* Your assessment is very important for improving the work of artificial intelligence, which forms the content of this project
Download The phosphorylation of proteins: a major mechanism for biological
Oxidative phosphorylation wikipedia , lookup
Point mutation wikipedia , lookup
Gene expression wikipedia , lookup
Clinical neurochemistry wikipedia , lookup
Expression vector wikipedia , lookup
Magnesium transporter wikipedia , lookup
Biochemical cascade wikipedia , lookup
Ancestral sequence reconstruction wikipedia , lookup
Metalloprotein wikipedia , lookup
Protein structure prediction wikipedia , lookup
Lipid signaling wikipedia , lookup
Ultrasensitivity wikipedia , lookup
Interactome wikipedia , lookup
Bimolecular fluorescence complementation wikipedia , lookup
Western blot wikipedia , lookup
Paracrine signalling wikipedia , lookup
Nuclear magnetic resonance spectroscopy of proteins wikipedia , lookup
Protein purification wikipedia , lookup
Signal transduction wikipedia , lookup
G protein–coupled receptor wikipedia , lookup
Protein–protein interaction wikipedia , lookup
Two-hybrid screening wikipedia , lookup
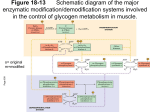
The phosphorylation of proteins: a major mechanism for biological regulation EDWIN G. KREBS Howard Hughes Medical Institute and the Department of Pharmacology, University of Washington School of Medicine, Seattle, WA 981 95,U. S. A . I am very. appreciative of the honour that has been ._ bestowed upon me by the Biochemical Society in inviting me t o give the fourteenth Hopkins Memorial Lecture. When 1 took mv first course in biochemistrv at the Universitv of Illinois i;l 1940, it was fashionabie for professors to acquaint students with the names of the great pioneers in the field. so at that time 1 became aware of some of Hopkins' contributions t o nutrition, particularly with respect t o the vitamins. Later, however, I came to realize the breadth of his work and thinking and the influence that he exerted on the development of many other aspects of biochemistry. My lecture this evening will be divided into several sections. First. I will present a brief historical account of the developnient of our present knowledge of protein phosphorylation. After that I would like to discuss the protein kinases, which constitute key elements of the protein phosphorylation-dephosphorylation machinery. Then, briefly, I will consider what is known about the effect of phosphorylation on the structure and function of proteins. This will lead into a section on the biological significance of protein phosphorylation, which is the part most closely related to the title of the lecture. Finally, I will close by attcnipting to forecast future developments in tlie field. Historv of protein phosphorvlation A textbook of biochemistry published in 1947 (Hawk ct a/.. 1947) had this to say about phosphoproteins, 'The phosphorus in these proteins is bound in ester linkage to the hydroxyamino acids, serine and threonine . . . . Examples of pliosplioproteins include casein from milk, ovovitellin from egg yolk. and other proteins associated with the feeding of the young.' The nutritional function of phosphoproteins is still considered to be important, of course, but these proteins are now known to have additional roles. One of these relates to the formation of phosphoproteins as transient intermediates involved i n the mechanism of action of certain enzymes and transport proteins: but the most noteworthy function of phosphoproteins is their role in dynamic regulatory processes within the cell. It is with the last aspect that this lecture will be conce rned. A realization that the phosphorylation and dephosphorylation of cellular proteins constitutes a major regulatory process was relatively slow in developing. Unlike the sudden and universal acceptance of allosteric control, which followed tlic exciting revelations by Monod and his co-workers (Monod etal., 1963) in the mid-l960s, a general appreciation of tlie significance of phosphorylationdephosphorylation occurred only after several decades of work carried out in a number of different laboratories. Let us go back to early studies on glycogen metabolism, which served to set the stage for the development of this field. I n tlie early 1940s the Coris' and Arda Green showed that the enzyme phosphorylase exists in two forms. which they designated as phosphorylase b and phosphorylase a (Cori & Green, 1943; Cori & Cori, 1945). Phosphorylase b Vol. 13 Fourteenth sir Frederick Gowland Hopkins Lecture Delivered on 20 December 1984 at St. Georgets Hospital Medical School, London DOCTOR EDWIN G. KREBS required high concentrations of 5'-AMP for activity and was thought to be a physiologically inactive form, whereas phosphorylase a was highly active in the absence of cofactors, and was considered t o be the active form of the enzyme. The Coris' showed that the two forms of phosphorylase are interconvertible in the intact cell, and they postulated that the interconversion reactions constituted a physiologically significant regulatory mechanism. It was difficult for them t o be very precise as t o how the regulatory system would work, because at that time the existence of a separate biosynthetic pathway for glycogen formation was unknown, and it was believed that phosphorylase catalysed glycogen biosynthesis as well as glycogen degradation. Certi Cori died during tlie 1950s, but Carl Cori passed away only this past October. As my former mentor he had a major influence on my decision to follow a career in biochemistry rather than internal medicine, and at this time I would like to pay tribute to him. Cori, like Hopkins, was one of the giants of 20th century biochemistry. His active research career overlapped that of Hopkins and those of all of us here today. An understanding of the mechanism involved in the 81 3 8 I4 BIOCHEMICAL SOCIETY TRANSACTIONS interconversion reactions of phosphorylase came approximately 10 years after their discovery, when, in the 1950s, independent work on liver phosphorylase by Earl Sutherland (also a Cori student) and muscle phosphorylase by E. H . Fischer and myself (Fischer & Krebs, 1955; Sutherland & Wosilait, 1955) showed that enzymecatalyscd protein phosphorylation-dephosphorylation reactions were involved. The enzyme catalysing the phosphorylation reaction was named phosphorylase kinase and that catalysing the dephosphorylation step was called phosphorylase phosphatase (Fig. 1). In 1959, evidence was obtained that phosphorylase kinase, like phosphorylase itself, is also regulated by phosphorylation-dephosphorylation and that cyclic AMP is somehow involved in the conversion of the non-activated form of the kinase t o a more active form (Krebs et al., 1959). In the early 1960s, Joseph Larner and his collaborators showed that the then recently discovered enzyme, glucogen synthase, constituted a third enzyme that undergoes reversible phosphorylation-dephosphorylation (Friedman & Larner, 1963). To this point the three known examples of enzyme regulation by phosphorylation-dephosphorylation, phosphorylase, phosphorylase kinase and the synthase, all involved glucogen metabolism, and it was considered by some that perhaps this was an esoteric type of control system restricted t o one limited area of carbohydrate metabolism. Preliminary reports involving possible examples of phosphorylatable enzymes in pathways other than those of glycogen metabolism appeared in the mid-1960s (Rizak, 1964; Medicino et al., 1966), but it was not until 1969, with the finding from Lester Reed’s laboratory that pyruvate dehydrogenase is regulated by phosphorylationdephosphorylation (Linn et al., 1 9 6 9 ~b. ) , that the field broke out of the more restricted area. This event more or less coincided with the finding that the enzyme that catalyses the phosphorylation and activation of phosphorylase kinase is a cyclic AMP-dependent protein kinase having a relatively broad specificity, thus making it a likely mediator for the many functions of cyclic AMP (Walsh et al., 1968; Kuo & Greengard, 1969). A period of rapid growth in the number of reports of enzymes undergoing phosphorylation ensued, and by 1984 at least 50 enzymes in this category were known. It should be emphasized, however, that some reports of enzymes ‘regulated’ by phospliorylation are based solely on work in vitro and niany of them do not meet the criteria that need to be satisfied before it is established that a given enzyme phosphorylation is physiologically significant (Krebs et al., 1985). In addition, the protein kinases themselves, essentially all of which undergo autophosphorylation reactions, are included in this number. and in most instances there is n o known Phosphorylase kinase 8 . Mq2’ A T P Phorphorylare phosphatase Phosphorylase a lacttvel physiological significance of these phosphorylations. Nonetheless, perhaps at least 20-25 enzymes can be listed tor which good evidence exists that their activities are regulated physiologically by this process. If one were t o tabulate the number of non-enzymic proteins known t o undergo dynamic pliosphorylation-dephosphorylation, the totals would be far greater than for the number of enzymes. Many of these proteins are known only by their M, values and may, in fact, turn out to be enzymes. Others, however, are well-defined non-enzymic proteins having a variety of biological functions. They arc found in virtually all parts of the cell. The protein kinascs If one considers the general process of protein phosphorylation-dephospliorylation as exemplified by the activation-inactivation cycle of phosphorylase (Fig. 1) it is clear that in order for protein phosphorylation to serve in a regulatory capacity, factors must be involved in regulating the forward and reverse reactions so that the proportion of the protein in its phosphorylated form can be varied. Regulation is exerted at the kinase level and at the phosphatase level, but in general we know more about the protein kinases and their control than we d o about the phosphatases. This picture may change, however, because a number of laboratories, most notably that of Philip Cohen in Dundee, are bringing the latter set of enzymes t o the fore. Today, however, I would like t o restrict my discussion to the protein kinascs and consider sonic of their properties. Classification of protein kinases. How many different protein kinases are there and how are they classified‘? With respect t o the latter question. the Nomenclature Committee of the International Union of Biochemistry has been struggling with the problem. and. indeed, has appointed a study panel t o make recommendations in this area. Because a given protein kinase usually catalyses the phosphorylation of a number of different proteins. as well as the fact that more often than not one protein serves as a substrate for more than one kinase. classical conventions for naming these enzymes are not applicable. As we will see in a moment, the protein kinases are usually classified and named o n the basis of what regulates their activities. As to how many protein kinases exist, I think it is much too early t o tell, but some idea as t o their number and diversity can be seen in Table 1 and Table 2. Perhaps the best-characterized protein kinases are the family of cyclic AMP-dependent kinases. These exist in several isoenzymic forms designated as type I. type II and type 11’. These enzymes share a common catalytic subunit and all of them exhibit the same specificity. There is also a cyclic GMP-dependent protein kinase. No isoenzymic forms for this kinase have been described. A very important group is made up of the Ca2+ (calmodu1in)-dependent protein kinases. These are distinct enzymes having different specificities. Of the enzymes in this category listed here, I have already mentioned phosphorylase kinase, an enzyme that is regulated not on1 by pliosphorylation~depliosphorylation but also by Cax ions. Then there are two types of myosin light chain kinase (MLCK), an enzyme first discovered by Pircs et al. (1974). Finally, there is a sonicwhat less specific Ca2+ (calmodu1in)-dependent protein kinase, which is designated as the multifunctional kinasc by many investigators. This latter enzyme was probably first detected by Schulnian & Grcengard (1978) in brain and other tissues and by Payne & Soderling (1980) as a glycogen synthase kinase. Another Ca2+ ion-dependent protein serine kinase, but one that does not utilize calmodulin, is protein kinase C, discovered by Niskizuka and co-workers (Inoue et al., 1977; Takai et al., 1977) and 1985 FOURTEENTH HOPKINS MEMORIAL LECTURE 815 Table 1 , Protein seririe (thrtwnine) kiriases and insulin-like growth factor (IGF-I) receptors. In addition t o the identified viral and cellular oncogen-encoded protein tyrosine kinases and the receptor kinases, investigators have noted the presence of protein tyrosine kinases in various animal tissues such as spleen and liver (Swarup etal., 1983; Wong & Goldber. 1983). Structural homologies betweeti proteiri kinases. AS a collaborative project involving several laboratories at the University of Washington, including those of Kenneth A. Walsh, Koiti Titani, Edniond H. Fischer and my own, the primary structures of a group of protein serine kinases are being examined. Today I would like t o summarize the results that have been obtained. The protein kinases that we have been interested in, each o f which has been sequenced at least in part, are shown in Table 3. They include type I and type I1 cyclic AMP-dependent protein kinases, cyclic GMP-dependent protein kinase, phosphorylase kinase and skeletal muscle MLCK. The cyclic AMP-dependent protein kinases are made up o f distinct cyclic AMP-binding regulatory and catalytic subunits, and have the quaternary structure R 2 C 2 . The Mr values of the several isoenzymic forms of the regulatory subunit (R) are all about 4 5 0 0 0 ; the catalytic subunit, which is identical for all types of cyclic AMP-dependent protein kinases, has an M, of 40000. When activated by cyclic AMP the inactive holoenzyme form undergoes dissociation t o yield the active catalytic subunit. For the closely related cyclic GMP-dependent protein kinase the regulatory and catalytic domains are on the same polypeptide chain, and this enzyme is activated simply by binding cyclic GMP. These relationships for the cyclic nucleotide-dependent kinases are shown in Fig. 2 . In this model the catalytic subunits of the cyclic AMPdependent protein kinase are shaded, as are the catalytic domains of the cyclic GMP-dependent protein kinase chains. Regulatory subunits or regulatory domains are unshaded. On binding of cyclic nucleotides dissociation and activation of the inactive holoen~yme occurs with the cyclic AMP-dependent kinase, but with the cyclic GMPdependent enzyme actication occurs without dissociation. In each case 2 mol of cyclic nucleotide are bound per mol of regulatory subunit or regulatory domain. Corbin ct a / . (1978) were the first t o show the correct stoichiometry for cyclic nucleotide binding to these enzymes. Phosphorylase kinase (Table 2 ) is a large molecule made up of four different types of subunits. This enzyme has an a4/34y464structure. The y-subunit is the catalytic subunit of this kinase; its M, value is 40 000. the same as that of the catalytic subunit of the cyclic AMP-dependent protein kinase. The 6-subunit is calmodulin. as shown by Cohen e l al. (1978). The a- and /3-subunits are involved in the activation of the enzyme by phosphorylation-dephosphorylation. In addition t o activation as a result of the binding of Regulatory agent(s) Cyclic AMP Cyclic GMP Ca2+(calmodulin) Diacylglycerol (Ca2+, phospholipid) Double-stranded RNA Haemin AcctylCoA. N A D H , pyruvate, ADP Unknown or nonexistent Specific enzymes Type I , type 11, and Type 11' cyclic AMP-dependent protein kinases Cyclic GMP-dependent protein kinase Phosphorylase kinasc Skeletal muscle MLCK Smooth muscle MLCK Multifunctional Ca2+ (ca1modulin)-dependen t protein kinase Protein kinase C eIF2 kinase 1 elF2 kinase 2 Pyruvic dehydrogenase kinase Casein kinase I Casein kinase 11 Glycogen synthase kinases 3 and4 Growth-associated protein kinase Others very much in the news nowadays (for a review, see Nishizuka, 1983). The principle effector controlling the activity of protein kinase C is diacylglycerol. Phorbol esters have been shown t o mimic diacylglycerol in this respect (Castagna ct al., 1982). There are other protein serine kinases regulated by various effectors including doublestranded RNA, haeniin, and metabolites involved in the pyruvate dehydrogenase reaction. Finally, there is a fairly large group of protein serine kinases for which no regulatory agents are known. These so-called 'independent' protein kinases include those listed in Table 1 as well as a number of others. It is not unlikely that regulators for iiiany of the enzymes in this group will eventually be discovered, o r it may turn out that these enzymes are regulated by other kinases. I n 1979 it was recognized that there are protein kinases (Table 2 ) that catalyse the phosphorylation of tyrosine residues i n proteins (for a review, see Sefton & Hunter, 1984). The first of thcm to be described was pp6OSm, the protein product of the Rous sarcoma oncogene, src. Later i t was found that at least six other viral oncogenes also encode protein tyrosine kinases, as d o their cellular counterparts. All of the enzymes in this group are struc~ . major group o f turally related t o the ~ ~ 6 0A" second protein tyrosine kinases consist of a set of hormones o r growth factor receptors, four of which are currently known; the enzynies in this group are the insulin, epidermal growth factor (EGF), platelet-derived growth factor (PDGF) Table 3. Propcrties of protein kinases Quaternary structure Mechanism of activation by effectors K,C, Dissociation Table 2. Protein tyrosine kiriusrs Regulatory agent(s) Specific enzymes Enzyme Mr ~~ Insulin FG I: PDGI: IGF-I Unknown or nonexistent Vol. 13 Insulin receptor EGF receptor PDGF receptor IGF-I-recep tor pp6V" and related oncogeneencoded kinases Tissue kinases Cyclic AMP-dependent protein kinase Cyclic GMP-dependent protein kinase Phosphorylase kinase MLCK 1.7 x l o s 1.6 x l o 5 Jlomodimer Binding 1.3 x 10' Binding o(qp4y.164 7 X lo4 Monomer Binding 816 BIOCHEMICAL SOCIETY TRANSACTIONS Cylic A M P h (Inactive) .4 Cyclic GMP- c- Fig. 2 . Activation of the qrclic AMP- and cyclic CMPdrpcndent protcin kinases Shaded portions represent catalytic subunits or domains. Non-shaded portions represent regulatory subunits or dorn ains. Ca2+ ions t o the endogenous calmodulin, this enzyme can bc further activated through the binding of additional Ca*+--calmodulin complex. No dissociation of phosphorylase kinase occurs when it is activated. Skeletal muscle MLCK is a simple monomeric enzyme having an M, of about 7 0 0 0 0 , which is activated as a result of binding the Ca'+-calmodulin complex. Let us look first at the cyclic nucleotide-binding domains of the cyclic AMP-dependent and cyclic GMPdependent protein kinases, which I will hereafter refer t o simply as the cA and CC kinases respectively (Fig. 3). In Fig. 3 bovine cG kinase has arbitrarily been chosen as the basis for sequence comparison with the other proteins (Takio et aL, 1984). This enzyme is depicted diagrammatically as a linear bar. The two cyclic nucleotide-binding domains are indicated by the shaded sections labelled A and B. The bars under the CC kinase represent the regulatory 85 cGK I 04 cAKRI I V A 8 10 0 13 8 / / / / / subunits of bovine types I and type I1 cA kinase and the bars under them the cyclic AMP-binding protein from Escheridria coli known as CAP, i.e. the catabolite gene activator protein. The numbers in Fig. 3 refer t o alignment scores, which serve as a useful index for indicating amino acid sequence homology (Dayhoff, 1983; Reimann et al., 1984). Without going into the derivation of these scores, suffice it t o say that an alignment score of 2 corresponds t o a probability o f 0.023 that random chance would lead t o a higher score. A score of 3 corresponds t o a probability o f 0.0014. Higher scores are clearly indicative of very significant homology. What has been done in Fig. 3 is t o use the second (B) cyclic GMP-binding donlain of the CG kinase as a basis for comparison, first with the other cyclic GMP-binding domain in the same polypeptide chain (A) and then with the cyclic AMP-binding sites in the other proteins. A score of 8.5 for domain A as compared with B indicates strong homology between the two domains in the cG kinase. Aligment scores of 13.8 and 10.2 show an even higher degree of homology of this segment with the corresponding segments of type I and type I1 R. Lesser, but still strong, homology of this domain with the first cyclic AMP-binding sites in R, and R!, is also apparent. Interestingly, the cyclic nucleotide-binding sites in the mammalian protein kinases are homologous t o the cyclic AMP-binding domain in C A P (Weber et al., 1982). This homology is greater when the B domain of CG kinase is used as a basis for calculating the scores rather than the A domain. Turning now t o the relationship that exists between the structures of the catalytic subunits or catalytic domains of protein kinases, we find that all of those that have been sequenced t o date are homologous. In Fig. 4 the catalytic subunit of the cA kinase is used as the basis for calculating alignment sources. The subunit has been divided more or less arbitrarily into three segments, each of which is compared with segments of other proteins lined up beneath it. Alignment scores of 17.3, 22.9 and 13.2 indicate very strong homology with the corresponding segments of the catalytic domain of the c C kinase (Takio et al., 1984). The catalytic or y-subunit of rabbit muscle phosphorylase kinase, sequenced by Erwin Reimann while on sabbatical leave in Kenneth Walsh's laboratory, is homologous t o the catalytic subunit throughout part of its structure but not in its C-terminal portion (Reimann et al., 1984). The catalytic domian of rabbit skeletal muscle MLCK, which resides in the C-terminal half of that molecule, is homologous to the catalytic subunit (K. Takio, D. K . Z c A K catalytic subunit cAK RI' 1 05 10 2 81 V / , Z / 4 1 17 3 cGK 62 I W 13 2 229 / i Z l 1 1 CAP 20 3 48 06 Phosphorylase kinase catalytic subunll ~ 1 1 1 30' 1 -09' CAP MLCK v I nucleotide-binding domains of' various proteins The second cyclic GMP-binding domain (B) of the cyclic GMP-dependent protein kinase (cGK) is compared with the first binding domain (A) of the same enzyme and with cyclic AMP-binding domains in other proteins. RI and RII refer t o type 1 and type 2 regulatory subunit of the cyclic AMPdependent protein kinase, respectively. CAP refers t o the catabolite gene activator protein. The numbers are Dayhoff alignment scores. *Alignment scores relevant t o segment A of cG kinase. P P E ~ " 'r I p 64 V 0 3 13 0 66 Fig. 3 . A mi n o acid sequence homologies between cyclic 94 / / 21 2 Fig. 4.Amino acid sequence homologics hctwivn catal.vtic domains of'various protein kinases cAK, catalytic subunit of cyclic AMP-dependent protein kinase; cGK, cyclic GMP-dependent protein kinase. MLCK, skeletal muscle myosin light chain kinase. The numbers are Dayhoff aligment scores. Segments of cAK are compared with segments of other proteins immediately under them. 1985 FOURTEENTlI HOPKINS MEMORIAL LECTURE 817 Blumenthal, A. M., Edelman, K. A. Walsh, E. G. Krebs, & K . Titani, unpublished work). Again, however, this homology is lost in the C-terminal portion. Finally, amino acid sequence homology exists between the catalytic subunit of tlie cA kinase and pp6OSm (Barker & Dayoff, 1982). Not shown are comparisons with other oncogeneencoded protein tyrosine kinases or the EGF receptor. However, inasmuch as all of the protein tyrosine kinases whose sequences are known show homology with pp6Oflcc, they are all related to the catalytic subunit (reviewed in Sefton & Hunter, 1984). It can be anticipated that tlie insulin receptor will also fall into this category. Phosphorylase kinase and MLCK are both regulated by CaZ+ and calmodulin, whereas the cA and cG kinases are regulated by cyclic nucleotides. It was hoped at first that a unique sequence, common to the first pair of en7ynies but lacking in the second, might stand out to help in the identification o f the calmodulin-binding site on these enLynies. At first glance this did not occur, although a very low degree of homology (alignment of 2.4) was found between the C-terminal segments of the y-subunit of phosphorylase kinase and MLCK. Other approaches were successful, however, in defining and localizing a calmodulin-binding site on the latter enzyme. If skeletal muscle MLCK is partially degraded by using proteases one can obtain catalytically active fragments, as has also been demonstrated by Mayr & Heilmayer (1983). The size and location in the molecule of some of the fragments generated by chyniotrypsin and trypsin are shown in Fig. 5. With chymotrypsin a fragment is obtained that is calniodulin-independent and has a molecular mass of about 35 000 daltons. This fragment contains the catalytic domain (shaded), i.e. the protein homologous t o the catalytic subunit of the cA kinase, as well as having small portions which show n o honiology with the catalytic subunit of the cyclic AMP-dependent protein kinase. With trypsin one generates catalytically active calmodulin-dependent fragments having molecular masses of 40000 and 60000 daltons. These fragments extend almost all the way t o the C-terminus of MLCK and also have regions beyond the catalytic domain that extend toward the N-terminus. Based on these findings. it was tempting t o postulate that the calmodulin-binding site in MLCK resides somewhere in the non-catalytic portions of the tryptic fragments that are missing in the chymotryptic fragment, i.e. either at their N-terminal or C-terminal ends. The latter turned out to be the case. Localization and definition of the calniodulin-binding site was finally determined by testing the ability of CNBr C 3 51K T40K T-60 K [ I I -& " I A/ -V M-13 0 Fig. 5. Frugmcnts derived from skeletal muscle M L C K Upper bar represents intact MLCK in which the shaded portion is that part of the molecule homologous with the catalytic subunit of the cyclic AMP-dependent protein kinase. C-35K, an M,-35 000 fragment generated by using ehymotrypsin; T-40K, a n M,40 000 fragment generated by using trypsin; C-60K, an M,-60 000-fragment generated by using trypsin; M-13, the C-terminal peptide from MLCK generated by using cyanogen bromide. Vol. 13 fragments of MLCK to inhibit MLCK activity under conditions in which added calmoddin is limiting. When a total CNBr digest of non-proteolysed MLCK was examined it was found to be inhibitory at low molar concentrations, halfmaximal inhibition inhibition occurring at a molarity of 4-5 nM, based o n the original MLCK concentration. A digest of a mixture of the 40 and 60 kilodalton tryptic fragments was similarly inhibitory. By contrast, the CNBr digest of the calmodulin-independent 35 kilodalton fragment showed a marked reduction in its ability t o inhibit activity, and the slight inhibition that was seen could have been due to a low level of contamination of the calmodulinindependent fragment with dependent fragment(s). When CNBr fragments from MLCK were exaniined individually, only one of them, fragment 13, consisting of the C-terminal 27 amino acids of the enLyme, was found to be inhibitory. Inhibition by this fragment, M-13, was only slightly less than that of the digest of intact MLCK. The adjacent fragment, M-12, or a mixture of M-l 1 and M-12, was not inhibitory. Donald Blumenthal from any laboratory has synthesized a peptide corresponding to M-13 and has determined that it is even more potent in inhibiting MLCK activity than M-13 generated by CNBr digestion. The lower inhibitory potency of M-13 relative to the synthetic peptide is probably due to modification of sensitive amino acid side chains in the peptide by the conditions used for CNBr digestion. Drs Blumenthal, Edelman, Takio and others involved in this work will be reporting on thc structure and properties of the calmodulin-binding site at tlie Biophysical Society Meeting in Baltimore in February (Blumenthal et al., 1985). Effect of phosphorylation on protein structure For protein phosphorylation to function in a regulatory capacity it is clear that the introduction or removal of covalently bound phosphate must cause structural changes in protein substrates that in turn lead t o functional changes. How much d o we really know about the chemical nature of this process? The demonstration of functional changes accompanying phosphorylation has been the sine qua non among investigators for acceptance that a given protein phosphorylation event is of regulatory significance, and such changes are readily seen, but in only one instance is much known about structural changes that underlie functional change. This exception is for the original phosphorylation-dephosphorylation enzyme, namely glycogen phosphorylase. As a result of X-ray crystallography studies o n the structure of phosphorylase b , carried out by Louise Johnson at Oxford University, and similar studies on the structure of phosphorylase a by Robert Fletterick and Neil Madsen in Canada, and their respective collaborators, we now have one example in which information is available with respect t o the actual structural change brought about by phosphorylation. In the conversion of phosphorylase b to phosphorylase a a single phosphate group is introduced on Ser-14, very close t o the N-terminus of each phosphorylase subunit, which contains 841 residues. Phosphorylation of the protein results in the formation of three new salt bridges, one of which is between the phosphate group and Arg-69 of the same subunit. Two of the salt bridges are intersubunit bridges. Fletterick & Madsen have calculated that the binding energy of these salt bridges is translated into a highly significant decrease in the allosteric constant for phosphorylase a as compared with phosphorylase b . They have spoken of the phosphate group as a 'covalently bound allosteric ligand', which, like 5'-AMP and other positive effectors, promotes a T to R conformational change (Sprang & Fletterick, 1980; N. B. Madsen, personal communication). 818 BIOCHEMICAL SOCIETY TRANSACTIONS The majority of proteins that are regulated by phosphorylation-dephosphorylation are oligomeric proteins, which, like phosphorylase. are known t o be regulated by the non-covalent binding o f various ligands. It is not unlikely that the findings with respect to the effect of phosphorylation on phosphorylase can be extended to other systems regulated in this manner. The biological significance of protein phosphorylation Given the tremendous potential for the regulation of protein function provided by allosteric control involving non-covalently bound ligands, why is another regulatory mechanism such as phosphorylation-dephosphorylation needed? Investigators puzzling over this question have invoked a number of possible reasons. For example, some think of the various forms of regulation on a time scale and consider allosteric control as being rapid and transient, whereas regulation due t o protein phosphorylation is viewed as being slower and niore enduring. Disregarding these temporal considerations, I like t o think of the two control systems as having evolved to serve two different situations, allosteric control being primarily designed to handle signals arising from within the cell, whereas control by phosphorylation is geared to the handling of signals arising from outside the cell. According t o this general concept, messages carried by hormones and other extracellular stiniuli would be transmitted as a result of the phosphorylation o f key functional proteins ultimately responsible for the cellular functions known t o be affected by the various outside agents (Fig. 6). The idea that protein phosphorylation-dephosphorylation is largely concerned with the handling of external signals is in keeping with the fact that protein phosphorylation is much more abundant in eukaryotic cells than it is in prokaryotic cells, suggesting that the process evolved to meet the needs of more complex organisms in which extracellular signals are especially important. By contrast, allosteric control mechanisms are fully developed in prokaryotes. Let us look at some of the spccit’ic systems in which protein phosphorylation serves such a function: Systems in which cyclic AMP is involved. The prototypical model for the regulation of cellular activity by hormones is the cyclic AMP system (Fig. 7). Here i t is known that one group o f hormones, including, for example. adrenocorticotropin, glucagon, P-adrenergic catecholamines and the gonadotropins, acting through their specific receptors, stimulate adenylate cyclase, which leads t o elevated cyclic AMP levels. A second group of horinones, exemplified by adenoisine and a2-adrenergic agents cause inhibition of adenylate cyclase leading t o decreased cyclic AMP levels. The changes in cyclic AMP concentration in turn alter the activity of the cyclic AMP-dependent protein kinase, thus far the only receptor protein defined for cyclic AMP in eukaryotic cells. Changes in the activity of the cyclic AMP-dependent kinase lead t o an increase or a decrease in the phosphorylation state of key cellular proteins. The last change then promotes some physiological Hormone I IIIIITrIIu:;ln~I1I:I::::::::.:::::::.::i 0 Cyclic A M P Cycl~ AMP-deprndsnt prolein kinas? I 0 Phosphorylailon o f key p r o i e i r ~ s I ~ + Physiological evenis Fig. I . General scheme for the rcgulation o j ’ cellular functions by hormones that cause an increase or a dccrease in cvclic A M P levels within the cell ATP Cyclic A M P 4j i Cyc18~AMP-dependent profejn kinabe n /-----\ Altered cellular functions Glycogen 1 Glucose 1 phosphate I Fig. 6 . llormones and extracellular stimuli transmit their signals through changes in the state of’ phosphorylation of’ Fig. 8 . Regulation o j glycogenol.vsis muscle cellular proteins by adrenalitw in 1985 FOURTEENTH HOPKINS MEMORlAL LECTURE event. A specific example of a cyclic AMP-niediated process involving protein phosphorylation is the classical glycogcnolytic cascade as it occurs in skeletal muscle stiniulated by adrenaline (Fig. 8). The particular target for tlie cyclic AMP- dependent protein kinase in the regulation of glycogenolysis is phosphorylase kinasc, which, as mentioned earlier, is activated by phosphorylation. Activated phosphorylase kinasc catalyses the conversion of pliospliorylasc h t o phosphorylase a and this event triggers glycogenolysis providing glucose 1-phosphate for glycolysis and ATP production. Sj~stcnisin w/ric/r Cu2+ions arid diacjl/g!jwrol scwc as scwrid rncsserrgers of honiional action. A second major system in which hormonal signals generate cellular 1-csponscs as a result of changes in the state of phospliorylation of key proteins involves second messengers that are fornied as a result of stimulation of the phosphatidylinositol (Ptdlns) cycle (Fig. 9). According to current concepts, many different extracellular agents. including, for example, niuscarinic cholincrgic hormones, a-adrenergic Iiorniones, 5-liydroxytryptaminc, histamine. and angiotensin trigger tlic breakdown of pliosphatidylinositol bisphosphatc and acceleration o f tlic Ptdlns cycle. This leads to elevated levels of two intracellular second messengers, inositol triphosphate and diacylglycerol. The first of. these niesscngcrs is responsible for the mobilization of free intracellular C;i2+ ions and the subsequent activation o f a set of at least four Ca2+ (caliiiodu1in)-dependent protein kinases and one phosphoprotein phosphatase. Diacylglyccrol accumulation causes activation of protein kinasc C. These cnzynies then niodulate protein phosphorylation i n a nicaningful nianner so as to produce tlie physiological effect of tlic hormone in question (for reviews. see Berridge. 1984: Nishizuka, 1984). Ssstcwrs iri which Ca" ion niobilization as a result oj electrical cxcitatioii resiiits in protein phosphorylation. Horniones arc not the only extracellular stimuli that give rise to enhanced intracellular protein phosphorylation. This plicnonienon is also seen in connection with the electrical stimulation of cells. Consider, for example, the protein phosphorylation events that occur in skeletal 819 muscle stimulated to contract as a result of the nerve impulse (Fig. 10). When muscle is stimulated electrically or by nerve inipulse Ca2+ ions are released from the sarcoplasinic reticulum and this leads t o contraction asa result o f t h e interaction of Ca2+ with the troponin- tropoinysin complex on thin filaments. The rise in muscle Ca2+ also activates two Ca2+ (ca1iiiodulin)-dcpendciit protein kinases. One of these enLynies. phosphorylasc kinasc, promotes glycogenolysis through tlie familiar pathway involving phosphorylase activation. Glycogenolysis, which ensues, serves to supply ATP that is needed for muscular work. The other protein kinase activated by Ca2+ is MLCK. Activation of this enzyme leads to phosphorylation of myosin light chains, which exerts a regulatory influence on actin -myosin interaction (Stull et ul., 1982). Mechanisnis oj' action of horniones whose receptors arc protein tyrosine kirmcs. The final system that I would like t o consider is the mechanism of intracellular transmission of signals generated by those hormones whose receptors are protein tyrosine kinases. When it became known that the EGF, PDGF, insulin and IGF-I receptors are all protein tyrosine kinases (reviewed in Hunter & Cooper, 1985), it was generally assumed that the mechanism of action of this set of horniones would be elucidated quickly. The obvious inference was that appropriate cellular proteins, which could account for the plciotropic actions of these hormones, would soon be identified as substrates for the receptor kinases and that at last the long sought (particularly in reference to insulin) niechanisni of action would be solved. Several years have gone by, however, and investigators have not as yet identified any universally accepted substrates for the receptor protein tyrosine kinases. For this reason some investigators are now having doubts as t o whether or not the protein kinase activity of the receptors is really important. My own bias remains that it is very important. A catalytic function o f a protein is not a trivial property. In work being carried out in my own laboratory, we have been guided by the hypothesis that protein tyrosine Extracellular stimulus 1 Phosvhorvlase Muscle i h t n MLCK Phosphory lase I I Fig. 9. Mechuriisni o j actiori of horniories o r othc-r c x t r u ccllitlor s t i m u l i that u c t through sccond rncsscwgcJrs of pro tciri pli o s p h o rjila t io ti getwra t cd by t h e p h o s p h t i d y lit1 o sitol (Ptdlri s ) i:v cle Vol. 13 9- act in-myosin M~~,~,,, interaction G lycoqenulysis Fig. 10. Role of protcin pliosphorvlatiori iri mediating t h c rcsporisc to ric'rvc s t i m u l i iri skelcJtul rnusclc 820 BIOCHEMICAL SOCIETY TRANSACTIONS Insulin I 0 t I / :,Ynraqsdne I 1 I I D ~ v r r s r ,m ~ t ~ ~ b eof fle~ c tcs Fig. 1 1. Ilortnoncs whose r e c e p t o r s are p r o t e i n t j frosine kinases a c t through the regulation of p r o t e i n sarine a n d tlireonine p h o s p l i o r y l a t i o n phosphorylation feeds into or regulates the much more abundant serine (threonine) phosphorylation that occurs in cells. This is illustrated using the insulin receptor as an example in Fig. 11. One mechanism by which this might occur would be a ‘mixed cascade’ systeni in which a specific protein serine kinase or a phosphatase serves as a substrate for a protein tyrosine kinase. Activation of the kinase (or phosphatase) as a result of its being phosphorylated on a tyrosine residue could then lead t o alteration of serine phosphorylation within the cell. Support for a communication between protein tyrosine kinases and changes in protein serine phosphorylation has been obtained by a number of laboratories in intact cell systems. For example it is known that insulin stimulates the serine phosphorylation of proteins such as ribosomal protein S6, acetyl-CoA carboxylase, and ATP-citrate lyase. Insulin stimulates the dephosphorylation of other proteins such as glycogen synthase and pyruvate dehydrogenase. Despite a considerable amount of work, however, we have not been successful in reconstructing a mixed cascade system using isolated components. In this connection it is of interest, however, that at this meeting there is a report (Tavare cf al., 1985) that insulin stimulates the phosphorylation of acetyl-CoA carboxylase on serine residues in Triton extracts of placental membranes. Elucidation of the steps involved in this and other insulin-responsive cell-free systems should eventually lead t o an understanding of how tyrosine phosphorylation affects serine phosphorylation. Barker, W. C. & Dayhoff, M. 0. (1982) Proc. Natl. Acad. Sci. lJ. S. A. 79,2836-2839 Berridge, M. J. (1984) Biochem, J . 220,345-360 Blunienthal, D. K., Takio, K., Edelnian, A . M., Charbonnc;iu. H., Walsh, K., Titani, K. & Krcbs, I<. G. (1985) Biophys. J . 47. Abstract M-POS23 Castagna, M., Takai, Y., Kaibuchi, R., Sana, K., Kikkaw;), U . & Nishizuka, Y. (1982)J. Biol. Chem. 257, 7847-7851 Cohen, P., Burchell, A., Foulkes, J . G. & Cohcn, P. T. W. (1978) FEBS Lett. 92,287-293 Corbin, J . D., Sugden, P. H., West, L.. I:lockhart, I). A , , Lincoln, T. M. & McCarthy, D. (1978)J. Biol. Chem. 253, 3997-4003 Cori, G. T . & Cori, C. 1:. (1945)J. Biol. Chem. 158, 321 -332 Cori,G. T. & Green, A. A. (1943)J. B i d . C/tein. 151, 31-38 Dayhoff, M. O., Barker, W. C. & Hunt. L. T. (1983) Methods Enzymol. 9 1 . 5 24 Fischer, E. H . & Krebs, E. G. (1955)J. Biol. Cheni. 216. 121 --I32 Friedman, D. L. & Larner, J . (1963) Biochemistry 2.669-675 Hawk, P. B., Oser, B. L. & Summerson, W. tl. (1947) Practical Physiological Chemistry. 12th edn., p. 169 and p. 178, The Blakiston Co., Philadelphia Hunter, T. & Cooper, J . A. (1985) Anrtu. Rev. Biochem. 54. 897930 Inoue, M.. Kishinioto, A., Takai, Y. & Nisliizuka, Y. (1977)J. Bid. Chem. 252,7610-7616 Krebs, I:. G., Graves, D. J. & I:ishccr, E . 11. (1959) J . Biol. C I i ~ i n . 234,2867--2873 Krebs, 1;. G., Blunicmthal, D. K.. 1:dclman. A . M. 6; Hales, <’. N. (1985) in Mechanisms of Receptor Re&atiori (Crookc. S. T. & Poste, G., eds.), Plenum Publishing Co., New York, in the press Kuo, J . 1.’. & Grccngard, P. (1969)J. Biol. Chem. 244, 3417-3419 Linn, T. C., Pettit, I:. H. Hucho, I:. & Reed. L. (1. ( 1 9 6 9 ~Proc. ) Natl. Acad. Sci. U. S. A . 6 4 , 227-234 Linn, T. C . , Pettit, I:. H. & Reed, L. J . (1969b) Proc. Natl. Acad. Sci. U. S. A. 6 2 , 234-241 Mayr, G. W . & Heiliiicycr, L. M . G., Jr. (1983) FEBS Lett. 157. 225 -23 1 Mcndicino, J . . Beaudreau, C. & Bhattachryya, R. N. (1966) Arch. Biochem. Biophys. 116,436-445 Monod, J . , Changeus, J.-P. & Jacob. 1:. (1963)J. Mol. Biol. 6 . 3063 29 Nishizuka, Y . (1983) Trends Biocherri. Sci. 8. 13-16 Nishizuka, Y. (1984) Nature (London) 308.693-697 Payne, M . 1;. & Soderling, T. R. (1980) J. Biol. Chem. 258. 2376-2382 Pires, E., Perry, S. V. & Thomas, M. A. W . (1974) FEBS Lett. 4 1 , 292-296 Reimann, F.. M . , Titani, K., Ericsson, L. H., Wade, R. 0 . . Fischcr, I;. 11. & Walsh, K. A. (1984) Biochemistry 23.4185-4192 Rizak, M. A. (1961) J. Biol G e m . 239. 392-395 Schulman, H. & Greengard, P. (1978)Proc. Natl. Acad. Sci. U.S. A . 75,5432-5436 Sefton, B. M. & Hunter, T. (1984) Adv. Cyclic Nucleoride Prorein Phosphorylation Res. 18. 195-226 Sprang, S . & Fletterick, R. J . (1980) Biophys. J. 32, 175--192 Stull, J . T., Blumenthal, D. K., Botterman, B. R., Klug, G. A., Manning, D. R. & Silver, P. J . (1982) in Colmodulin and Intracellular Ca‘+ Receptors (Kakiuchi, S . , Hidaka, H. & Means, A,, eds.), pp. 219-238, Plenum Publishing Co., New York Sutherland, E. W. & Wosilait, W. D. (1955) Nature (London) 175, 169-171 Swarup, G . , Dasgupta, J. D. & Garbers, D. L. (1983)J. Biol. Chem. 258,10341-10347 Takai, Y., Kishimoto, A,, Inoue, M. & Nishizuka. Y . (1977)J. Biol. Chem. 252,7603-7609 Takio, K., Wade, R. D., Smith, S. B., Krcbs, E. G., Walsh. K . A. & Titani, K. (1984) Biochemistry 23,4207-4218 Tavare, J . M., Smyth, J . E., Barthwick, A. C., Brownsey, R. W. & Denton, R. M. (1985) Biochem. SOC. nuns. 13,734-735 Walsh, D. A., Perkins, J . P. & Krebs, E. G . (1968)J. Biol. Chem. 243,3763-3765 Weber, J . T., Takio, K. & Steitz, T. A. (1982) Proc. Natl. Acad. Sci. U.S. A . 79,7679-7683 Wong, T . W. & Goldberg, A. R . ( 1 983) R o c . Natl. Acad Sci. U.S. A . 80,2529-2533 1985