loss of cell material during migration. However, for fibroblasts migrating in vitro, a large fraction of integrins has been found to be released from the cell and left on the substratum. In addition, the formation of migration tracks resulting from the release of cellular material onto glass surfaces and artificial matrices has been described for a number of cell types including fibroblasts of different organisms (Fuhr et al., 1998; Richter et al., 2000), mammalian thymoma and sarcoma cells, cytotoxic T-lymphocytes and primary chondrocytes (Zimmermann et al., 2001). Migration tracks are hardly visible by conventional light microscopy and have originally been considered matrix fibrils Mechanisms of rear detachment during cell migration 721 (Halfter et al., 1990). Later, Palecek et al. (1996) showed that fibroblasts leave behind migration tracks which contain integrin macroaggragates, i.e., integrin-containing membranous patches (Regen and Horwitz, 1992). These structures apparently result from a process which has also been described as ™membrane ripping∫ during the migration of fibroblasts (Bard and Hay, 1975; Chen, 1981; Regen and Horwitz, 1992) (for review see (Lauffenburger and Horwitz, 1996)). The formation of integrin-macroaggregates by membrane ripping is thought to depend on the gradual disruption of the actin cytoskeleton thereby inducing the fragmentation of cylindrical cell extensions into periodic chains of pearls (Bar-Ziv et al., 1999). In contrast to fibroblasts, cells migrating faster and more persistent such as keratinocytes and neutrophils were believed not to form migration tracks (Palecek et al., 1996). Recently, however, it was demonstrated that migrating keratinocytes leave behind migration tracks (Fig. 3a) containing large numbers of macroaggregates (Fig. 3c) that could be classified due to their size, distribution within the migration tracks and their origin (Kirfel et al., 2003). Type I macroaggregates are spherical and tubular structures with a diameter of about 100 nm which both are arranged like ™pearls on a string∫ and apparently derive from ripping of retracting fibers, i.e. the tubular membrane extensions at the rear end of keratinocytes (Fig. 3c). Comparable but in detail different structures had also been described for fibroblasts, lymphocytes and chondrocytes (Fuhr et al., 1998; Zimmermann et al. 2001). Dendritically organized tubular structures as described by Zimmermann et al. (2001) for these cell types were absent from keratinocyte migration tracks. The absence of these dendritic structures from keratinocyte migration tracks was assumed to be due to a migration velocity-dependent increase in the forces between cells and substrate. Accordingly, low velocity produces low forces which might favor the formation of branched structures, whereas high velocity/high forces abolish branching. Type II macroaggregates are spherical structures of about 50 nm which are left behind in form of clusters in the gaps between the retracting fibers (Fig. 3c) and were assumed to derive from the fragmentation of former hemidesmosomes. So far, there have been no reports on comparable structures for other cell types (for review see (Zimmermann et al., 2001)). By immunolabeling it was shown that type I macroaggregates contain high amounts of b1 integrin (Fig. 3b) that is a regular component of focal adhesions and part of the fibronectin and laminin receptors expressed on the surface of migrating keratinocytes. Both types of macroaggregates were shown to be linked to the substratum by regularly arranged fibrous material consisting of ECM proteins such as laminin and fibronectin (Kirfel et al., 2003). It can be concluded that the anchorage of the macroaggregate-resident integrins to ECM proteins is maintained during the rear release process thereby contributing to the formation of migration tracks. Keratinocyte macroaggregates lack, however, any cytosolic proteins including actin and the adhesion complex constituents talin and vinculin (Kirfel et al., 2003; Rigort et al., this issue). This points to a cytosolic cleavage mechanism between integrins and their adaptor proteins that might be mediated by calpain proteases or by modulators of integrin-adaptor affinity such as calcineurin. There is accumulating evidence from current investigations on keratinocytes that macroaggregates are completely membrane-covered structures that contain besides integrins a variety of other membrane proteins but also the complete set 722 G. Kirfel et al. EJCB Fig. 3. Characteristic migration tracks are formed during rear detachment of epidermal keratinocytes pointing away from the cell rear with its numerous retracting fibers (a). These tracks consist of macroaggregates containing high amounts of cellular b1 integrin as visualized by immunofluorescence (b). Transmission electron microscopy revealed that two types of macroaggregates are formed. Type I macroaggregates are tubular and spherical structures with a diameter of about 100 nm that are arranged like pearls on a string and seem to derive from membrane ripping at the tips of retracting fibers (c, arrows). Type II macroaggregates are spherical structures with a diameter of about 50 nm that are found as clusters between the type I macroaggregates (c, ovals). Bars: (a, b) 20 mm; (c) 2 mm. of membrane lipids such as cholesterol and ceramides. The release of macroaggregates might represent an additional strategy of rear detachment allowing efficient cell migration. However, it cannot be excluded that macroaggregates fulfill additional physiological tasks and might act as guiding structures for trailing cells or represent a special type of paracrine signaling machinery involved in chemotactic steering of neighboring cells. Banyard, J., Bao, L., Zetter, B. R., 2003. Type XXIII collagen, a new transmembrane collagen identified in metastatic tumor cells J. Biol. Chem. 278, 20989 ± 20994. Bard, J. B. L., Hay, E. D., 1975. The behavior of fibroblasts from the developing avian cornea. Morphology and movement in situ and in vitro. J. Cell Biol. 67, 400 ± 418. Bar-Ziv, R., Tlusty, T., Moses, E., Safran, S. A., Bershadsky, A., 1999. Pearling in cells: a clue to understanding cell shape. Proc. Natl. Acad. Sci. USA 96, 10140 ± 10145. Beckerle, M. C., Burridge, K., DeMartino, G. N., Croall, D. E., 1987. Colocalization of calcium-dependent protease II and one of its substrates at sites of cell adhesion. Cell 51, 569 ± 577. Bhatt, A., Kaverina, I., Otey, C., Huttenlocher, A., 2002. Regulation of focal complex composition and disassembly by the calcium-dependent protease calpain. J. Cell Sci. 115, 3415 ± 3425. Black, R. A., Rauch, C. T., Kozlosky, C. J., Peschon, J. J., Slack, J. L., Wolfson, M. F., Castner, B. J., Stocking, K. L., Reddy, P., Srinivasan, S., Nelson, N., Boiani, N., Schooley, K. A., Gerhart, M., Davis, R., Fitzner, J. N., Johnson, R. S., Paxton, R. J., March, C. J., Cerretti, D. P., 1997. A metalloproteinase disintegrin that releases tumournecrosis factor-a from cells. Nature 385, 729 ± 733. Blasi, F., 1999 The urokinase receptor: a cell surface, regulated chemokine. APMIS 107, 96 ± 101. Blobel, C. P., 1997. Metalloprotease-disintegrins: links to cell adhesion and cleavage of TNF-a and notch. Cell 90, 589 ± 592. References Alfandari, D., Cousin, H., Gaultier, A., Smith, K., White, J. M., Darribere, T., DeSimone, D. W., 2001. Xenopus ADAM 13 is a metalloprotease required for cranial neural crest-cell migration. Curr. Biol. 11, 918 ± 930. Arribas, J., Coodly, L., Vollmer, P., Kishimoto, T. K., Rose-John, S., Massague¬, J., 1996. Diverse cell surface protein ectodomains are shed by a system sensitive to metalloprotease inhibitors. J. Biol. Chem. 271, 11376 ± 11382. Aruffo, A., Stamenkovic, I., Melnick, M., Underhill, C. B., Seed. B., 1990. CD44 is the principal cell surface receptor for hyaluronate. Cell 61, 1303 ± 1313. EJCB Buck, C. A., Horwitz, A. F., 1987. Cell surface receptors for extracellular matrix molecules. Annu. Rev. Cell Biol. 3, 179 ± 205. Buxbaum, J. D., Liu, K.-N., Luo, Y., Slack, J. L., Stocking, K. L., Peschon, J. J., Johnson, R. S., Castner, B. J., Cerretti, D. P., Black, R. A., 1998. Evidence that tumor necrosis factor-a converting enzyme is involved in regulated a-secretase cleavage of the Alzheimer amyloid protein precursor. J. Biol. Chem. 273, 27765 ± 27767. Carragher, N. O., Frame, M. C., 2004. Focal adhesion and actin dynamics: a place where kinases and proteases meet to promote invasion. Trends Cell Biol. 14, 241 ± 249. Chapman, H. A., Wei, Y., Simon, D. I., Waltz, D. A., 1999. Role of urokinase receptor and caveolin in regulation of integrin signaling. Thromb. Haemost. 82, 291 ± 297. Chen, M. S., Almeida, E. A., Huovila, A. P., Takahashi, Y., Shaw, L. M., Mercurio, A. M., White, J. M., 1999. Evidence that distinct states of the integrin a6b1 interact with laminin and an ADAM. J. Cell Biol. 144, 549 ± 561. Chen, W. T., 1981. Mechanisms of retraction of the trailing edge during fibroblast movement. J. Cell Biol. 90, 187 ± 200. Cooray, P., Yuan, Y., Schoenwaelder, S. M., Mitchell, C. A., Salem, H. H., Jackson, P. P., 1996. Focal adhesion kinase (pp125FAK) cleavage and regulation by calpain. Biochem. J. 318, 41 ± 47. Cram, E. J., Schwarzbauer, J. E., 2004. The talin wags the dog: new insights into integrin activation. Trends Cell Biol. 14, 55 ± 57. Cramer, L. P., Siebert M., Mitchison. T. J., 1997. Identification of novel graded polarity actin filament bundles in locomoting heart fibroblasts ± implications for the generation of motile force. J. Cell Biol. 136, 1287 ± 1305. Crowley, E., Horwitz, A. F., 1995. Tyrosine phosphorylation and cytoskeleton tension regulate the release of fibroblast adhesions. J. Cell Biol. 131, 525 ± 537. DeMali, K. A., Wennerberg, K., Burridge, K., 2003. Integrin signaling to the actin cytoskeleton. Curr. Opin. Cell Biol. 15, 572 ± 582. DiMilla, P. A., Barbee, K., Lauffenburger, D. A., 1991. Mathematical model for the effects of adhesion and mechanics on cell migration speed. Biophys. J. 60, 15 ± 37. Dourdin, N., Bhatt, A. K., Dutt, P., Greer, P. A., Arthur, J. S., Elce, J. S., Huttenlocher, A., 2001. Reduced cell migration and disruption of the actin cytoskeleton in calpain-deficient embryonic fibroblasts. J. Biol. Chem., 276, 48382 ± 48388. Du, X., Saido, T. C., Tsubuki, S., Indig, F. E., Williams, M. J., Ginsberg, M. H., 1995. Calpain cleavage of the cytoplasmic domain of the integrin b3 subunit. J. Biol. Chem. 270, 26146 ± 26151. Dunlevy, J. R., Couchman, J. R., 1993. Controlled induction of focal adhesion disassembly and migration in primary fibroblasts. J. Cell Sci. 105, 489 ± 500. Frame, M. C., Fincham, V. J., Carragher, N. O., Wyke, J. A., 2002. v-Src×s hold over actin and cell adhesions. Nat. Rev. Mol. Cell Biol. 3, 233 ± 245. Franzke, C. W., Tasanen, K., Borradori, L., Huotari, V., BrucknerTuderman, L., 2004. Shedding of collagen XVII/BP180: structural motifs influence cleavage from cell surface. J. Biol. Chem. 279, 24521 ± 24529. Franzke, C. W., Tasanen, K., Sch‰cke, H., Zhou, Z., Tryggvason, K., Mauch, P., Zigrino, C., Sunnarborg, S., Lee, D. C., Fahrenholz, F., Bruckner-Tuderman, L., 2002. Transmembrane collagen XVII, an epithelial adhesion protein, is shed from the cell surface by ADAMs. EMBO J. 21, 5026 ± 5035. Fuhr, G., Richter, E., Zimmermann, H., Hitzer, H., Niehus, H., Hagedorn, R., 1998. Cell traces ± footprints of individual cells during locomotion and adhesion. Biol. Chem. 379, 1161 ± 1173. Galbraith, C., Sheetz, M., 1997. A micromachined device provides a new bend on fibroblast traction forces. Proc. Natl. Acad. Sci. USA 94, 9114 ± 9118. Giancotti, F. G., 2000. Complexity and specificity of integrin signaling. Nat. Cell Biol. 2, E13-E14. Glading, A., Lauffenburger, D. A., Wells, A., 2002. Cutting to the chase: calpain proteases in cell motility. Trends Cell Biol. 12, 46 ± 54. Goldsmith, E. C., Carver, W., McFadden, A., Goldsmith, J. G., Price, R. L., Sussman, M., Lorell, B. H., Cooper, G., Borg, T. K., 2003. Mechanisms of rear detachment during cell migration 723 Integrin shedding as a mechanism of cellular adaptation during cardiac growth. Am. J. Physiol. Heart Circ. Physiol. 284, H2227 ± H2234. Grondahl-Hansen, J., Lund, L. R., Ralfkiaer, E., Ottevanger, V., Dano, K., 1988. Urokinase- and tissue-type plasminogen activators in keratinocytes during wound reepithelialization in vivo. J. Invest. Dermatol. 90, 790 ± 795. Halfter, W., Liverani, D., Vigny, M., Monard, D., 1990. Deposition of extracellular matrix along the pathways of migrating fibroblasts. Cell Tissue Res. 262, 467 ± 481. Han, J., Rose, D. M., Woodside, D. G., Goldfinger, L. E., Ginsberg, M. H., 2003. Integrin alpha 4 beta 1-dependent T cell migration requires both phosphorylation and dephosphorylation of the alpha 4 cytoplasmic domain to regulate the reversible binding of paxillin. J. Biol. Chem. 278, 34845 ± 34853. Hendey, B., Klee, C. B., Maxfield, F. R., 1992. Inhibition of neutrophil chemotaxis on vitronectin by inhibitors of calcineurin. Science 258, 296 ± 299. Huttenlocher, A., Sandborg, R. R., Horwitz, A. F., 1995. Adhesion in cell migration. Curr. Opin. Cell Biol. 7, 697 ± 706. Huttenlocher, A., Palecek, S. P., Lu, Q., Zhang, W., Mellgren, R. L., Lauffenburger, D. A., Ginsberg, M. H., Horwitz, A. F., 1997. Regulation of cell migration by the calcium-dependent protease calpain. J. Biol. Chem. 272, 32719 ± 32722. Hynes, R. O., 1992. Integrins: versatility, modulation and signalling in cell adhesion. Cell 69, 11 ± 25. Jay, P. V., Pham, P. A., Wong, S. A., Elson, E. L., 1995. A mechanical function of myosin II in cell motility. J. Cell Sci. 108, 387 ± 393. Jones, J. C., Hopkinson, S. B., Goldfinger, L. E., 1998. Structure and assembly of hemidesmosomes. Bioessays 20, 488 ± 494. Kaverina, I., Krylyshkina, O., Small, J. V., 2002. Regulation of substrate adhesion dynamics during cell motility. Int. J. Biochem. Cell Biol. 34, 746 ± 761. Kiosses, W. B., Shattil, S. J., Pampori, N., Schwartz, M. A., 2001. Rac recruits high-affinity integrin alphavbeta3 to lamellipodia in endothelial cell migration. Nat. Cell Biol. 3, 316 ± 320. Kirfel, G., Rigort, A., Borm, B., Schulte, C., Herzog, V., 2003. Structural and compositional analysis of the keratinocyte migration track. Cell Motil. Cytoskeleton 55, 1 ± 13. Larsen, M., Tremblay, M. L., Yamada, K. M., 2003. Phosphatases in cellmatrix adhesion and migration. Nat. Rev. Mol. Cell Biol. 4, 700 ± 711. Lauffenburger, D. A., Horwitz, A. F., 1996. Cell migration: a physically integrated molecular process. Cell 84, 359 ± 369. Lawson, M. A., Maxfield, F. R., 1995. Ca(2 )- and calcineurindependent recycling of an integrin to the front of migrating neutrophils. Nature 377, 75 ± 79 Lee, J., Ishihara, A., Oxford, G., Johnson, B., Jacobson, K., 1999. Regulation of cell movement is mediated by stretch-activated calcium channels. Nature 400, 382 ± 386. Lesley, J., Hyman, R., Kincade, P. W., 1993. CD44 and its interaction with extracellular matrix. Adv. Immunol. 54, 271 ± 335. Lesley, J., Hyman, R., 1998. CD44 structure and function. Front. Biosci. 3, D616 ± 630. Liu, S., Kiosses, W. B., Rose, D. M., Slepak, M., Salgia, R., Griffin, J. D., Turner, C. E., Schwartz, M. A., Ginsberg, M. H., 2002. A fragment of paxillin binds the alpha 4 integrin cytoplasmic domain (tail) and selectively inhibits alpha 4-mediated cell migration. J. Biol. Chem. 277, 20887 ± 20894. Matsumoto, K., Nakamura, T., Kramer, R. H., 1994. Hepatocyte growth factor/scatter factor induces tyrosine phosphorylation of focal adhesion kinase (p125FAK) and promotes migration and invasion by oral squamous cell carcinoma cells. J. Biol. Chem. 269, 31807 ± 31813. Maxfield, F. R., 1993. Regulation of leukocyte locomotion by Ca . Trends Cell Biol. 3, 386 ± 391. McCawley, L. J., Matrisian, L. M., 2001. Matrix metalloproteinases: They×re not just for matrix anymore! Curr. Opin. Cell Biol. 13, 534 ± 540. Mitchison, T. J., Cramer, L. P., 1996. Actin-based cell motility and cell locomotion. Cell 84, 371 ± 379. 724 G. Kirfel et al. Miyamoto, S., Akiyama, S. K., Yamada, K. M., 1995. Synergistic roles for receptor occupancy and aggregation in integrin transmembrane function. Science 267, 883 ± 885. Mondino, A., Resnati, M., Blasi, F., 1999. Structure and function of the urokinase receptor. Thromb. Haemost. 82 (Suppl.), 19 ± 22. Murphy, G., Gavrilovic, J., 1999. Proteolysis and cell migration: creating a path? Curr. Opin. Cell Biol. 11, 614 ± 621. Okamoto, I., Kawano, Y., Tsuiki, H., Sasaki, J., Nakao, M., Matsumoto, M., Suga, M., Ando, M., Nakajima, M., Saya, H., 1999. CD44 cleavage induced by a membrane-associated metalloprotease plays a critical role in tumor cell migration. Oncogene 18, 1435 ± 1446. Ossowski, L., Aguirre-Ghiso, J. A., 2000. Urokinase receptor and integrin partnership: coordination of signaling for cell adhesion, migration and growth. Curr. Opin. Cell Biol. 12, 613 ± 620. Palecek, S. P., Schmidt, C. E., Lauffenburger, D. A., Horwitz, A. F., 1996. Integrin dynamics on the tail region of migrating fibroblasts. J. Cell Sci. 109, 941 ± 952. Palecek, S. P., Huttenlocher, A., Horwitz, A. F., Lauffenburger, D. A., 1998. Physical and biochemical regulation of integrin release during rear detachment of migrating cells. J. Cell Sci. 111, 929 ± 940. Peschon, J. J., Slack, J. L., Reddy, P., Stocking, K. L., Sunnarborg, S. W., Lee, D. C., Russell, W. E., Castner, B. J., Johnson, R. S., Fitzner J. N., Boyce, R. W., Nelson, N., Kozlosky, C. J., Wolfson, M. F., Rauch, C. T., Cerretti, D. P., Paxton, R. J., March, C. J., Black, R. A., 1998. An essential role for ectodomain shedding in mammalian development. Science 282, 1281 ± 1284. Perrin, B. J., Huttenlocher, A., 2002. Calpain. Int. J. Cell Biol. 34, 722 ± 725. Preissner, K. T., Kanse, S. M., May, A. E., 2000. Urokinase receptor: a molecular organizer in cellular communication. Curr. Opin. Cell Biol. 12, 621 ± 628. Regen, C. M., Horwitz, A. F., 1992. Dynamics of b1 integrin-mediated adhesive contacts in mobile fibroblasts. J. Cell Biol. 119, 1347 ± 1359. Richter, E., Hitzler, H., Zimmermann, H., Hagedorn, R., Fuhr, G., 2000. Trace formation during locomotion of L929 mouse fibroblasts continuously recorded by interference reflection microscopy (IRM). Cell Motil. Cytoskeleton 47, 38 ± 47. Rigort, A., Gr¸newald, J., Herzog, V., Kirfel, G., 2004. Release of integrin macroaggregates as a mechanism of rear detachment during keratinocyte migration. Eur. J. Cell Biol. 83, 725 ± 733. Romer, J., Lund, L. R., Eriksen, J., Pyke, C., Kristensen, P., Dano, K., 1994. The receptor for urokinase-type plasminogen activator is expressed by keratinocytes at the leading edge during re-epithelialization of mouse skin wounds. J. Invest. Dermatol. 102, 519 ± 522. Ruoshlahti, E., Pierschbacher, M. D., 1987. New perspectives in cell adhesion. Science 238, 491 ± 497. Salo, T., Makela, M., Kylmaniemi, M., Autio-Harmainen, H., Larjava, H., 1994. Expression of matrix metalloproteinase-2 and -9 during early human wound healing. Lab. Invest. 70, 176 ± 182. Schwartz, M. A., Ginsberg, M. H., 2002. Networks and crosstalk: integrin signalling spreads. Nat. Cell Biol. 4, E65-E68. EJCB Seiki, M., 2002. The cell surface: the stage for matrix metalloproteinase regulation of migration. Curr. Opin. Cell Biol. 14, 624 ± 632. Sheetz, M. P., 1994. Cell migration by graded attachment to substrates and contraction. Semin. Cell Biol. 5, 149 ± 155. Shi, M., Dennis, K., Peschon, J. J., Chandrasekaran, R., Mikecz, K., 2001. Antibody-induced shedding of CD44 from adherent cells is linked to the assembly of the cytoskeleton. J. Immunol. 167, 123 ± 131. Sieg, D. J., Hauck, C. R., Ilic, D., Klingbeil, C. K., Schaefer, E., Damsky, C. H., Schlaepfer, D. D., 2000. FAK integrates growth-factor and integrin signals to promote cell migration. Nat. Cell Biol. 2, 249 ± 256. Simon, D. I., Wei, Y., Zhang, L., Rao, N. K., Xu, H., Chen, Z., Liu, Q., Rosenberg, S., Chapman, H. A., 2000. Identification of a urokinase receptor-integrin interaction site: Promiscuous regulator of integrin function. J. Biol. Chem. 275, 10228 ± 10234. Sjaastad, M. D., Nelson, W. J., 1997. Integrin-mediated calcium signaling and regulation of cell adhesion by intracellular calcium. Bioessays 19, 47 ± 55. Sonnenberg, A., 1993. Integrins and their ligands. Curr. Top. Microbiol. Immunol. 184, 7 ± 26. Stossel, T. P., 1993. On the crawling of animal cells. Science 260, 1086 ± 1094. Webb, D. J., Donais, K., Whitmore, L. A., Thomas, S. M., Turner, C. E., Parsons, T. J., Horwitz, A. F., 2004. FAK-Src signalling through paxillin, ERK and MLCK regulates adhesion disassembly. Nature Cell Biol. 6, 154 ± 161. Wei, Y., Lukashev, M., Simon, D. I., Bodary, S. C., Rosenberg, S., Doyle, M. V., Chapman, H. A., 1996. Regulation of integrin function by the urokinase receptor. Science 273, 1551 ± 1555. Werb, Z., Yan, Y.,1998. A cellular striptease act. Science 282, 1279 ± 1280. Wolfsberg, T. G., Straight, P. D., Gerena, R. L., Huovila, A. P. J., Primakoff, P., Myles, D. G., White, J. M., 1995. ADAM, a widely distributed and developmentally regulated gene family encoding membrane proteins with a disintegrin and metalloprotease domain. Dev. Biol. 169, 378 ± 383. Yamada, K. M., Gailit, J., Clark, R. A. F., 1996. Integrins in wound repair, in: Clark, R. A. F. (Ed.), The Molecular and Cellular Biology of Wound Repair. Plenum Press, New York, pp. 311 ± 338. Yamamoto, S., Higuchi, Y., Yoshiyama, K., Shimizu, E., Kataoka, M., Hijiya, N., Matsuura, K., 1999. ADAM family proteins in the immune system. Immunol. Today 20, 278 ± 284. Yoshinaga-Ohara, N., Takahashi, A., Uchiyama, T., Sasada, M., 2002. Spatiotemporal regulation of moesin phosphorylation and rear release by rho and serine/threonine phosphatase during neutrophil migration. Exp. Cell Res. 278, 112 ± 122. Zimmermann, H., Richter, E., Reichle, C., Westphal, I., Geggier, P., Rehn, U., Rogaschewski, S., Bleiss, W., Fuhr, G. R., 2001. Mammalian cell traces ± morphology, molecular composition, artificial guidance and biotechnological relevance as a new type of ™bionanotube™. Appl. Phys. A 73, 11 ± 26.