* Your assessment is very important for improving the work of artificial intelligence, which forms the content of this project
Download Neuronal polarity: from extracellular signals to
Phosphorylation wikipedia , lookup
Organ-on-a-chip wikipedia , lookup
Endomembrane system wikipedia , lookup
Biochemical switches in the cell cycle wikipedia , lookup
Cell growth wikipedia , lookup
Extracellular matrix wikipedia , lookup
Rho family of GTPases wikipedia , lookup
Protein phosphorylation wikipedia , lookup
Node of Ranvier wikipedia , lookup
Cytokinesis wikipedia , lookup
REVIEWS Neuronal polarity: from extracellular signals to intracellular mechanisms Nariko Arimura and Kozo Kaibuchi Abstract | After they are born and differentiate, neurons break their previous symmetry, dramatically change their shape, and establish two structurally and functionally distinct compartments — axons and dendrites — within one cell. How do neurons develop their morphologically and molecularly distinct compartments? Recent studies have implicated several signalling pathways evoked by extracellular signals as having essential roles in a number of aspects of neuronal polarization. Dendritic spine A small (sub-micrometre) membranous extrusion that protrudes from a dendrite and forms one half of a synapse. Filopodia Finger-like exploratory cell extensions found in crawling cells and growth cones. They are composed largely of filamentous actin bundles. Department of Cell Pharmacology, Graduate School of Medicine, Nagoya University, 65, Tsurumai, Showa, Nagoya, Aichi 466-8550, Japan. Correspondence to K.K. e-mail: kaibuchi@med. nagoya-u.ac.jp doi:10.1038/nrn2056 The axons and dendrites of neuronal cells differ from each other in the composition of their proteins and organelles. Axons are typically long and thin, with a uniform width, and they branch at right angles from the cell body. Dendrites are relatively short; as they emerge from the cell body they appear thick, but become thinner with increased distance from the cell body and then undergo Y-shaped branching1,2. Axons contain synaptic vesicles from which they release neurotransmitters at axon terminals in response to electrical signals from the cell body. Dendrites, especially dendritic spines, contain receptors for these neurotransmitters, as well as organelles and signalling systems3. These two distinct cellular structures are fundamental for neuronal function, as they enable neurons to receive and transmit electrical signals. However, the molecular mechanisms that underlie this neuronal polarization were unclear until a decade ago. Many experiments using cultured embryonic hippocampal neurons have revealed that, as they develop, neurons initially generate several equivalent neurites, but then begin to polarize so that one neurite becomes an axon while the remaining neurites become dendrites4 (FIG. 1; see below). This early asymmetric neurite outgrowth (axon specification) is regulated by signalling molecules that have established roles in cytoskeletal rearrangements and protein trafficking. A balance of positive and negative signals regulates these cellular functions, which result in the formation of a single axon. Here we provide an overview of recent progress in the study of axon specification. Development of neuronal polarization Cultured rat embryonic hippocampal neurons have frequently been used to study the establishment of neuronal polarity. Banker and colleagues developed a culture system of embryonic hippocampal neurons and described in detail the morphological changes that occur 194 | MARCH 2007 | VOLUME 8 during polarization4. They divided the morphological events into five stages (FIG. 1). First, after being dissociated from embryonic rat brains, hippocampal neurons form several thin filopodia (stage 1). After several hours, the neurons form a number of immature neurites, so-called ‘minor processes’ (stage 2). These neurites are morphologically equal, and undergo repeated, random growth and retraction. Half a day after plating, one of these minor processes begins to extend rapidly, becoming much longer than the other neurites (stage 3; see Supplementary information S1 (movie))5,6. This extended process becomes an axon; the other minor processes continue to undergo brief spurts of growth and retraction, maintaining their net length, for up to a week, when they then become mature dendrites (stage 4). During this process, dendrites become thicker and shorter than the axon and begin to establish dendritic components and to construct premature dendritic spines (stage 5). When the axon and dendrites are mature, neurons form synaptic contacts that enable the transmission of electrical activity. Although culturing hippocampal neurons has became the most popular tool to monitor neuronal polarity, the morphological changes seen in immature neurons in different brain areas, tissues and organisms are not necessarily identical in vivo and in vitro (BOX 1). Specification by intrinsic signalling How is a single axon specified among equally potential neurites in cultured hippocampal neurons? A possible scenario is that signalling factors (morphogens) form positive and negative feedback loops that can interchangeably affect one another7. Neurite extension and retraction are controlled by positive and negative signalling molecules respectively, which diffuse within neurites to regulate the behaviour of actin filaments and microtubules, or to influence cargo transport7. The extension of www.nature.com/reviews/neuro © 2007 Nature Publishing Group REVIEWS a Stage 1 Stage 2 Stage 3 Stage 4 Dendrite Immature neurite Stage 5 Dendritic spine Axon Growth cone b c d Stage 1 Stage 3 Stage 5 Figure 1 | Dramatic change in neuronal morphology during neuronal polarization. a | Schematic representation of neuronal polarization in cultured rat embryonic hippocampal neurons. Shortly after plating, the neurons form small protrusion veils and a few spikes (stage 1). These truncated protrusions have growth cones at their tips, and develop into several immature neurites (stage 2). One neurite then starts to break the initial morphological symmetry, growing at a rapid rate, and immediately establishing the polarity (stage 3). A few days later, the remaining neurites elongate and acquire the characteristics of dendrites (stage 4). Approximately seven days after plating, neurons form synaptic contacts through dendritic spines and axon terminals, and establish a neuronal network (stage 5). b–d | Immunocytochemical images of the neurons in stages 1, 3 and 5. The arrowheads indicate the axon. Red staining represents actin filaments, whereas microtubules are shown by green staining. The blue staining in (d) shows synapsin 1, which concentrates at the presynaptic terminal. Panel a modified, with permission, from REF. 4 © (1988) Society for Neuroscience. neurites is driven by four main steps: an increase in the amount of plasma membrane (by vesicle recruitment and fusion); an increase in the local concentration and activation of signalling molecules (such as phosphatidylinositol 3-kinase (PI3K) and Rho GTPase) and their receptors; an increase in the dynamics of actin filaments; and the enhancement of microtubule formation (FIG. 2). Immediately after a small amount of growth, the opposite reaction is induced: microtubule catastrophe, a decrease in the dynamics of actin filaments and enhanced endocytosis occur. The study of neurite motility indicates that these signalling cascades do indeed form an extension–retraction cycle in the minor processes (FIG. 2). Moreover, each neurite seems to release negative feedback signals that feedback to the cell body or metabolize molecules that are required for axon specification, either of which might antagonize axon specification in other neurites7. Before polarization, therefore, positive and negative signals seem to be intricately balanced. When this balance is broken by a positive cue, this leads to the activation of a continuous self-activation system (positive feedback loop)7,8, and a single minor process elongates to become an axon. Concurrently, this self-activation system creates strong negative feedback signals that prevent other neurites from forming a second axon7. Direct evidence for the existence of negative feedback signals comes from the observation that growth and/or elongation of a single neurite driven by laminin-coated beads is inhibited by the contact with — and subsequent elongation of — a second neurite with laminin beads (see below)9. This result indicates the potential existence of negative feedback signals that propagate from the tips of neurites to the cell body; such signals could be induced by the activation of transmembrane receptors. This hypothesis addresses the issue of why only a single neurite is selected to become an axon, and indicates that promoting neurite elongation might lead to neuronal polarization. Box 1 | Similarities and differences among different cell types, and between in vivo and in vitro Rho GTPase This enzyme has a molecular weight of ~21 kDa and generally serves as a molecular switch for various cellular signalling events. Advances in live imaging techniques have revealed that just after cell division, neurons recognize the asymmetric extracellular milieu and move towards their destined position with a leading process at the front side and a tailing process at the rear side. The timing of axon and dendrite formation varies greatly in each type of neuron. For example, the Purkinje cells in the cerebellum, like the pyramidal cells in the neocortex or hippocampus, are generated near the ventricular zone, grow an axon (but not minor processes) towards the basal surface, and migrate toward pre-determined positions following the leading processes that enwrap the glial fibres139. Young cerebellar granule cells migrate along the pia matter, and then form axons bilaterally and migrate towards the inner layer. In both Purkinje cells and cerebellar granule cells, nascent dendrites develop at a later stage139. In C. elegans, the hermaphrodite-specific motorneuron bears several short protrusions, and finally establishes a single axon but not multiple dendritic processes15. Given the variety of polarized morphologies and the different modes of neuronal migration in every organism, it is clear that cell-type-specific components play a crucial part. Therefore, at present, it is difficult to explain every type of neuronal polarization by components and pathways identified from studies of hippocampal cultures. More importantly, a recent study compared the morphological change in the early development of retinal ganglion cells in vivo and in vitro, and showed that in vivo, axons emerge directly from polarized cells in the absence of other immature neurites that are observed in vitro125. It is possible that this discrepancy of morphological changes between in vivo and in vitro is caused by the presence of the extracellular signals; these signals could inhibit immature neurite formation (FIG. 2). The common denominator of polarization in vivo and in vitro seems to be the breaking of symmetry, which first allows the compartmentalization of the nascent axon, and finally leads to the molecularly and morphologically distinct axon and dendrite both in vitro and in vivo2. Further analyses both in vivo and in vitro are required for a better understanding of the mechanism leading to neuronal polarization. NATURE REVIEWS | NEUROSCIENCE VOLUME 8 | MARCH 2007 | 195 © 2007 Nature Publishing Group REVIEWS a Stage 2 Negative regulation • Membrane elimination • Degradation of proteins • Decrease in dynamics of F-actin • Microtubule catastrophe Retraction Phosphatase Rho GAP Negative feedback signals b Stage 3 Negative feedback signals Axon specification Rho GTPases and GEF PI3K Centrosome Extension Positive regulation • Membrane recruitment • Protein transport • Increase in dynamics of F-actin • Microtubule assembly Positive feedback loop Extracellular signals, receptors, adhesion molecules. Transport of key regulators Figure 2 | A tentative model for axon specification in neuronal polarization. a | During stage 2, immature neurites extend and retract randomly to maintain their overall length. Neurite extension is driven by four main steps: an increase in the amount of plasma membrane by vesicle recruitment and fusion; the local concentration and activation of signalling molecules (such as Rho GTPase and phosphatidylinositol 3-kinase (PI3K)), an increase in actin dynamics; and an increase in microtubule formation. After extension, some signalling molecules (such as GTPase-activating proteins and phosphatases) counteract the positive regulation, induce microtubule catastrophe, decrease actin dynamics and decrease the amount of plasma membrane by endocytosis and by preventing vesicle fusion. b | When the balance between positive and negative signals is upset (in the transition from stage 2 to stage 3) by extracellular signals, (auto-) activation of receptors or adhesion molecules and by the recruitment of signalling molecules, one neurite elongates rapidly. Continuous elongation is supported by a positive feedback loop and sustains the activation cycle. The inhibitory signals that mutually antagonize neurite extension (negative feedback signals; red arrows in a and b) are progressively generated at the growing axon more than at the other neurites (a thicker red arrow in b), and interfere with their specification into axons. F-actin, filamentous actin; GAP, GTPase-activating protein; GEF, guanine nucleotide exchange factor. Extracellular matrix Any material that is part of a tissue yet not part of any cell. The main components of the extracellular matrix are various glycoproteins, proteoglycans and hyaluronic acid. Cell-autonomous A function not driven by extracellular signals, but only by an intrinsic program within cells. Planar polarization The coordinated organization of cells within the plane of a single-layered sheet of cells. Plus end The site of microtubule polymerization. It allows the assembly of GTP-bound tubulin dimers. Flexibility in specification. Even after the axon is specified, other neurites have the potential to change their fate during development. Goslin et al. reported that transecting the axons of hippocampal neurons early in development could cause the polarity to alternate; a minor process starts to become an axon, instead of the transected axon8. This change depends on the difference in the length of the cut axon compared to the neurites. If the cut axon is at least 10 μm shorter than the other minor processes, the shortened axon becomes a dendrite, and the longest minor process begins to grow rapidly and eventually becomes an axon8. Axon–dendrite plasticity is known to be retained in stage 4 neurons10, indicating that, to some degree, the identities of axons and dendrites are flexible during development. Extracellular signals and polarity Neurons in the brain and nervous system are surrounded not only by other cells, but also by extracellular matrix, and these extracellular signals are potential cues for specifying axon formation. It has been reported that UNC-6 (also known as netrin in mammals), a secreted protein that mediates axon guidance and cell migration11–14, functions in 196 | MARCH 2007 | VOLUME 8 axon formation to orient asymmetric neuronal growth in Caenorhabditis elegans15. In wild-type C. elegans, the hermaphrodite-specific motorneuron (HSN) extends multiple ventrally directed neurites from its cell body during the third larval stage (L3), and eventually directs a single axon ventrally and then anteriorly. In unc-6 or unc-40 (a receptor for UNC-6) mutants, neurons abnormally extend several immature neurites in all directions during L3, and finally form a single axon directed anteriorly. Interestingly, another repulsive guidance cue, semaphorin 3A, is also known to act as a dendritic chemoattractant and regulates the oriented growth of apical dendrites in cortical neurons16. Axon guidance cues seem to stimulate and orient the asymmetrical growth of neurites and guide the mature axon. Another secreted protein, WNT, has been reported to determine neuronal polarity along the anterior–posterior body axis17,18. WNT and its receptor frizzled (FZ) control various developmental processes, including asymmetric cell division, cell fate determination and tissue polarity19. In C. elegans, the mechanosensory posterior lateral microtubule neuron (PLM) extends a long anterior process that forms a chemical synapse, and a shorter posterior process that does not form a synapse. In lin-44/wnt or lin-17/fz mutants, the longer PLM process extends posteriorly to its cell body instead of anteriorly. The synaptic vesicle protein synaptobrevin 1 (SNB-1) in PLM is abnormally enriched in the longer posterior process in lin-44 or lin-17 mutants. Interestingly, when the posterior and anterior processes are identical in length in lin-17 mutants, synaptic vesicles are localized in both PLM processes17, indicating that these PLM neurons have two functional axon-like processes. Given that the lin-44/wnt defect causes LIN-17/FZ protein to mislocalize cell-autonomously18, secreted LIN-44/ WNT seems to act directly on the PLM mechanosensory neuron through LIN-17/FZ localization, and to specify the anterior process as an axon. WNT signalling cascades inhibit the activity of glycogen synthase kinase 3β (GSK3β) during planar polarization19,20. In migrating mammalian astrocytes, cell division cycle 42 (Cdc42)-dependent inactivation of GSK3β induces the interaction of adenomatous polyposis coli (APC) protein with microtubule plus ends specifically at the leading edges, and makes the centrosome turn towards the front side of migrating cells in a dynein- or dynactin-dependent manner21. It might be interesting to examine whether extracellular signals such as WNT also regulate neuronal polarity through GSK3β and dynein. The neurotrophic factors nerve growth factor (NGF), brain-derived neurotrophic factor (BDNF) and neurotrophin 3 (NT3) are also candidate extracellular polarity-regulating cues, and have been proposed to accelerate neuronal polarization by enhancing axon growth. PI3K is known to mediate downstream signalling from tyrosine receptor kinases (Trks), which are receptors of neurotrophic factors22. It has been reported that the ganglioside-converting enzyme plasma membrane ganglioside sialidase (PMGS), which promotes the activation of TrkA at the tips of neurites, is necessary for axon outgrowth23. As PMGS accumulates in a single immature neurite during stage 2, its activity seems essential for the www.nature.com/reviews/neuro © 2007 Nature Publishing Group REVIEWS Centrosome The main microtubule organizing centre, which consists of two centrioles surrounded by pericentriolar material. It is responsible for microtubule nucleation and anchoring, and forms the radial microtubule organization. Pleckstrin homology (PH) domain A sequence of ~100 amino acids that is present in many signalling molecules and that binds to lipid products of phosphatidylinositol 3-kinase. Ubiquitin–proteasome system Ubiquitin is a small regulatory protein. Ubiquitylation refers to the post-translational modification of a protein by the covalent attachment of one or more ubiquitin monomers. After a chain of at least four ubiquitin peptides are attached to a lysine on a substrate protein, the substrates are degraded by the proteasome. polarized activation of TrkA and the subsequent activation of PI3K. How PMGS becomes restricted to the single process before polarization is still unclear; nevertheless, these results indicate that receptors of neurotrophic factors are crucial in neuronal polarization. Many extracellular matrix and cell adhesion molecules, such as laminin or neuron–glia cell adhesion molecule (NgCAM), enhance neurite outgrowth and axon elongation. One study tested whether these growthpromoting proteins could selectively enhance axon elongation and specification24. When neurons were plated on alternating stripes of poly-d-lysine (which promotes cell adhesion through ionic interactions) and either laminin or NgCAM, any minor processes growing on the stripe coated with laminin or NgCAM elongated rapidly and became an axon, whereas the processes growing only poly-d-lysine remained shorter. Similar substrate preference was also observed in an experiment using laminincoated beads in stage 2 neurons9. Extracellular signals might therefore accelerate the growth of minor processes to determine the neurite identity (BOX 1). Intracellular signalling and polarity The observations described above indicate that extracellular cues lead to a polarized intracellular response that allows neurons to determine axon and dendrite fate during development. However, embryonic neurons in culture (in the absence of a gradient of physiological extracellular cues) also polarize in a few days. Therefore, cell-autonomous signalling cascades, which are also stimulated in response to extracellular signals, are thought to underlie neuronal polarization. PI3K and phosphatidylinositol-3,4,5-trisphosphate. Accumulating evidence indicates the importance of PI3K and its lipid product, phosphatidylinositol-3,4,5trisphosphate (PtdIns(3,4,5)P3), in neuronal polarization9,15,23,25–27 (BOX 2; TABLE 1). PI3K and PtdIns(3,4,5)P3 exhibit a polarized activation and distribution during chemotaxis in neutrophils28,29, Dictyostelium discoideum30,31 and fibroblasts32, and this polarity is required for protrusion of the leading edge membrane in cell migration33,34. In cultured hippocampal neurons, PI3K Box 2 | Key regulators and experimental criteria in neuronal polarity Among the various molecules involved in neuronal polarity, which molecules have a central role? Accumulating evidence indicates that evolutionarily conserved proteins such as small GTPases, phosphatidylinositol 3-kinase (PI3K) and the partitioning defect (PAR) complex control neuronal polarity. We list in TABLE 1 the prominent molecules reported as regulators of neuronal polarity, with their subcellular distribution and the effect of up- or downregulated activity. The criteria used to score the effect on polarization varies greatly. For the integration of information, we suggest that it would be better to develop some common criteria. The axon or dendrite formation should be determined by several specific markers, such as tau 1, synaptic markers and cytoskeletal markers (TABLE 1). In particular, morphology should not be used as the only determinant for axons, and a minor neurite should not be called a ‘dendrite’ before differentiation. Early neuronal polarization is divided into 3 steps: symmetry breaking and elongation of a selective neurite; stabilization of the broken symmetry by a positive or negative feedback loop; and compartmentalization of the axon and somatodendrite. It will be of great importance to examine which molecules are coupled to each step. NATURE REVIEWS | NEUROSCIENCE activity concentrates at the tip of the newly specified axon during stage 3 (REF. 25), and inhibiting PI3K activity prevents axon specification9,25,26. Local activation of PI3K generates PtdIns(3,4,5)P3 at the membrane, which recruits proteins that contain the complementary pleckstrin homology (PH) domain. The local activation of minor processes using laminin-coated beads results in the accumulation of PtdIns(3,4,5)P3 at the tips of immature neurites, and induces the dramatic growth of an axon from a neurite9. Overexpression of Pten (phosphatase and tensin homologue deleted on chromosome 10), the product of which dephosphorylates PtdIns(3,4,5)P3, disrupts the development of polarity25. These results indicate that the polarized activation of PI3K and the accumulation of PtdIns(3,4,5)P3 at the tip of a single minor process (future axon) are required for axon specification. PI3K, Akt and GSK3 β . Stimulation of developing neurons with BDNF or NT3 also activates the PI3K– PtdIns(3,4,5)P3–Akt–GSK3β signalling pathway (TABLE 1). In response to stimulation by neurotrophic factors, PI3K is activated through Ras (FIG. 3) and insulin receptor substrate (IRS)22,35. PI3K, through PtdIns(3,4,5)P3, activates phosphoinositide-dependent kinase 1 (PDK1)36. PDK1, and other kinases such as integrin-linked kinase, phosphorylates the protein kinase Akt (also known as protein kinase B) at threonine 308 and serine 473 (REF. 37). Activated Akt phosphorylates GSK3β on serine 9. GSK3β is constitutively active, and its phosphorylation by Akt inactivates the kinase. As Akt localizes to the tips of the axons in hippocampal neurons, the phosphorylated, inactive GSK3β is also restricted at the tips of growing axons in cultured hippocampal neurons38,39. Overexpression of constitutively active GSK3β disrupts axon elongation, whereas knockdown of GSK3β and the use of specific inhibitors or transfection of the constitutively active form of Akt into neurons38,40 cause the formation of multiple axons38,39, indicating that the activity of Akt and GSK3β regulates neuronal polarity. One study has reported that the inhibition of GSK3β at later stages of development causes dendrites to change suddenly to axons38. This indicates the significance of the PI3K–PtdIns(3,4,5)P3–Akt– GSK3β signalling pathway in neuronal polarity, especially during the transition from minor processes and dendrites to axons. However, a recent study used neurons derived from knock-in mice in which the two isoforms of GSK3 (GSK3α and GSK3β) were unable to be phosphorylated by Akt to show that GSK3β inhibition during neuronal polarization is not mediated by Akt-dependent phosphorylation41. Although the authors have not mentioned which molecules are responsible for GSK3β inhibition, membrane transport and sorting machinery might be involved in GSK3β-dependent neuronal polarization41 (BOX 2; TABLE 1). How are these signalling molecules localized at the tip of the axon? Interestingly, it has been reported that inhibition of the ubiquitin–proteasome system induces symmetric Akt distribution and multiple axons42. This indicates that localized protein degradation regulates protein stability and neuronal polarization. VOLUME 8 | MARCH 2007 | 197 © 2007 Nature Publishing Group REVIEWS Table 1 | Neuronal polarity-regulating molecules in cultured hippocampal neurons Protein (function) Subcellular localization PI3K (kinase; produces Activited at the tip of PtdIns(3,4,5)P3) future axon (st.2,3) *Effect of upregulated activity Myr-p110: multiple axons PTEN (PtdIns(3,4,5)P3 degradating enzyme) Uniform (EGFP– PTEN) WT: unpolarized R-Ras (small GTPase) Single neurite (st.2); axon (st.3) WT, DA (Q87L): multiple axons H-Ras (small GTPase) ND RAP1B (small GTPase) Tip of single minor process (st.2); tip of axon (st.3) Cdc42 (small GTPase) Tip of single minor process (st.2); tip of axon (st.3) Rac1 (small GTPase) ND Akt (mediates the signals of growth factors) Tips of minor processes (st.2); tip of axon (st.3) GSK3β (glycogen synthesis) Tips of all neurites (st.2); tip of axon (st.3); pGSK3β: tip of axon (st.3); cell body PAR3 (involved in asymmetric cell division and cell polarization) PAR6 (involved in asymmetric cell division and cell polarization) aPKC (involved in asymmetric cell division and cell polarization) SAD (presynaptic differentiation) Tips of minor processes (st.2); tip of axon (st.3) MARK2 (microtubule affinity-regulating kinase) CRMP2 (mediator in semaphorin 3A signalling; cargo receptor) *Effect of downregulated activity Inhibitor (LY294002 etc.): no axon, or delay of polarization siRNA: increased number of axons siRNA: unpolarization; R-RasGAP-Myr: prevention of axon formation WT, DA (V12): multiple DN(N17): no axon axons siRNA: loss of DA (V12): superpolarity, no axon numerary axons, or reduced number of minor processes siRNA: no axon; DA (L28): supernumerary axons; N17: no effect V12: unpolarized DA (V12): increased the length of minor neurite, or unpolarized WT, Myr-Akt: multiple axons, or increased number of neurites WT, S9A: no axon or unpolarized Criteria used to score effects Morphological and molecular (tau-1) Association with other molecules PtdIns(3,4,5)P3, Akt Morphological and molecular (PAR3, tau-1, MAP2, pAkt, synapsin 1, GAP43) Morphological and molecular (tau-1, synapsin 1, GAP43, FM4-64 uptake, MAP2, pAkt, pGSK3β) Morphological and molecular (tau-1) Morphological and molecular (tau-1, MAP2, PAR3, p-aPKC, pAkt) Morphological and molecular (tau-1, MAP2, PAR3, p-aPKC, pAkt, synapsin 1) Morphological and molecular (tau-1, MAP2, synapsin 1) PI3K, Akt, GSK3β Refs 9,25, 38,40 25,38 GSK3β, Akt 51 PI3K, MEK, GSK3β, CRMP2 PI3K, Cdc42, PAR3, PAR6, aPKC, Akt 40 26 PI3K, PAR3, PAR6, aPKC, Akt 26,63 STEF 26,63 Morphological and molecular (tau-1, MAP2, pAkt, synapsin 1, GAP43) Morphological shRNA, inhibitor and molecular GID5–6 peptide: (tau-1, MAP2, pAkt, multiple axon synapsin 1, GAP43, GSK3α/β knock-in synaptophysin) mice: no effect delC, delN: no axon, Morphological and or increased length molecular (tau-1, of minor processes MAP2, synapsin 1) PI3K, Ras, GSK3β, tau, MAP1B 38,40, 42 PAR3, PI3K, Akt, CRMP2, tau, BDNF, NT3, MAP1B, APC 38,39, 41,42, 83 PAR6, aPKC, STEF 25,26, 63 WT, delCREB: unpolarized Morphological and molecular (tau-1, MAP2, synapsin 1) PAR3, aPKC, Cdc42, STEF 25,26, 63 25,26, 63 DN (N17): reduced the length of minor process, or unpolarized shRNA: neuronal death Tip of axon (st.3) WT, 4N-1: unpolarized, or multiple axon-like neurites ND ND ND Inhibitor (Bis): unpolarized Morphological and molecular (tau-1, synapsin 1, MAP2) PAR3, PAR6 Diffuse (st.3) ND Morphological and molecular (tau-1, MAP2) Tau 94 All neurites, or tip of longest neurite (st.2); tip of axon (st.3) Diffuse (st.2); distal part of axon (st.3) WT or kinase dead mutant: unpolarized KO: immature axon formation, enhancement of dendritic formation siRNA: multiple axon-like neurites Tau, aPKC 89 WT: multiple axons, or increased length of axon Morphological and molecular (tau-1, synapsin 1) siRNA: unpolarized, Morphological or decreased length and molecular (tau-1, synapsin 1, of axon synaptophysin, MAP2) Tubulin, kinesin 1, numb, GSK3β, Akt, PI3K, BDNF, NT3 39,98, 99,104, 132 *Descriptions of ‘effect of up- or downregulated activity’ were extracted from each reference. 4N-1, deletion construct containing first coiled–coil domain of PAR3; APC, adenomatosis polyposis coli; BDNF, brain-derived neurotrophic factor; Bis, bisindolylmaielmide; CRMP2, collapsin response mediator protein; DA, dominant active; delCREB, CRIB domain deletion mutant; delC, C-terminal deletion mutant; delN, N-terminal deletion mutant; DN, dominant negative; EGFP, enhanced green fluorescent protein; FM4-64, fluorescent membrane marker; GAP43, growth-associated protein 43; KO, knockout mice; L28, mutant where amino acid at 28 has been replaced with Leu; MAP1B, mitogen-activated protein 1B; MARK2, microtubule affinity-regulating kinase 2; MEK, mitogen-activated protein kinase kinase; Myr-p110, myristylated p110, which is the subunit of PI3K containing the kinase domain; N17, mutant where amino acid at 17 has been replaced with Asn; ND, not determined; NT3, neurotrophin 3; pAkt, phosphorylated Akt; p-aPKC, phosphorylated atypical protein kinase C; PAR3, partitioning defect 3; pGSK3β, phosphorylated glycogen synthase kinase 3β; PI3K, phosphatidylinositol 3-kinase; PtdIns(3,4,5)P3, phosphatidylinositol-3,4,5-trisphosphate; PTEN, phosphatase and tensin homologue deleted on chromosome 10; Q87L, amino acid Gln in position 87 is mutated to Leu; RAP1B, Ras-related protein 1b; S9A, constitutive active mutant with Ser in position 9 mutated to Ala; shRNA, short hairpin RNA; siRNA, small interfering RNA; st., stage; STEF, SIF and TIAM1-like exchange factor; V12, mutant where amino acid at 12 has been replaced with Val; WT, wild type. 198 | MARCH 2007 | VOLUME 8 www.nature.com/reviews/neuro © 2007 Nature Publishing Group REVIEWS RNA interference (RNAi). A method by which double-stranded RNA that is encoded on an exogenous vector can be used to interfere with normal RNA processing, causing rapid degradation of the endogenous RNA and thereby precluding translation. This provides a simple way of studying the effects of the absence of a gene product in simple organisms and in cells. Guanine nucleotide exchange factor (GEF). A protein that facilitates the exchange of GDP for GTP in the nucleotide-binding pocket of a GTP-binding protein. Adaptor protein A protein that contributes to cellular function by recruiting other proteins to a complex. Such molecules often contain several protein–protein interaction domains. PI3K and RAP1. Ras-related protein 1B (RAP1B), a Ras superfamily GTPase, localizes to the tips of prospective axons before the accumulation of Cdc42 and the partitioning defect (PAR) complex (TABLE 1; see below)26. Rap1b overexpression induces multiple axon-like neurites and the accumulation of the PAR complex in each neurite. Suppression of Rap1b expression by RNA interference (RNAi) causes the complete loss of axons. The concentration of RAP1B into a single neurite seems to contribute to the recruitment of essential molecules for axon specification, and therefore could be a decisive step. The Rap1b RNAi-induced axonal loss is partially rescued by expressing an active form of Cdc42, whereas axonal loss in response to treatment with a PI3K inhibitor is rescued by the active form of RAP1B. So, RAP1B seems to function upstream of Cdc42 (REF. 43) and the PAR complex, and downstream of PI3K, probably in a PtdIns(3,4,5)P3-dependent manner44 (FIG. 3). It is noteworthy that RAP1 is activated by the neurotrophin receptor TrkA, which is itself activated at the tip of growing axons23. Active TrkA recruits guanine nucleotide exchange factors (GEFs) of RAP1 (such as C3G) and adaptor proteins (such as CRK and fibroblast growth factor receptor substrate 2), and stimulates RAP1 (REFS 45–49). Further studies are needed to elucidate how RAP1B is concentrated and activated in prospective axons. Small GTPases of the Ras family. Ras proteins (H-Ras, K-Ras, N-Ras and R-Ras), which are small GTPases that regulate cell growth and differentiation50, are also reported as activators of PI3K40,51 during neuronal polarization. Active, GTP-bound Ras interacts with several effector proteins; the best characterized are PI3K and Raf 52–55 (FIG. 3). In D. discoideum, active Ras and PI3K localized at the leading edge regulate directional cell movement, indicating that Ras regulates cell polarity56. Consistent with this, an essential role for Ras in neuronal polarization has been reported40,51. Overexpression of wild-type Ras induced multiple axons in cultured hippocampal neurons, whereas ectopically expressed dominantnegative Ras inhibited axon formation40,51. Inhibition of PI3K or mitogen-activated protein kinase kinase (MEK), which lies downstream of Raf, suppresses the Ras-induced formation of multiple axons. This indicates that Ras functions upstream of PI3K and the mitogen-activated protein kinase (MAPK) cascade in the establishment and maintenance of neuronal polarity. Dominant-negative A mutant form of a molecule that can form a heteromeric complex with the normal molecule, knocking out the activity of the entire complex. Growth cones Hand-like structures at the tip of growing neurites, axons, immature neurites and dendrites. Cytochalasin D An actin depolymerizing chemical compound. Rho GTPases and their regulators During initiation of axon growth, the growth cone undergoes net elongation and retraction driven by the rapid polymerization and depolymerization of actin filaments57–59. Applying cytochalasin D to stage 2 neurons causes multiple axon-like neurites59, indicating that the reorganization of actin filaments is necessary for determining a single axon. Among many other molecules, Rho GTPases have been reported as important regulators of axon specification26,60–63. Activated Rho GTPases activate specific effector molecules to change cell morphology or motility64. NATURE REVIEWS | NEUROSCIENCE Among the 18 types of Rho GTPase, RhoA, Cdc42 and Rac1 have been the most extensively characterized65. In neuroblastoma cells, such as PC12 cells or N1E-115 cells, the activation of Cdc42 or Rac1 enhances neurite elongation, whereas RhoA activation is associated with inhibition of neurite formation66–69. NGF-induced neurite outgrowth in PC12 cells involves several Rho GTPase effector molecules: p21-activated kinase (PAK)70, p35 (a neuron-specific regulator for cyclin-dependent kinase 5)71, myotonic dystrophy kinase-related Cdc42-binding kinase (MRCK)72, neural Wiskott–Aldrich syndrome protein (N-WASP)73, and IQGAP3 (REF. 74). These effector molecules influence neurite outgrowth through the rearrangements of microtubules or actin filaments. Rho GTPase regulators. Numerous Rho GTPase regulatory mechanisms are implicated in neuronal polarization. The Rac1-specific GEF T-lymphoma and metastasis 1 protein (TIAM1)75 and SIF and TIAM1like exchange factor (STEF; also known as TIAM2)76 have been identified as regulators of neuronal polarity upstream of Rac1 (REFS 62,63) (FIG. 3). In hippocampal neurons, TIAM1 accumulates at the tip of the prospective axon. Overexpression of Tiam1 induces multiple axon-like neurites, whereas the depletion of Tiam1 inhibits axon formation by preventing actin filament reorganization62. Interestingly, a distinct pathway has been delineated in which local activation of a novel Rac-specific GEF, dedicator of cytokinesis 7 (DOCK7), regulates Rac activity to inactivate the microtubuledestabilizing protein stathmin (also known as OP18) and promote axon formation77. The DOCK7 protein is asymmetrically distributed in non-polarized stage 2 hippocampal neurons and selectively expressed in the axon. Knockdown of Dock7 expression prevents axon formation, whereas overexpression induces the formation of multiple axons77. So, DOCK7, and TIAM1 or STEF, trigger the activation of Rac to regulate microtubule and actin remodelling, respectively. In addition, it has been reported that the cytoplasmic dynein light chain TCTEX1 functions in initial neurite sprouting and axon outgrowth through the activation of Rac61. So, Rac and its GEFs are necessary for establishing neuronal polarity in response to upstream signals. The PAR complex regulates polarity The prototypic par genes were identified in C. elegans for their roles in directing asymmetric cell division during early development78. The PAR protein complex, PAR3–PAR6–aPKC (atypical protein kinase C), is crucial for establishing and maintaining the anterior–posterior polarity at the single-cell embryo stage in C. elegans, as well as in epithelial cells and neuroblast cells of Drosophila melanogaster. In hippocampal neurons, the PAR3–PAR6–aPKC complex becomes concentrated into a single axon from all minor processes during the transition between stages 2 and 3 (REFS 25,79) (TABLE 1). Inhibition of aPKC activity prevents axon formation25, whereas phosphorylated (active) aPKC can be seen at the tips of growing axons26. Furthermore, inhibitors of PI3K prevent polarization and cause mislocalization of PAR3 VOLUME 8 | MARCH 2007 | 199 © 2007 Nature Publishing Group REVIEWS Guidance cues WNT Netrin Receptors Adhesion molecules Plasma membrane Positive signal Negative signal Rho GEF Ras PI3K RhoA ILK PAK Rac1 RAP1B Rho-kinase Raf Positive feedback loop PIP3 PTEN PDK1 IQGAP3 GAP Cdc42 GEF Akt N-WASP Cdc42 p35/CDK5 MAPK Rac GEF PAR6 aPKC PAR3 GSK3β MEK MRCK SRA1 (STEF/TIAM1) WAVE 1 MAPKAP-K1 MARK2 LIMK APC CRMP-2 Tau MAP1B Microtubules KLC Transport β-Catenin MLC Cell adhesion Arp2/3 Cofilin F-actin Stathmin CREB L1 Gene expression Figure 3 | Simplified model of positive and negative signals. In response to the activation of cell surface receptors by extracellular ligands or adhesion molecules, Ras becomes activated. Ras activates phosphatidylinositol 3-kinase (PI3K) and other signalling molecules. PI3K-mediated production of phosphatidylinositol-3,4,5-trisphosphate (PtdIns(3,4,5)P3; or PIP3) tethers PtdIns(3,4,5)P3-binding molecules and activates Ras-related protein 1b (RAP1B) and integrin-linked kinase (ILK), or phosphoinositide-dependent kinase 1 (PDK1). RAP1B activates cell division cycle 42 (Cdc42) at the tips of growing axons. As partitioning defect 6 (PAR6) specifically associates with the GTP-bound form of Cdc42, the PAR3–PAR6–aPKC (atypical protein kinase C) complex becomes activated. PAR3 can then interact with, and activate, the Rac guanine nucleotide exchange factors (GEFs) T-lymphoma and metastasis 1 protein (TIAM1) and SIF and TIAM1-like exchange factor (STEF), and thereby activate Rac1. Active Rac1 and Cdc42 interact with effector molecules, such as neural Wiskott–Aldrich syndrome protein (N-WASP), myotonic dystrophy kinase-related Cdc42-binding kinase (MRCK), p21-activated kinase (PAK), IQGAP3, p35–cyclin-dependent kinase 5 (CDK5), and specifically Rac1-associated protein 1 (SRA1)1– WASP-family verprolinhomologous protein 1 (WAVE1), and regulate filamentous actin (F-actin) reorganization. As Rac1 can activate PI3K, this forms a positive feedback loop. Specific GTPase-activating proteins (GAPs) for Rac1 and Cdc42 will inactivate both GTPases respectively and therefore can break this feedback loop. Activated ILK or PDK1 phosphorylates Akt (also known as protein kinase B) which, in turn, phosphorylates and inactivates glycogen synthase kinase 3β (GSK3β). Microtubule affinity-regulating kinase 2 (MARK2) is inhibited by aPKC. As GSK3β or MARK2 activity promotes microtubule destabilization, inactivation of either of these kinases has a positive effect on microtubule formation and neurite outgrowth. Inactive GSK3β also activates phosphatase and tensin homologue deleted on chromosome 10 (PTEN). In some cases, Rho is activated in response to certain ligands or guidance cues including WNT and netrin, and targets PTEN to membranous areas and enhances its activity. Active PTEN decreases the levels of PtdIns(3,4,5)P3 at the leading edge of the neurite, and prevents positive regulatory signal transduction. The function of some downstream molecules of Rho kinase, GSK3β and mitogen-activated protein kinase-activated protein kinase 1 (MAPKAP-K1) — such as β-catenin, myosin light chain (MLC) and cyclic AMP-response element binding protein (CREB) — were examined in non-neuronal cells, and their exact function in neuronal polarity needs to be addressed in the future. APC, adenomatosis polyposis coli; ARP2/3, actinrelated protein 2/3; CRMP2, collapsin response mediator protein 2; KLC, kinesin light chain; LIMK, LIM domain kinase; MAP1B, microtubule-associated protein 1B; MEK, mitogen-activated protein kinase kinase. 200 | MARCH 2007 | VOLUME 8 www.nature.com/reviews/neuro © 2007 Nature Publishing Group REVIEWS APP-containing vesicles WAVE 1 APP SRA1 GAP43 JIPs PIP3-containing vesicles Tubulin hetrodimer α β CRMP2 aPKC CRMP2 PIP3 PAR6 PAR3 PIP3BP KAP3 KLC KIF5 KIF3 – GAKIN + Rac1 by activating STEF. Ectopic expression of Stef and Par3 induces multiple axon-like neurites but impairs axon maturation63, indicating that axonally localized Rac1 and PAR3 activity is required for axon formation but is not sufficient for axon maturation. Given that GTP-bound Rac1 can activate PI3K84–86, this might form the basis for the positive feedback loop that results in the continued activation of PI3K and downstream molecules (FIG. 3). Microtubule Centrosome Figure 4 | Transport of key regulators in axon formation by kinesin. When one neurite begins to adopt the fate of an axon, kinesin family member 5 (KIF5) strictly concentrates in the growing axon. Consistent with this, several key molecules that are involved in axon formation start to accumulate into the distal parts or tips of axons. These molecules are directly or indirectly associated with kinesins. Cargo, such as amyloid precursor protein (APP), growth-associated protein 43 (GAP43), the SRA1–WAVE1 complex (where SRA1 is specifically Rac1-associated protein 1 and WAVE1 is WASPfamily verprolin-homologous protein 1) and tubulin heterodimers, that cannot directly interact with kinesins, binds to ‘cargo receptors’ such as c-Jun amino-terminal kinase (JNK)-interacting proteins (JIPs) and collapsin response mediator protein 2 (CRMP2), which subsequently dock to kinesin light chain (KLC). The molecules are transported specifically towards the axon terminal and promote axon specification. The partitioning defect (PAR3)–PAR6–aPKC (atypical protein kinase C) complex is transported towards the axon terminal by kinesin 2 (which comprises KIF3 and kinesin superfamily-associated protein 3 (KAP3)), through the direct binding of PAR3 to KAP3. Phosphatidylinositol3,4,5-trisphosphate (PtdIns(3,4,5)P3) is associated with PtdIns(3,4,5)P3-binding protein (PIP3BP), and is transported by the guanylate kinase-associated kinesin (GAKIN) to the prospective axon. and PAR6 (REF. 25), indicating that the localization and activity of the PAR complex are regulated downstream of PI3K, and are required for neuronal polarization. A study in D. melanogaster indicates that PAR6, aPKC and bazooka (as PAR3 is known in D. melanogaster) are not involved in axon or dendrite specification in this species80. Physiological circumstance or another, unknown signal cascade in D. melanogaster might compensate for the PAR complex-induced phenotype observed in cultured rat hippocampal neurons (BOX 2). Lamellipodia Sheet-like extensions at the edge of a cell that contain a crosslinked filamentous actin meshwork, and that are often associated with cell migration. Microtubule-associated proteins (MAPs). MAPs, including tau and MAP1B, are high molecular weight proteins that comprise four distinct polypeptide chains. MAPs are distributed along the lengths of microtubules, and have a stabilizing role. From Cdc42 to Rac through PAR. Recent data indicate that the PAR complex mediates Cdc42-induced Rac activation through STEF or TIAM1 (REF. 63) in hippocampal neurons (FIG. 3). The PAR complex associates with GTPbound Cdc42 more than with GTP–Rac1 through a Cdc42/Rac-interactive binding (CRIB) domain of PAR6 (REFS 81,82) (FIG. 3; TABLE 1). Recent research has shown that STEF and TIAM1 directly interact with the carboxy (C)-terminal region of PAR3 (REFS 63,83) and form a complex with PAR3–PAR6–aPKC–GTP-bound Cdc42 (REF. 63). The isolated STEF-binding fragment of PAR3 is sufficient to induce lamellipodia independently of Cdc42 and PAR6, indicating that PAR3 can activate NATURE REVIEWS | NEUROSCIENCE PAR complex localization. The PAR complex is transported from the cell body to the axon terminal by the plus-end-directed kinesin motor protein kinesin 2 (which comprises kinesin family member 3 (KIF3) and kinesin superfamily-associated protein 3 (KAP3))79,87, and begins to concentrate in a growing axon during the transition from stage 2 to 3 (FIG. 4). Signals, which could be evoked by upstream molecules (which might also be transported by motor proteins or the centrosome; see below), stabilize the PAR complex at the tip of the future axon, thereby generating a localized positive feedback loop. This positive feedback loop might be a driving force for initial axon sprouting and axonal maturation. By contrast, in epithelial cells and mature neurons, an opposite role of Rac1 has been reported. In these cells, Rac1 is activated by the depletion of PAR3 by RNAi83,88. One possible explanation is that knockdown of PAR3 in these cells seems to prevent the recruitment of TIAM1 to the cell–cell contact site between epithelial cells or to the dendritic spine in neurons, and thereby promotes the inappropriately localized activation of Rac1. PAR complex and MARK2. Another function of the PAR complex in neuronal polarization has been reported recently89 (FIG. 3). Microtubule affinity-regulating kinase 2 (MARK2; also known as PAR1b) is a serine/threonine protein kinase that phosphorylates microtubule-associated proteins (MAPs) such as tau90. The depletion of MARK2 by RNAi was reported to induce multiple axons in hippocampal neurons89 (TABLE 1). By contrast, ectopic expression of MARK2 increases tau phosphorylation and prevents axon formation. This defect is rescued by the ectopic expression of the PAR complex, as aPKC phosphorylates MARK2 on threonine 595 and inactivates its kinase activity91–93. A point mutant of MARK2, in which the aPKC phosphorylation site is replaced by alanine, also causes the defect of axon formation, but this defect cannot be corrected by the ectopic expression of PAR3, PAR6 or aPKC. These results indicate that aPKC in complex with PAR3 and PAR6 negatively regulates MARK2 activity at the tip of the axon to prevent MAP phosphorylation and thereby assemble microtubules. A study of SAD kinases — which phosphorylate tau and contain a kinase domain related to that of MARK2 (REF 94) — found that the neurons from SAD kinaseknockout mice showed a loss of axons and abnormally orientated dendrites. SAD kinase is neither phosphorylated nor downregulated by aPKC89. Although their mechanism of regulation is distinct, both MARK2 and SAD kinase seem to regulate neuronal polarity through microtubule reorganization. VOLUME 8 | MARCH 2007 | 201 © 2007 Nature Publishing Group REVIEWS CRMP2 and axon elongation The collapsin response mediator protein (CRMP; also known as TOAD, ULIP and DRP) family (FIG. 3,4), which displays significant homology to C. elegans UNC-33, is expressed at high levels in the developing nervous system95,96. unc-33 mutants exhibit severely uncoordinated movement and abnormalities in axon guidance and axon growth in many neurons97. We have shown that CRMP2 is involved in the regulation of axon formation during neuronal polarization98. CRMP2 is enriched in the growing axons of cultured hippocampal neurons during stage 3, and overexpression of Crmp2 induces the formation of multiple axons. As the induced axon-like processes possess synaptophysin-positive synaptic terminals, CRMP2 is thought to induce and to maintain the mature surplus axon98. Furthermore, overexpression of Crmp2 can alter an established dendrite to become an axon during stage 4, indicating that overexpressed Crmp2 confers axonal identity not only on immature neurites but also on established dendrites98. These observations indicate that CRMP2 has a crucial role in axon formation of hippocampal neurons, thereby establishing and maintaining neuronal polarity. Potential CRMP2 effectors. Several CRMP2 binding proteins have been identified. CRMP2 interacts with tubulin heterodimers and promotes microtubule assembly in vitro99. It binds and colocalizes with numb proteins — which function in Notch-induced cell fate determination100,101 and in clathrin-mediated endocytosis102,103 — in central regions of axonal growth cones and, in turn, regulates the endocytosis of L1, a neuronal cell adhesion molecule104. CRMP2 associates with the SRA1–WAVE1 complex105 (where SRA1 is specifically Rac1-associated protein 1 (REF. 106); also known as CYFIP1 (REF. 107)) and WAVE1 is WASP-family verprolin-homologous protein 1 (REF. 108)), and regulates actin filament stability during lamellipodia formation105 (FIG. 4). So, CRMP2 seems to promote neurite elongation and axon specification by regulating microtubule assembly, endocytosis of adhesion molecules and reorganization of actin filaments. GSK3β phosphorylates CRMP2. We and others have found that GSK3β phosphorylates CRMP2 at threonine 514 and inactivates it39,109,110. In cultured hippocampal neurons, approximately 30% of CRMP2 is constitutively phosphorylated at threonine 514, and this phosphorylation is decreased by GSK3β inhibitors. GSK3βphosphorylated CRMP2 is localized in the distal part of the growing axon, but clearly excluded from the axonal growth cones39, indicating that there is a pool of nonphosphorylated (on threonine 514) CRMP2 in the growing axonal tips. Phosphorylation of CRMP2 lowers its ability to interact with tubulin dimer and numb99,111, and therefore this CRMP2 loses its activity to enhance microtubule formation and L1 endocytosis. The expression of a non-phosphorylated form of CRMP2 efficiently induces the formation of multiple axons, and can also counteract the inhibitory effects of constitutively active GSK3β on neuronal polarization, indicating that GSK3β regulates neuronal polarity by phosphorylating CRMP2 (REF. 39). NT3 and BDNF inhibit GSK3β activity, and, therefore, 202 | MARCH 2007 | VOLUME 8 CRMP2 phosphorylation. CRMP2 depletion prevents NT3-induced axon outgrowth39. Taken together, the regulation of GSK3β and CRMP2 activities is of crucial importance in neuronal polarization. GSK3β and other MAPs. GSK3β also phosphorylates tau112,113, MAP1B114 and APC115. These three substrates stabilize microtubules in the absence of phosphorylation, but are prevented from doing so by GSK3βmediated phosphorylation115,116. One study reported that APC phosphorylation by GSK3β is prevented downstream of NGF and PI3K at the tip of the axon in neurons117. Hippocampal neurons derived from mice lacking both tau and MAP1B show axon loss at stage 3 (REF. 118). As microtubule stabilization and protrusion into the actin meshwork at the distal area of the growth cone promote axon elongation57,59,119, GSK3β seems to have a central role as a negative regulator of neuronal polarization, and inactivation of GSK3β downstream of neurotrophic factors increases the stability of microtubules by increasing the activity of MAPs and vesicle trafficking41 (FIG. 3). It has been reported that partial elimination of GSK3α/β activity results in axon branch formation, whereas complete elimination of GSK3α/β results in a marked inhibition of axon growth120. GSK3α/β activity might be tightly regulated spatially and temporally, so that neurites can undergo dynamic growth and retraction through microtubule reorganization during early development. In addition, it has been found that casein kinase 2 and GSK3β synergistically phosphorylate PTEN at its C terminus, which inhibits its activity121. As GSK3β is constitutively active, the PTEN activity is probably usually downregulated, and therefore upregulation of the PTEN activity by inactivation of GSK3β might provide negative feedback regulation, which counteracts the PI3K activity before and after axon specification in minor processes (FIG. 3). Further work is needed to elucidate the relationship between GSK3β and PTEN in neuronal polarization. Microtubules and neuronal polarity As mentioned earlier, MAPs and regulators of microtubule organization are crucial in neuronal polarization. How do microtubules regulate axon specification? The position of centrosomes in the cell body has been reported to be important for axon determination by influencing where the nascent axon will eventually protrude122–124. Lefcort and Bentley123 first demonstrated a correlation between centrosome and axon formation in grasshopper neurons in 1989, and this was generalized by using granule cells and hippocampal neurons124. Zmuda and Rivas122 reported that the centrosome consistently appeared to be repositioned to the base of the newly emerging axon during transition from a unipolar to bipolar morphology in granule cells. Another recent paper shows that neurons that contain multiple centrosomes have multiple axons; neurons without centrosomes fail to grow stable axons124. Directional microtubule assembly and membrane transport towards specific neurites can be observed in stage 2 neurons124. Therefore, the position of the centrosome promotes the outgrowth of one neurite to become an axon over other minor processes. www.nature.com/reviews/neuro © 2007 Nature Publishing Group REVIEWS Initial segment The proximal part of an axon, the neck of the axonal shaft. It is reported that the position of the centrosome is regulated by Cdc42, cytoplasmic dynein, GSK3β and certain microtubule-associated molecules in a variety of cells21. As some essential cascades for polarity seem to be conserved from worm to mammal, the position of the centrosome might be regulated by extracellular signals and downstream signalling cascades that involve the PAR complex and GSK3β in developmental neurons. This issue, however, still remains somewhat ambiguous, because different studies using distinct cell types and experimental conditions have reported that the centrosome does not localize to the site of the emerging axon125 (BOX 1). Further studies are required to more precisely determine the specific role of the centrosome in neuronal polarity. Transport of key regulators A distinct set of proteins that are responsible for either axon or dendrite formation contributes to the functional differences in each compartment2,126. Kinesin 1 (previously called ‘kinesin’ or ‘conventional kinesin’) is a microtubule plus-end-directed motor127,128 that comprises two kinesin heavy chains (KIF5; also known as KHC) and two kinesin light chains (KLC)129,130 (FIG. 4). KIF5 contains the motor domain and KLC contains the binding domain for the cargo (see below). KIF5 preferentially accumulates in a mature axon, especially in its initial segment, through the association of the motor domain with microtubules in the initial segment131. This preferential localization of KIF5 into a single neurite is observed before neurons become polarized6. KIF5 was not seen to accumulate in minor processes undergoing growth spurts in stage 2 neurons. Dominant-negative KIF5 or the depletion of KLC by RNAi prevent axon specification132, which indicates that at least the basic activity of kinesin 1 is required for axonal growth. Kinesin cargos. The specific nature of kinesin 1 transport enables the cargos of kinesin 1 to target a single neurite (FIG. 4). Several cargo proteins have been reported to be transported by kinesin 1 during the early stages of neuronal development, such as growth-associated protein 43 (GAP43)133 and amyloid precursor protein (APP)134. c-Jun amino-terminal kinase (JNK)-interacting proteins (JIPs)135 and CRMP2 (REFS 105,132,136) function as ‘cargo receptors’ to link other cargo vesicles or molecules with kinesin 1. The CRMP2–kinesin 1 complex is evolutionarily 1. 2. 3. 4. 5. 6. Fukata, Y., Kimura, T. & Kaibuchi, K. Axon specification in hippocampal neurons. Neurosci. Res. 43, 305–315 (2002). Craig, A. M. & Banker, G. Neuronal polarity. Annu. Rev. Neurosci. 17, 267–310 (1994). Nimchinsky, E. A., Sabatini, B. L. & Svoboda, K. Structure and function of dendritic spines. Annu. Rev. Physiol. 64, 313–353 (2002). Dotti, C. G., Sullivan, C. A. & Banker, G. A. The establishment of polarity by hippocampal neurons in culture. J. Neurosci. 8, 1454–1468 (1988). Ruthel, G. & Hollenbeck, P. J. Growth cones are not required for initial establishment of polarity or differential axon branch growth in cultured hippocampal neurons. J. Neurosci. 20, 2266–2274 (2000). Jacobson, C., Schnapp, B. & Banker, G. A. A change in the selective translocation of the Kinesin-1 motor 7. 8. 9. conserved from worms to mammals132,134, and regulates the transport of tubulin heterodimers or SRA1–WAVE1 to the distal part of the growing axon to influence the organization of microtubules and actin filaments105,132. GSK3β phosphorylates KLC and causes the release of kinesin 1 from membrane-bound organelles137. It will be important to determine what kind of signals and posttranslational modifications (such as phosphorylation) regulate the activity of kinesin motors or cargo receptors, and ensure that cargo transport is tightly regulated. It has been reported that PtdIns(3,4,5)P3 could be transported by the guanylate kinase-associated kinesin (GAKIN) to the prospective axon, where it regulates neuronal polarity formation27. Similarly, PtdIns(3,4,5)P3 was seen to be transported to the neurite terminal in laminin bead-induced neurite elongation (N.A. and K.K., unpublished observations). Furthermore, a novel protein shootin 1 is preferentially transported towards axon terminals, where it is required for the activity of PI3K138. So, two independent mechanisms maintain PtdIns(3,4,5)P3 at the tips of axons: the production by PI3K at the axon tip, and the recruitment of PtdIns(3,4,5)P3 by GAKIN. Taken together, the kinesin family of proteins has important roles in neuronal polarity by recruiting the key molecules into a single neurite to establish neuronal polarization. Conclusion and perspectives Many papers concerning neuronal polarity using hippocampal cultures have been published recently. In particular, evolutionarily conserved proteins, such as PI3Kassociated molecules and small GTPases, have now been reported to be involved in neuronal polarity, as observed in other migrating cells or polarized cells (BOX 2). The function of some of these proteins, for example PI3K, guidance cues and signalling molecules, have also been confirmed in vivo16. However, at present the functions of other molecules implicated in neuronal polarity have not been fully evaluated and are mainly based on in vitro data. In order to confirm gene functions in polarity, the information derived from in vivo systems are becoming more important (BOX 1). Nevertheless, neuronal cultures will remain a useful tool to examine neuronal migration and polarization. In vivo and in vitro studies — when based on common criteria — will further complement each other and will lead to a new and integrated insight for understanding the molecular machinery required to establish neuronal polarity. domain marks the initial specification of the axon. Neuron 49, 797–804 (2006). Describes that the motor domain of kinesin 1 accumulates in a single neurite before polarization of a neuron. Andersen, S. S. & Bi, G. Q. Axon formation: a molecular model for the generation of neuronal polarity. Bioessays 22, 172–179 (2000). An excellent review that extensively discusses the positive and negative feedback loops in neuronal polarization. Goslin, K. & Banker, G. Experimental observations on the development of polarity by hippocampal neurons in culture. J. Cell Biol. 108, 1507–1516 (1989). Menager, C., Arimura, N., Fukata, Y. & Kaibuchi, K. PIP3 is involved in neuronal polarization and axon formation. J. Neurochem. 89, 109–118 (2004). NATURE REVIEWS | NEUROSCIENCE 10. Bradke, F. & Dotti, C. G. Differentiated neurons retain the capacity to generate axons from dendrites. Curr. Biol. 10, 1467–1470 (2000). 11. Wadsworth, W. G., Bhatt, H. & Hedgecock, E. M. Neuroglia and pioneer neurons express UNC-6 to provide global and local netrin cues for guiding migrations in C. elegans. Neuron 16, 35–46 (1996). 12. Serafini, T. et al. The netrins define a family of axon outgrowth-promoting proteins homologous to C. elegans UNC-6. Cell 78, 409–424 (1994). 13. Hong, K. et al. A ligand-gated association between cytoplasmic domains of UNC5 and DCC family receptors converts netrin-induced growth cone attraction to repulsion. Cell 97, 927–941 (1999). 14. Hedgecock, E. M., Culotti, J. G. & Hall, D. H. The unc-5, unc-6, and unc-40 genes guide circumferential migrations of pioneer axons and VOLUME 8 | MARCH 2007 | 203 © 2007 Nature Publishing Group REVIEWS 15. 16. 17. 18. 19. 20. 21. 22. 23. 24. 25. 26. 27. 28. 29. 30. 31. 32. 33. 34. 35. 36. 37. 38. mesodermal cells on the epidermis in C. elegans. Neuron 4, 61–85 (1990). Adler, C. E., Fetter, R. D. & Bargmann, C. I. UNC-6/ Netrin induces neuronal asymmetry and defines the site of axon formation. Nature Neurosci. 9, 511–518 (2006). Whitford, K. L., Dijkhuizen, P., Polleux, F. & Ghosh, A. Molecular control of cortical dendrite development. Annu. Rev. Neurosci. 25, 127–149 (2002). Prasad, B. C. & Clark, S. G. Wnt signaling establishes anteroposterior neuronal polarity and requires retromer in C. elegans. Development 133, 1757–1766 (2006). Hilliard, M. A. & Bargmann, C. I. Wnt signals and frizzled activity orient anterior-posterior axon outgrowth in C. elegans. Dev. Cell 10, 379–390 (2006). Logan, C. Y. & Nusse, R. The Wnt signaling pathway in development and disease. Annu. Rev. Cell Dev. Biol. 20, 781–810 (2004). Montcouquiol, M., Crenshaw, E. B., 3rd & Kelley, M. W. Noncanonical Wnt signaling and neural polarity. Annu. Rev. Neurosci. 29, 363–386 (2006). Etienne-Manneville, S. & Hall, A. Rho GTPases in cell biology. Nature 420, 629–635 (2002). Huang, E. J. & Reichardt, L. F. Trk receptors: roles in neuronal signal transduction. Annu. Rev. Biochem. 72, 609–642 (2003). Da Silva, J. S., Hasegawa, T., Miyagi, T., Dotti, C. G. & Abad-Rodriguez, J. Asymmetric membrane ganglioside sialidase activity specifies axonal fate. Nature Neurosci. 8, 606–615 (2005). Esch, T., Lemmon, V. & Banker, G. Local presentation of substrate molecules directs axon specification by cultured hippocampal neurons. J. Neurosci. 19, 6417–6426 (1999). Shi, S. H., Jan, L. Y. & Jan, Y. N. Hippocampal neuronal polarity specified by spatially localized mPar3/mPar6 and PI 3-kinase activity. Cell 112, 63–75 (2003). A key paper that describes the effect of PI3K and the PAR complex on neuronal polarization. Schwamborn, J. C. & Puschel, A. W. The sequential activity of the GTPases Rap1B and Cdc42 determines neuronal polarity. Nature Neurosci. 7, 923–929 (2004). Horiguchi, K., Hanada, T., Fukui, Y. & Chishti, A. H. Transport of PIP3 by GAKIN, a kinesin-3 family protein, regulates neuronal cell polarity. J. Cell Biol. 174, 425–436 (2006). Rickert, P., Weiner, O. D., Wang, F., Bourne, H. R. & Servant, G. Leukocytes navigate by compass: roles of PI3Kγ and its lipid products. Trends Cell Biol. 10, 466–473 (2000). Servant, G. et al. Polarization of chemoattractant receptor signaling during neutrophil chemotaxis. Science 287, 1037–1040 (2000). Meili, R. et al. Chemoattractant-mediated transient activation and membrane localization of Akt/PKB is required for efficient chemotaxis to cAMP in Dictyostelium. EMBO J. 18, 2092–2105 (1999). Jin, T., Zhang, N., Long, Y., Parent, C. A. & Devreotes, P. N. Localization of the G protein βγ complex in living cells during chemotaxis. Science 287, 1034–1036 (2000). Haugh, J. M., Codazzi, F., Teruel, M. & Meyer, T. Spatial sensing in fibroblasts mediated by 3’ phosphoinositides. J. Cell Biol. 151, 1269–1280 (2000). Van Haastert, P. J. & Devreotes, P. N. Chemotaxis: signalling the way forward. Nature Rev. Mol. Cell Biol. 5, 626–634 (2004). Weiner, O. D. Regulation of cell polarity during eukaryotic chemotaxis: the chemotactic compass. Curr. Opin. Cell Biol. 14, 196–202 (2002). Yamada, M. et al. Insulin receptor substrate (IRS)-1 and IRS-2 are tyrosine-phosphorylated and associated with phosphatidylinositol 3-kinase in response to brainderived neurotrophic factor in cultured cerebral cortical neurons. J. Biol. Chem. 272, 30334–30339 (1997). Alessi, D. R. et al. Characterization of a 3-phosphoinositide-dependent protein kinase which phosphorylates and activates protein kinase Bα. Curr. Biol. 7, 261–269 (1997). Burgering, B. M. & Coffer, P. J. Protein kinase B (c-Akt) in phosphatidylinositol-3-OH kinase signal transduction. Nature 376, 599–602 (1995). Jiang, H., Guo, W., Liang, X. & Rao, Y. Both the establishment and the maintenance of neuronal polarity require active mechanisms: critical roles of GSK-3β and its upstream regulators. Cell 120, 123–135 (2005). 39. Yoshimura, T. et al. GSK-3β regulates phosphorylation of CRMP-2 and neuronal polarity. Cell 120, 137–149 (2005). This work, together with that of reference 38, provides the evidence that the PI3K/Akt/GSK3β signal cascade has essential roles in neuronal polarization, especially in the determination of axon and dendrite fate. 40. Yoshimura, T. et al. Ras regulates neuronal polarity via the PI3-kinase/Akt/GSK-3β/CRMP-2 pathway. Biochem. Biophys. Res. Commun. 340, 62–68 (2006). 41. Gartner, A., Huang, X. & Hall, A. Neuronal polarity is regulated by glycogen synthase kinase-3 (GSK-3β) independently of Akt/PKB serine phosphorylation. J. Cell Sci. 119, 3927–3934 (2006). 42. Yan, D., Guo, L. & Wang, Y. Requirement of dendritic Akt degradation by the ubiquitin-proteasome system for neuronal polarity. J. Cell Biol. 174, 415–424 (2006). 43. Hogan, C. et al. Rap1 regulates the formation of E-cadherin-based cell–cell contacts. Mol. Cell Biol. 24, 6690–6700 (2004). 44. Lova, P. et al. A selective role for phosphatidylinositol 3,4,5-trisphosphate in the Gi-dependent activation of platelet Rap1B. J. Biol. Chem. 278, 131–138 (2003). 45. Kao, S., Jaiswal, R. K., Kolch, W. & Landreth, G. E. Identification of the mechanisms regulating the differential activation of the mapk cascade by epidermal growth factor and nerve growth factor in PC12 cells. J. Biol. Chem. 276, 18169–18177 (2001). 46. Gotoh, T. et al. Identification of Rap1 as a target for the Crk SH3 domain-binding guanine nucleotidereleasing factor C3G. Mol. Cell Biol. 15, 6746–6753 (1995). 47. Sasagawa, S., Ozaki, Y., Fujita, K. & Kuroda, S. Prediction and validation of the distinct dynamics of transient and sustained ERK activation. Nature Cell Biol. 7, 365–373 (2005). 48. Vossler, M. R. et al. cAMP activates MAP kinase and Elk-1 through a B-Raf- and Rap1-dependent pathway. Cell 89, 73–82 (1997). 49. York, R. D. et al. Rap1 mediates sustained MAP kinase activation induced by nerve growth factor. Nature 392, 622–626 (1998). 50. Hancock, J. F. Ras proteins: different signals from different locations. Nature Rev. Mol. Cell Biol. 4, 373–384 (2003). 51. Oinuma, I., Katoh, H. & Negishi, M. R-Ras controls axon specification upstream of GSK-3β through integrin-linked kinase. J. Biol. Chem. 282, 303–318 (2007). 52. Dickson, B., Sprenger, F., Morrison, D. & Hafen, E. Raf functions downstream of Ras1 in the Sevenless signal transduction pathway. Nature 360, 600–603 (1992). 53. Rodriguez-Viciana, P. et al. Phosphatidylinositol-3-OH kinase as a direct target of Ras. Nature 370, 527–532 (1994). 54. Rodriguez-Viciana, P., Warne, P. H., Vanhaesebroeck, B., Waterfield, M. D. & Downward, J. Activation of phosphoinositide 3-kinase by interaction with Ras and by point mutation. EMBO J. 15, 2442–2451 (1996). 55. Vojtek, A. B., Hollenberg, S. M. & Cooper, J. A. Mammalian Ras interacts directly with the serine/ threonine kinase Raf. Cell 74, 205–214 (1993). 56. Sasaki, A. T., Chun, C., Takeda, K. & Firtel, R. A. Localized Ras signaling at the leading edge regulates PI3K, cell polarity, and directional cell movement. J. Cell Biol. 167, 505–518 (2004). 57. Dent, E. W. & Gertler, F. B. Cytoskeletal dynamics and transport in growth cone motility and axon guidance. Neuron 40, 209–227 (2003). 58. Baas, P. W. & Buster, D. W. Slow axonal transport and the genesis of neuronal morphology. J. Neurobiol. 58, 3–17 (2004). 59. Bradke, F. & Dotti, C. G. The role of local actin instability in axon formation. Science 283, 1931–1934 (1999). 60. Bito, H. et al. A critical role for a Rho-associated kinase, p160ROCK, in determining axon outgrowth in mammalian CNS neurons. Neuron 26, 431–441 (2000). 61. Chuang, J. Z. et al. The dynein light chain Tctex-1 has a dynein-independent role in actin remodeling during neurite outgrowth. Dev. Cell 9, 75–86 (2005). 62. Kunda, P., Paglini, G., Quiroga, S., Kosik, K. & Caceres, A. Evidence for the involvement of Tiam1 in axon formation. J. Neurosci. 21, 2361–2372 (2001). 63. Nishimura, T. et al. PAR-6-PAR-3 mediates Cdc42induced Rac activation through the Rac GEFs STEF/ Tiam1. Nature Cell Biol. 7, 270–277 (2005). 204 | MARCH 2007 | VOLUME 8 64. 65. 66. 67. 68. 69. 70. 71. 72. 73. 74. 75. 76. 77. 78. 79. 80. 81. 82. 83. 84. 85. This work, together with that of reference 62, provides evidence that the activity of Cdc42 and Rac1 is essential for neuronal polarization and neuronal maturation. Ridley, A. J. et al. Cell migration: integrating signals from front to back. Science 302, 1704–1709 (2003). Govek, E. E., Newey, S. E. & Van Aelst, L. The role of the Rho GTPases in neuronal development. Genes Dev. 19, 1–49 (2005). An excellent review that extensively describes a variety of signalling cascades that mediate Rho GTPases and the associating factors. Sebok, A. et al. Different roles for RhoA during neurite initiation, elongation, and regeneration in PC12 cells. J. Neurochem. 73, 949–960 (1999). Kranenburg, O. et al. Activation of RhoA by lysophosphatidic acid and Gα12/13 subunits in neuronal cells: induction of neurite retraction. Mol. Biol. Cell 10, 1851–1857 (1999). Kozma, R., Sarner, S., Ahmed, S. & Lim, L. Rho family GTPases and neuronal growth cone remodelling: relationship between increased complexity induced by Cdc42Hs, Rac1, and acetylcholine and collapse induced by RhoA and lysophosphatidic acid. Mol. Cell Biol. 17, 1201–1211 (1997). Tigyi, G. et al. Lysophosphatidic acid-induced neurite retraction in PC12 cells: control by phosphoinositideCa2+ signaling and Rho. J. Neurochem. 66, 537–548 (1996). Daniels, R. H., Hall, P. S. & Bokoch, G. M. Membrane targeting of p21-activated kinase 1 (PAK1) induces neurite outgrowth from PC12 cells. EMBO J. 17, 754–764 (1998). Nikolic, M., Chou, M. M., Lu, W., Mayer, B. J. & Tsai, L. H. The p35/Cdk5 kinase is a neuron-specific Rac effector that inhibits Pak1 activity. Nature 395, 194–198 (1998). Chen, X. Q., Tan, I., Leung, T. & Lim, L. The myotonic dystrophy kinase-related Cdc42-binding kinase is involved in the regulation of neurite outgrowth in PC12 cells. J. Biol. Chem. 274, 19901–19905 (1999). Banzai, Y., Miki, H., Yamaguchi, H. & Takenawa, T. Essential role of neural Wiskott–Aldrich syndrome protein in neurite extension in PC12 cells and rat hippocampal primary culture cells. J. Biol. Chem. 275, 11987–11992 (2000). Wang, S. et al. IQGAP3, a novel effector of Rac1 and Cdc42, regulates neurite outgrowth. J. Cell Sci. 23 Jan 2006 (doi:10.1242/jcs.03356). Habets, G. G. et al. Identification of an invasioninducing gene, Tiam-1, that encodes a protein with homology to GDP–GTP exchangers for Rho-like proteins. Cell 77, 537–549 (1994). Hoshino, M. et al. Identification of the stef gene that encodes a novel guanine nucleotide exchange factor specific for Rac1. J. Biol. Chem. 274, 17837–17844 (1999). Watabe-Uchida, M., John, K. A., Janas, J. A., Newey, S. E. & Van Aelst, L. The Rac activator DOCK7 regulates neuronal polarity through local phosphorylation of stathmin/Op18. Neuron 51, 727–739 (2006). Cowan, C. R. & Hyman, A. A. Asymmetric cell division in C. elegans: cortical polarity and spindle positioning. Annu. Rev. Cell Dev. Biol. 20, 427–453 (2004). Nishimura, T. et al. Role of the PAR-3-KIF3 complex in the establishment of neuronal polarity. Nature Cell Biol. 6, 328–334 (2004). Rolls, M. M. & Doe, C. Q. Baz, Par-6 and aPKC are not required for axon or dendrite specification in Drosophila. Nature Neurosci. 7, 1293–1295 (2004). Lin, D. et al. A mammalian PAR-3–PAR-6 complex implicated in Cdc42/Rac1 and aPKC signalling and cell polarity. Nature Cell Biol. 2, 540–547 (2000). Johansson, A., Driessens, M. & Aspenstrom, P. The mammalian homologue of the Caenorhabditis elegans polarity protein PAR-6 is a binding partner for the Rho GTPases Cdc42 and Rac1. J. Cell Sci. 113, 3267–3275 (2000). Chen, X. & Macara, I. G. Par-3 controls tight junction assembly through the Rac exchange factor Tiam1. Nature Cell Biol. 7, 262–269 (2005). Tolias, K. F., Cantley, L. C. & Carpenter, C. L. Rho family GTPases bind to phosphoinositide kinases. J. Biol. Chem. 270, 17656–17659 (1995). Keely, P. J., Westwick, J. K., Whitehead, I. P., Der, C. J. & Parise, L. V. Cdc42 and Rac1 induce integrinmediated cell motility and invasiveness through PI3K. Nature 390, 632–636 (1997). www.nature.com/reviews/neuro © 2007 Nature Publishing Group REVIEWS 86. Chan, T. O. et al. Small GTPases and tyrosine kinases coregulate a molecular switch in the phosphoinositide 3-kinase regulatory subunit. Cancer Cell 1, 181–191 (2002). 87. Shi, S. H., Cheng, T., Jan, L. Y. & Jan, Y. N. APC and GSK-3β are involved in mPar3 targeting to the nascent axon and establishment of neuronal polarity. Curr. Biol. 14, 2025–2032 (2004). 88. Zhang, H. & Macara, I. G. The polarity protein PAR-3 and TIAM1 cooperate in dendritic spine morphogenesis. Nature Cell Biol. 8, 227–237 (2006). 89. Chen, Y. M. et al. Microtubule affinity-regulating kinase 2 functions downstream of the PAR-3/PAR-6/ atypical PKC complex in regulating hippocampal neuronal polarity. Proc. Natl Acad. Sci. USA 103, 8534–8539 (2006). 90. Drewes, G., Ebneth, A., Preuss, U., Mandelkow, E. M. & Mandelkow, E. MARK, a novel family of protein kinases that phosphorylate microtubule-associated proteins and trigger microtubule disruption. Cell 89, 297–308 (1997). 91. Suzuki, A. et al. aPKC acts upstream of PAR-1b in both the establishment and maintenance of mammalian epithelial polarity. Curr. Biol. 14, 1425–1435 (2004). 92. Hurov, J. B., Watkins, J. L. & Piwnica-Worms, H. Atypical PKC phosphorylates PAR-1 kinases to regulate localization and activity. Curr. Biol. 14, 736–741 (2004). 93. Kusakabe, M. & Nishida, E. The polarity-inducing kinase Par-1 controls Xenopus gastrulation in cooperation with 14–3-3 and aPKC. EMBO J. 23, 4190–4201 (2004). 94. Kishi, M., Pan, Y. A., Crump, J. G. & Sanes, J. R. Mammalian SAD kinases are required for neuronal polarization. Science 307, 929–932 (2005). 95. Arimura, N., Menager, C., Fukata, Y. & Kaibuchi, K. Role of CRMP-2 in neuronal polarity. J. Neurobiol. 58, 34–47 (2004). 96. Goshima, Y., Nakamura, F., Strittmatter, P. & Strittmatter, S. M. Collapsin-induced growth cone collapse mediated by an intracellular protein related to UNC-33. Nature 376, 509–514 (1995). 97. Hedgecock, E. M., Culotti, J. G., Thomson, J. N. & Perkins, L. A. Axonal guidance mutants of Caenorhabditis elegans identified by filling sensory neurons with fluorescein dyes. Dev. Biol. 111, 158–170 (1985). 98. Inagaki, N. et al. CRMP-2 induces axons in cultured hippocampal neurons. Nature Neurosci. 4, 781–782 (2001). A key paper showing that overexpression of Crmp2 induces multiple axons in cultured neurons. Most importantly, this paper is one of the early reports showing that the polarity-regulating molecules could interchange the fate of a neurite from dendrite to axon. 99. Fukata, Y. et al. CRMP-2 binds to tubulin heterodimers to promote microtubule assembly. Nature Cell Biol. 4, 583–591 (2002). 100. Frise, E., Knoblich, J. A., Younger-Shepherd, S., Jan, L. Y. & Jan, Y. N. The Drosophila Numb protein inhibits signaling of the Notch receptor during cell–cell interaction in sensory organ lineage. Proc. Natl Acad. Sci. USA 93, 11925–11932 (1996). 101. Spana, E. P. & Doe, C. Q. Numb antagonizes Notch signaling to specify sibling neuron cell fates. Neuron 17, 21–26 (1996). 102. Berdnik, D., Torok, T., Gonzalez-Gaitan, M. & Knoblich, J. A. The endocytic protein α-Adaptin is required for numb-mediated asymmetric cell division in Drosophila. Dev. Cell 3, 221–231 (2002). 103. Santolini, E. et al. Numb is an endocytic protein. J. Cell Biol. 151, 1345–1352 (2000). 104. Nishimura, T. et al. CRMP-2 regulates polarized Numb-mediated endocytosis for axon growth. Nature Cell Biol. 5, 819–826 (2003). 105. Kawano, Y. et al. CRMP-2 is involved in kinesin-1dependent transport of the Sra-1/WAVE1 complex and axon formation. Mol. Cell Biol. 25, 9920–9935 (2005). 106. Kobayashi, K. et al. p140Sra-1 (specifically Rac1associated protein) is a novel specific target for Rac1 small GTPase. J. Biol. Chem. 273, 291–295 (1998). 107. Schenck, A. et al. CYFIP/Sra-1 controls neuronal connectivity in Drosophila and links the Rac1 GTPase pathway to the fragile X protein. Neuron 38, 887–898 (2003). 108. Miki, H., Suetsugu, S. & Takenawa, T. WAVE, a novel WASP-family protein involved in actin reorganization induced by Rac. EMBO J. 17, 6932–6941 (1998). 109. Cole, A. R. et al. GSK-3 phosphorylation of the Alzheimer epitope within collapsin response mediator proteins regulates axon elongation in primary neurons. J. Biol. Chem. 279, 50176–50180 (2004). 110. Uchida, Y. et al. Semaphorin3A signalling is mediated via sequential Cdk5 and GSK3β phosphorylation of CRMP2: implication of common phosphorylating mechanism underlying axon guidance and Alzheimer’s disease. Genes Cells 10, 165–179 (2005). 111. Arimura, N. et al. Phosphorylation by Rho kinase regulates CRMP-2 activity in growth cones. Mol. Cell. Biol. 25, 9973–9984 (2005). 112. Hanger, D. P., Hughes, K., Woodgett, J. R., Brion, J. P. & Anderton, B. H. Glycogen synthase kinase-3 induces Alzheimer’s disease-like phosphorylation of tau: generation of paired helical filament epitopes and neuronal localisation of the kinase. Neurosci. Lett. 147, 58–62 (1992). 113. Mandelkow, E. M. et al. Glycogen synthase kinase-3 and the Alzheimer-like state of microtubule-associated protein tau. FEBS Lett. 314, 315–321 (1992). 114. Lucas, F. R., Goold, R. G., Gordon-Weeks, P. R. & Salinas, P. C. Inhibition of GSK-3beta leading to the loss of phosphorylated MAP-1B is an early event in axonal remodelling induced by WNT-7a or lithium. J. Cell Sci. 111, 1351–1361 (1998). 115. Zumbrunn, J., Kinoshita, K., Hyman, A. A. & Nathke, I. S. Binding of the adenomatous polyposis coli protein to microtubules increases microtubule stability and is regulated by GSK3 β phosphorylation. Curr. Biol. 11, 44–49 (2001). 116. Mandelkow, E. M. et al. Tau domains, phosphorylation, and interactions with microtubules. Neurobiol. Aging 16, 355–362; discussion 362–353 (1995). 117. Zhou, F. Q., Zhou, J., Dedhar, S., Wu, Y. H. & Snider, W. D. NGF-induced axon growth is mediated by localized inactivation of GSK-3β and functions of the microtubule plus end binding protein APC. Neuron 42, 897–912 (2004). 118. Takei, Y., Teng, J., Harada, A. & Hirokawa, N. Defects in axonal elongation and neuronal migration in mice with disrupted tau and map1b genes. J. Cell Biol. 150, 989–1000 (2000). 119. Baas, P. W. Microtubules and neuronal polarity: lessons from mitosis. Neuron 22, 23–31 (1999). 120. Kim, W. Y. et al. Essential roles for GSK-3 in neurotrophin-induced and hippocampal axon growth. Neuron 52, 981–996 (2006). 121. Al-Khouri, A. M., Ma, Y., Togo, S. H., Williams, S. & Mustelin, T. Cooperative phosphorylation of the tumor suppressor phosphatase and tensin homologue (PTEN) by casein kinases and glycogen synthase kinase 3β. J. Biol. Chem. 280, 35195–35202 (2005). 122. Zmuda, J. F. & Rivas, R. J. The Golgi apparatus and the centrosome are localized to the sites of newly emerging axons in cerebellar granule neurons in vitro. Cell Motil. Cytoskeleton 41, 18–38 (1998). 123. Lefcort, F. & Bentley, D. Organization of cytoskeletal elements and organelles preceding growth cone emergence from an identified neuron in situ. J. Cell Biol. 108, 1737–1749 (1989). 124. de Anda, F. C. et al. Centrosome localization determines neuronal polarity. Nature 436, 704–708 (2005). 125. Zolessi, F. R., Poggi, L., Wilkinson, C. J., Chien, C. B. & Harris, W. A. Polarization and orientation of retinal ganglion cells in vivo. Neural Develop. 1, 1–21 (2006). An important paper showing the morphological and molecular differences between retinal ganglion cells in vivo and in vitro. NATURE REVIEWS | NEUROSCIENCE 126. Hirokawa, N. & Takemura, R. Molecular motors and mechanisms of directional transport in neurons. Nature Rev. Neurosci. 6, 201–214 (2005). 127. Brady, S. T. A novel brain ATPase with properties expected for the fast axonal transport motor. Nature 317, 73–75 (1985). 128. Vale, R. D., Reese, T. S. & Sheetz, M. P. Identification of a novel force-generating protein, kinesin, involved in microtubule-based motility. Cell 42, 39–50 (1985). 129. Kuznetsov, S. A. et al. The quaternary structure of bovine brain kinesin. EMBO J. 7, 353–356 (1988). 130. Bloom, G. S., Wagner, M. C., Pfister, K. K. & Brady, S. T. Native structure and physical properties of bovine brain kinesin and identification of the ATPbinding subunit polypeptide. Biochemistry 27, 3409–3416 (1988). 131. Nakata, T. & Hirokawa, N. Microtubules provide directional cues for polarized axonal transport through interaction with kinesin motor head. J. Cell Biol. 162, 1045–1055 (2003). 132. Kimura, T. et al. Tubulin and CRMP-2 complex is transported via Kinesin-1. J. Neurochem. 93, 1371–1382 (2005). 133. Ferreira, A., Niclas, J., Vale, R. D., Banker, G. & Kosik, K. S. Suppression of kinesin expression in cultured hippocampal neurons using antisense oligonucleotides. J. Cell Biol. 117, 595–606 (1992). 134. Kamal, A., Stokin, G. B., Yang, Z., Xia, C. H. & Goldstein, L. S. Axonal transport of amyloid precursor protein is mediated by direct binding to the kinesin light chain subunit of kinesin-1. Neuron 28, 449–459 (2000). 135. Verhey, K. J. et al. Cargo of kinesin identified as JIP scaffolding proteins and associated signaling molecules. J. Cell Biol. 152, 959–970 (2001). 136. Tsuboi, D., Hikita, T., Qadota, H., Amano, M. & Kaibuchi, K. Regulatory machinery of UNC-33 Ce-CRMP localization in neurites during neuronal development in Caenorhabditis elegans. J. Neurochem. 95, 1629–1641 (2005). 137. Morfini, G., Szebenyi, G., Elluru, R., Ratner, N. & Brady, S. T. Glycogen synthase kinase 3 phosphorylates kinesin light chains and negatively regulates kinesin-based motility. EMBO J. 21, 281–293 (2002). 138. Toriyama, M. et al. Shootin1: a protein involved in the organization of an asymmetric signal for neuronal polarization. J. Cell Biol. 175, 147–157 (2006). 139. Solecki, D. J., Govek, E. E., Tomoda, T. & Hatten, M. E. Neuronal polarity in CNS development. Genes Dev. 20, 2639–2647 (2006). Acknowledgements We thank S. Kuroda (University of Tokyo) for critical reading and helpful discussion on the manuscript, T. Miyata (Nagoya University) for the helpful discussion, and L. Van Aelst (Cold Spring Harbour Laboratory) for sending a preprint of work in the press. We also thank T. Yoshimura, A. Hattori, C. Lee, A. Shimada, N. Mishima and the members of our laboratory for preparing the manuscript. Competing interests statement The authors declare no competing financial interests. DATABASES The following terms in this article are linked online to: Entrez Gene: http://www.ncbi.nlm.nih.gov/entrez/query. fcgi?db=gene aPKC | CRMP2 | DOCK7 | FZ | GSK3β | N-WASP | PAR3 | PAR6 | RAP1B | STEF | TIAM1 | UNC-6 | WNT FURTHER INFORMATION Kaibuchi’s laboratory: http://www.med.nagoya-u.ac.jp/ Yakuri/yakuri_en_index.html SUPPLEMENTARY INFORMATION See online article: S1 (movie) Access to this links box is available online. VOLUME 8 | MARCH 2007 | 205 © 2007 Nature Publishing Group
















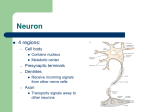
![Neuron [or Nerve Cell]](http://s1.studyres.com/store/data/000229750_1-5b124d2a0cf6014a7e82bd7195acd798-150x150.png)





