* Your assessment is very important for improving the work of artificial intelligence, which forms the content of this project
Download Vesicular transport of newly synthesized opsin from the Golgi
Node of Ranvier wikipedia , lookup
Cell nucleus wikipedia , lookup
Cell encapsulation wikipedia , lookup
Magnesium transporter wikipedia , lookup
Lipid bilayer wikipedia , lookup
Membrane potential wikipedia , lookup
Chemical synapse wikipedia , lookup
Organ-on-a-chip wikipedia , lookup
Signal transduction wikipedia , lookup
Model lipid bilayer wikipedia , lookup
Cytokinesis wikipedia , lookup
SNARE (protein) wikipedia , lookup
List of types of proteins wikipedia , lookup
Vesicular Transport of Newly Synthesized Opsin from
the Golgi Apparatus toward the Rod Outer Segment
Ulrrostrucrurol Immunocyrochemicol and Auroradiographic Evidence
in Xenopus Retinas
David 5. Papermasrer, Barbara G. Schneider, and Joseph C. Desharse*
Each day, rod photoreceptors of the vertebrate retina synthesize rhodopsin and insert it into new membranes of the rod outer segment (ROS). The authors determined which components of the rod cell
transport opsin from the Golgi to the ROS by a combined EM autoradiographic and immunocytochemical
study using radiolabeled amino acid precursors and antiopsin antibodies. Radiolabeled proteins in the
ellipsoid region of Xenopus laevis retinal rods were localized by comparison of the distribution of silver
grains with the predicted distribution generated by a hypothetical source: grain matrix. Sources of decay
were not uniformly distributed. Small vesicles compressed between mitochondria and clustered beneath
the connecting cilium that joins the inner to the outer segment contained more than 30% of the radiolabel
and had a specific activity 17 times higher than the surrounding cytoplasm. Opsin was localized immunocytochemically on thin sections of retinas embedded in Lowicryl K4M (Polysciences; Warrington,
PA) by reaction sequentially with biotinyl-rabbit antifrog opsin, biotinyl-sheep antirabbit F(ab')2, and
avidin-ferritin. Golgi apparatus, intermitochondrial vesicles, and vesicles that clustered beneath the
connecting cilium were prominently labeled. Subellipsoid smooth endoplasmic reticulum was labeled at
background levels. These results demonstrate that intracellular vesicular membranes transport newly
synthesized opsin from the Golgi to the base of the connecting cilium of X. laevis retinas. Antibody
labeled the outer segment plasma membrane at a 10-fold greater density than the contiguous inner
segment plasma membrane. The polarized distribution of opsin apparently involves not only vectorial
transport of opsin in the inner segment but also restrictions to the randomization of opsin inserted into
the inner and outer segment plasma membrane. Invest Ophthalmol Vis Sci 26:1386-1404, 1985
cell surfaces.1 4 The polarized budding of virus-infected
cells probably involves vesicular transport of virus
membrane proteins to the cell surface.5"13 Similar
pathways may function in the transport of newly synthesized membrane proteins in photoreceptor cells.
Rod photoreceptor cells offer, however, several advantages for the study of the biosynthesis, processing, and
sorting of membrane proteins. The major protein synthesized in the entire retina is the visual pigment apoprotein, opsin. Its synthesis is a normal physiologic
function of the rod cell.
Rod photoreceptors assemble an extraordinary
amount of new outer segment disk membranes. Under
normal circadian light cycles, about 80 disks are formed
per day in Xenopus laevis tadpole rods. 1415 Each disk
is about 6 nm in diameter or nearly the size of a human
red cell and contains 106 rhodopsins/disk. This corresponds to the generation of about 4500 /um2 of new
The generation and maintenance of cell polarity in
epithelial and neuronal tissues suggests that specific cell
membrane constituents are uniquely transported or are
otherwise restricted to sites of function in these cells.
In epithelial cells, vesicles may participate in the transport of hormones, immunoglobulins, and serum proteins across the cell and from sites of synthesis to unique
From the Department of Pathology, VA Medical Center, West
Haven, and Yale Medical School, New Haven, Connecticut; and the
Department of Anatomy and Cell Biology, *Emory University School
of Medicine, Atlanta, Georgia.
Supported in part from NIH grants EY-03239, EY-00845, GM21714, EY-02414, EY-03222, and the Veterans Administration.
During portions of this research DP and JB were recipients of RCDA
grants EY-00017 and EY-00169, respectively.
Submitted for publication: October 11, 1984.
Reprint requests: David S. Papermaster, MD, Department of Pathology/113, VA Medical Center, West Haven, CT 06516.
1386
Downloaded From: http://iovs.arvojournals.org/pdfaccess.ashx?url=/data/journals/iovs/933115/ on 06/18/2017
No. 10
VESICULAR TRANSPORT OF OPSIN / Popermosrer er ol.
1387
F. Disk Assembly
Fig. 1. Diagram of X. laevis rod photoreceptor inner
and outer segment illustrating the cellular membranes
involved in the biosynthesis
and transport of opsin. PostGolgi vesicles vectorially
transport newly synthesized
opsin past the closely packed
mitochondria in the ellipsoid
region. Vesicles apparently
fuse with the grooves of the
periciliary ridge complex
near the base of the connecting cilium. Opsin may then
proceed along the ciliary
plasma membrane to become
incorporated into the new
disks forming at the base of
the outer segment.
ROS
E. Ciliary
Transport
D. Insertion at
Plasma Membrane
of the PRC
Basal Bodies
C. Vesicular Transport
Ellipsoid
_
SER
B. GolghTerminol
Processing,
Glycosylation and
Packaging
photoreceptor membrane daily.15 It represents an average rate of 3.1 /xm2/min of rod outer segment (ROS)
membrane addition, a rate which is comparable to that
occurring at the growth cones of actively elongating
neurites.16
Rod outer segments contain no cellular constituents
for protein synthesis. Synthesis of disk membrane proteins occurs in the inner segment. Since rhodopsin is
a typical intrinsic membrane protein within ROS disks,
we wished to determine which cell constituents might
participate in its transport across the inner segment.
Prior autoradiographic and radiobiochemical studies
showed that most of the radiolabeled protein migrated
from the Golgi past the ellipsoid—a mitochondria rich
domain between the nucleus and the ROS—to arrive
eventually in newly formed basal.ROS disks.17"19 Photoreceptor inner and outer segments are joined by a
narrow connecting cilium. No membranes were seen
in the interior of the cilium, yet radiolabeled proteins
were shown to congregate beneath its base and silver
grains were present over cytoplasm beneath the cilium
and within the cilium interior. This was interpreted as
possible evidence for a soluble form of opsin as an
intermediate during its transport and final passage
through the cilium.20
Subcellular fractionation of frog retinas demonstrated, however, that newly synthesized opsin was isolated only on easily sedimented membrane fractions.
The cytosol fractions of retinal homogenates did not
R E R : Synthesis and
Core Glycosylation
Myoid
contain opsin.21 Vesicles were postulated as possible
carriers of newly synthesized opsin from the Golgi to
the base of the connecting cilium.22 In amphibian retinas, vesicles and cisternae are observed in the inner
segment, especially in the ellipsoid and in the cytoplasm
adjacent to the basal body of the connecting cilium.23"25
Additional evidence favoring a precursor role for the
periciliary vesicles was obtained by freeze fracture
analysis of their structure. Besharse and Pfenninger23
showed that intramembranous particles in the periciliary vesicles had 10-nm diameters that were comparable to particles in outer segment disks. Their diameters were distinct from those in adjacent mitochondria
and inner segment plasma membranes. To evaluate
further the possible function of these vesicles, we began
a joint effort of electron microscopic autoradiographic
and immunocytochemical analysis of thin sections of
X. laevis juvenile, tadpole, and adult retinas. Our results
indicate that radiolabeled protein is closely associated
with ellipsoid and periciliary vesicle membranes after
2 hr of incorporation. Antibodies to opsin bind to these
post-Golgi vesicles in the ellipsoid. Together, these results indicate that some of these vesicles contain newly
synthesized opsin destined for the ROS.* These observations are introduced in Figure 1.
* Portions of these results were presented in preliminary form at
a meeting of the American Society for Cell Biology (Papermaster
DS, Schneider BG, and Besharse JC: J Cell Biol 83:275a, 1979).
Downloaded From: http://iovs.arvojournals.org/pdfaccess.ashx?url=/data/journals/iovs/933115/ on 06/18/2017
1388
INVESTIGATIVE OPHTHALMOLOGY & VISUAL SCIENCE / October 1985
Materials and Methods
Xenopus laevis tadpoles, juveniles, and adults were
maintained at 20-25 °C under a 12 hr light: 12 hr dark
cycle. Some tadpoles were maintained for 6 days in
darkness and exposed to light for 30 to 120 min. Tadpoles at larval stages 54-56 26 were injected with a single
dose of 50 ;uCi of L-[(N)-4,5-3H] leucine (New England
Nuclear; Boston, MA) with a specific activity of 54.6
Ci/mmol. Postmetamorphic juveniles (3.5 cm long)
received a mixture of tritiated amino acids consisting
of 100 j*Ci of L-[(N)-4,5-3H] leucine (specific activity,
50 Ci/mmol) from ICN Chemical and Radioisotope
Division, Irvine, California, and 50 ixd each of L[2,3,4-3H] valine (specific activity, 11.1 Ci/mmol) and
L-[ring-2,3,4,5,6-3H] phenylalanine (specific activity,
98.6 Ci/mmol) from New England Nuclear. Radioactive amino acids were delivered by intraperitoneal
injections using 30-gauge disposable needles at 15 to
60 min prior to light onset under red light (Wratten
No. 2 filter, Kodak; Rochester, NY). Fixations were in
darkness just before light onset or at 30, 60, and 120
min after light onset. Eye cups prepared by surgical
removal of cornea, iris, and lens were fixed either in
2% paraformaldehyde and 2.5% glutaraldehyde in 0.1
M cacodylate buffer at pH 7.4 (tadpoles), the same
aldehydes in 0.067 M cacodylate buffer (postmetamorphic juveniles), or 4% paraformaldehyde in 0.1 M
cacodylate buffer (tadpoles). The last fixation was carried out to control for possible nonspecific binding of
radiolabeled amino acids.27 Portions of the eye were
embedded in Spurr's medium (postmetamorphic juveniles)28 or Epon-Araldite (tadpoles) for autoradiography,15 and in bovine serum albumin (BSA)29-30 or
Lowicryl K4M (Polysciences; Warrington, PA) 3132 for
immunocytochemistry. These investigations were carried out in accordance with the ARVO Resolution on
the Use of Animals in Research.
EM Autoradiography
Thin sections (silver-gold interference colors) of retina were obtained; they were assumed to be 100 nm
thick for purposes of quantitative analysis. Sections
were coated on glass slides with a monolayer of Ilford
L-4 emulsion using the flat substrate method.33 Following exposure in black dessicator boxes, sections were
developed with Kodak D19 or Microdol X, Phenidonascorbic acid, or with Agfa-Gevaert fine grain developer
after gold latensification.34 Although the fine grain developers provided an excellent qualitative understanding of the distribution of radioactivity, the detailed
quantitative analysis presented in the results was con-
Vol. 26
ducted on sections developed with Microdol X because
such material has been thoroughly characterized with
regard to resolution and use in quantitative studies.35
We used a resolution value in units of half-distance
(HD = 157 nm) appropriate for our section thickness.35
Comparable analyses (not reported in results) were
carried out using tissues fixed in paraformaldehyde only
and on paraformaldehyde-glutaraldehyde fixed tissue
developed with Kodak D19 or Phenidon-ascorbic acid.
Although each of the three analyses involved fewer observed silver grains than the results described in Table
1, the results were consistent with those described in
Table 1 for Microdol X development of tissues fixed
with mixed aldehydes.
Thin sections passing through a plane approximately
parallel to the long axis of photoreceptors were used
to obtain a collection of micrographs for quantitative
analysis. The inner segment and basal outer segment
region of all cells visible in sections was photographed
and printed at a final magnification of XI 8,000. The
use of all visible cells rather than a selected midcellular
sample yielded a random collection of cells sectioned
in different axial planes.
In order to estimate the relative degree of labeling
of various sources within photoreceptor inner segments, we used the hypothetical grain distribution
method of Blackett and Parry36'37 as developed further
by Salpeter et al.38 In this method a hypothetical
sourcergrain matrix was generated using masks designed to take into account the extent to which radioactivity in a structure would be expected to contribute
silver grains over all adjacent structures. This matrix
was generated by identifying possible real sources of
radioactivity (rows in Table 1). Silver grains resulting
from hypothetical disintegrations were assigned to grain
compartments (columns in Table 1). Observed silver
grains were then tabulated using the same definition
of grain compartments as was used for generation of
the hypothetical source-grain matrix; the sources corresponding to real grains were, of course, unknown.
Although direct comparison of the hypothetical distribution with the real distribution was useful and was
formally analogous to comparisons made using the
probability circle method of Williams,39 the real value
of this approach was to obtain an estimate of the source
density of radioactivity within the individual organelles
identified as sources (Tables 2 and 3). The source densities were estimated by using a computer program
which systematically varied a series of multipliers
(source density values) that altered the hypothetical
source-grain matrix until the x 2 value in comparing
real and expected distributions was minimized. The
final parameters were then considered to be source
Downloaded From: http://iovs.arvojournals.org/pdfaccess.ashx?url=/data/journals/iovs/933115/ on 06/18/2017
1389
VESICULAR TRANSPORT OF OPSIN / Popermosrer er ol.
No. 10
Table 1. Localization of 3H-labeled protein in the ellipsoid of rod inner segments (source: grain matrix)
Grain compartments
PM
Cytoplasm
Mitochondria
Mitochondriacytoplasm
Vesiclecytoplasm
10
258
6.1
18
19
3.0
140
33
20.0
114
84
3.8
33
3
2.2
327
44
1.9
46
10
4.7
214
2006
7.8
29
8
6.4
205
402
8.2
3
4
1.2
21
8
13.5
794.2
314.1
394.8
411.1
2288.5
658.6
153.4
16.1
60.6
6.4
76.3
8.0
79.4
8.4
442.1
46.3
Observed %
74
7.7
66
6.9
108
11.3
150
15.7
x2
41.1
.5
13.1
62.8
Grain source
EC-RIM
Extracellular
ROS
PM
Cytoplasm
Mitochondria
Vesicles
661
9
8.1
61
53
2.1
Total
Expected %
ROS-RIM
Vesicle-PM
Total
6
3
2.6
6
928
966
9
4.9
2625
45.2
50.7
31.5
4943.5
127.3
13.2
9.8
1.0
6.1
0.6
955
236
24.7
179
18.8
101
10.6
41
4.3
955
100
96.1
21.0
848.7*
199.7*
1283.0
328
51.3
100
The source compartments (left column) were denned as those structures
seen in the ellipsoid region (mitochondria, smooth membrane vesicles and
cisternae, cytoplasm, and plasma membrane) as well as the region surrounding
the ellipsoid (extracellular, ROS) which could also contribute grains to the
ellipsoid region. The grain compartments (columns) consisted of the external
rim regions (EC, ROS) as well as mitochondria and cytoplasm. The remaining
grain compartments consisted of membranous components identified asjunctional items with cytoplasm. Thus, they were identified only in association with
surrounding cytoplasm. In each case, the structure was identified by use of a
circle with a 1 HD radius. An exception was the plasma membrane grain compartment which included grains within 1 HD inside or outside of the compartment. The center of the circle including an entire filamentous grain was
taken as the locus of that grain. The source: grain compartment matrix ("crossfire matrix") was generated using the validated masks for 3H published as Figure
4 in Salpeter et al.38 These masks show the expected contribution of radioactivity
in each source to silver grains in each grain compartment if radioactivity were
uniformly distributed. Because mask sources rarely fell over vesicles or plasma
membrane which were of principal concern in our analysis, sampling was improved by separate analysis of vesicles alone followed by normalization of the
expected grains from these sources to the entire matrix by a procedure devised
by Salpeter et al.38 The column totals represent the expected distribution of
silver grains over the grain compartments. Sources for individual real silver
grains were of course unknown. Observed grains were tabulated using the same
definitions for grain compartments as was used in generating the matrix. Note
that the high x 2 and low probability indicate significant differences between
observed and expected. The asterisks indicate some of the individual grain
compartments which contribute to the high x 2 . EC: extracellular; PM: plasma
membrane; ROS: rod outer segment; RIM: rim region immediately outside of
the ellipsoid. See Figure 4 for further definition of the cellular regions analyzed.
density values necessary to account for the observed
distribution of silver grains.
Retinas of 10 adults were embedded in Lowicryl K4M31
(Polysciences) and were examined by immunocytochemistry. Eleven retinas from tadpole, juvenile, and
adult Xenopus were also embedded in BSA30 and comparably studied.40 Thin sections were labeled sequentially for 15 min with the following: (1) biotinyl-rabbit
antifrog opsin, 0.1 to 0.4 mg/ml (affinity purified F(ab')2
EM Immunocytochemistry
Xenopus adults were killed under dim red light after
11-12 hr of dark adaptation or after light adaptation.
Table 2. Computed grain distribution compared to observed using x 2 distribution
Computed
distribution
Observed
x2
EC-RIM
ROSRIM
PM
Cytoplasm
Mitochondria
Mitochondriacytoplasm
Vesiclecytoplasm
VesiclePM
73.8
74
0.0007
65.9
66
0.0001
109.8
108
0.0312
153.9
150
0.0971
238.7
236
0.0299
168.7
179
0.6306
104.2
101
0.1031
40.0
41
0.0199
Using a computer program developed by Besharse and Schmidt similar to
that described by Land and Salpeter (1978), we estimated the density of radioactivity in each source (source density) which would be necessary to yield a
real distribution of silver grains like that observed. The basis of the computer
program is a x 2 minimization routine which modifies the rows of the matrix
with multipliers (source density values) until the hypothetical grains in the
grain compartments yield a distribution which gives the lowest possible x 2
when compared to the real distribution of silver grains. Our computer program
differs from that of Land and Salpeter (appendix to reference 38) principally
Total
955
955
0.9126
in that it is written in BASIC and can be used on the Apple II microcomputers.
In a validation test, both the Land and Salpeter program and our own program
gave the same answers to a series of sample problems.
Note at the bottom of Table 2 that in the final iteration of the program the
X2 values are minimal and the new computed distribution is not significantly
different from the observed distribution. The source density values can be regarded as multipliers for each source which when applied to the grain compartments alter the expected distribution.
Downloaded From: http://iovs.arvojournals.org/pdfaccess.ashx?url=/data/journals/iovs/933115/ on 06/18/2017
1390
INVESTIGATIVE OPHTHALMOLOGY G VISUAL SCIENCE / Ocrober 1985
Table 3. Computed source densities for
source compartments
Source
Computed
source
density*
Extracellular
ROS
PM
Cytoplasm
Mitochondria
Vesicles
0.031 ±0.02
0.114 ±0.04
1.243 ±0.68
0.412 ±0.04
0.044 ±0.01
6.903 ± 0.68
Arecrf
%
Activity^
Relative
specific
activity§
18.8
6.6
1.0
19.6
53.1
0.9
3.0
3.9
6.7
41.7
12.1
32.6
0.162
0.589
6.433
2.132
0.228
35.73
%
* Computer generated source densities which yield the computed grain distribution shown in Table 2. Source densities were generated as described in
Table 2, and are reported as the density value ± a probable error estimate
calculated according to the procedure of Salpeter et al.43
t Stereometric estimates derived from the grain source row totals in Table I.
$ This is calculated as the source density X total hypothetical grains in source
compartment divided by total observed grains.
§ This is calculated as % activity/% area.
fragment or biotinyl-IgG 22 ; (2) biotinyl-F(ab')2 of
sheep antirabbit F(ab') 2 , 0.1 mg/ml (affinity purified
IgG); and (3) avidin-ferritin (0.03 mg/ml in 0.1 M TrisHC1 pH 7.4.30-41 The rabbit antifrog opsin sera used in
this study were the same as the sera designated serum
1 and serum 2 previously.29 Data presented in Table
4 and Figures 5-8 are derived from duplicate experiments using antiserum 2. Figure 9 was from an experiment using antiserum 1. Sections were stained with
aqueous uranyl acetate and bismuth subnitrate42 after
the immunocytochemical sequence. Controls consisted
of replacement of the first-stage antibody with biotinylpreimmune or biotinyl-nonimmune rabbit F(ab') 2 , or
biotinyl IgG for the corresponding antiopsin antibody.
Vol. 26
Preimmune sera were obtained from rabbits subsequently immunized; nonimmune sera were obtained
from unimmunized rabbits.
Quantitative distribution of labeling was estimated
morphometrically by point counting of duplicate experiments of retinas obtained after 1 hr of light exposure
of images magnified at least XI 00,000. The specific
data for Table 4 were obtained by random sampling
to eliminate observer bias in selection of labeled areas.
On duplicate grids from duplicated experiments, a
centrally located section was sampled by use of a random number table (from 1 to 5) to select the cell by
counting from the left grid bar. If the cell thus selected
was a cone, the adjacent rod was photographed. Images
were recorded on 35-mm film and each roll contained
an image of a calibration grid to determine the magnification. The values for outer segment plasma membrane labeling were obtained from all rods in the sections which were oriented so that the plane of section
had passed through the connecting cilium and its projecting microtubules in the ROS. The ciliary shaft separated the plasma membrane from the disks (Fig. 7a).
Linear labeling densities along the rod outer and inner
segment plasma membrane were obtained by projecting the image on to a lattice with a Bellco plaque viewer
(Bellco; Vineland NJ) and sampling at intersections of
the membrane and the lattice lines. A second lattice
was superimposed and aligned along the plasma membrane so that the intersections with the first lattice were
centered in the square of the second lattice. The number of ferritin particles within a square were counted
and counts from five squares were collected to determine the mean density by the equation: 7VF(c) = NP/dPc
Table 4. Immunocytochemical labeling densities of the inner and outer segment membranes
of rod photoreceptors
Ferritins/fim2
Ferritins/nm
Cellular site
Antibody
Biotinyl-affinity
purified antiopsin
No. 2
Biotinylnonimmune IgG
ROS
plasma
membrane
Ellipsoid
plasma
membrane*
Myoid
plasma
membrane
55
±5
N = 17
6
±2
N = 9
7 ± 1
N = 20
0.5 ± 0.2
N = 15
0.9 ± 0.2
N = 17
0.5 ± 0.2
N = 15
* In one experiment, sections of a retina from a dark-adapted tadpole were
heavily labeled on the ellipsoid plasma membrane. All sections from those
blocks labeled in that pattern but subsequent experiments with other animals
could not reproduce this phenomenon.
t The labeling density of Golgi membranes varied greatly in micrographs
not selected at random (range 200 to 1000 ferritins//im2). All data in this table
are means of duplicate experiments whose labeling densities were determined
Rod outer
segments
1189±81
N = 9
5 ± 0.5
N = 16
Mitochondria
Golgi
membranes
SER
Interphotoreceptor
matrix
8± 2
N = 19
353 ± 29f
N = 13
38 ± 10*
N = 10
8±4
N = 20
5± 1
N = 17
10 ± 2
N = 10
10 ± 2
N = 14
2 ±0.5
N = 16
by random sampling (see text for details). N = the number of micrographs
counted to determine the mean and standard error.
% See Mercurio and Holtzman43 for discussion of the relative contributions
of membrane density surface area to the observed area density in the subellipsoid
SER. When corrected for the high membrane density of this closely apposed
set of membranes, the labeling density approaches background.
Downloaded From: http://iovs.arvojournals.org/pdfaccess.ashx?url=/data/journals/iovs/933115/ on 06/18/2017
VESICULAR TRANSPORT OF OPSIN / Popermosrer er ol.
No. 10
where NF is the sum of ferritin counts in each of the
small squares, d is the lattice spacing of the small square
(in micrometers corresponding to the magnification of
the image) and Pc is the number of lattice points intersecting the plasma membrane. These densities were
summed from all micrographs (N = 9 to 20) to determine the mean density ± standard error (SE) of the
sample analyzed. Area densities of the Golgi, smooth
endoplasmic reticulum (SER), mitochondria, and ROS
were obtained as previously described.30
Results
EM Autoradiography
At 2 hr 15 min postinjection, we found that considerable radioactivity was still contained in the myoid
region of the inner segment in close association with
the Golgi complex and, to a lesser extent, with the rough
endoplasmic reticulum (RER) (Fig. 2). Radiolabeled
proteins were also observed in the ellipsoid and clustered beneath the periciliary ridge complex at the base
of the connecting cilium (Figs. 3, 4). By 3 or 4 hr after
injection, extensive transfer of radioactivity from inner
to outer segment had occurred. This time course and
path of transport of radiolabeled rod proteins in X.
laevis retinas is similar to earlier observations of renewal of ROS proteins in R. pipiens.ll-l9A3M
The principal question that we wished to answer by
detailed analysis of sources of radioactive decay was
whether or not radioactivity in the ellipsoid region of
the inner segment, destined for ultimate incorporation
into ROS disks, was associated with the rich array of
vesicular cytomembranes observed in that region.
Qualitative evaluation, particularly of the vesicle-rich
periciliary region (Fig. 3), suggested association of radioactivity with abundant vesicles seen there. A small
amount of radioactivity was already associated with
ROS basal disks by 2 hr and 15 min of incorporation.
The 2 hr or 2 hr and 15 min time point was chosen
for detailed analysis because radioactivity was abundant
in the ellipsoid and transfer to the ROS had just begun.
Within the ellipsoid, autoradiographic grains were
associated with vesicles between mitochondria and
vesicles clustered about the base of the connecting cilium (Fig. 3). However, most grains were associated with
other organelles (see Fig. 4 and Table 1, row labeled
Observed) including cytoplasm, mitochondria, and the
perimitochondrial space. Because of the close spatial
packing of vesicular membranes and mitochondria
within the ellipsoid (Fig. 4) and the low level of resolution attainable with autoradiography relative to the
size of vesicles, we used quantitative procedures to assess the sources of radioactive decay. In a preliminary
1391
analysis, we used the probability circle method 39 in
which an expected distribution of silver grains based
on the hypothesis of uniform labeling was generated.
We found that the actual distribution of silver grains
differed from a uniform distribution with a high degree
of probability (P < 0.001). This analysis showed that
the compartments with major deviations from a uniform distribution were mitochondria, vesicles, and
plasma membrane. The latter compartments, of necessity, also included the immediately adjacent cytoplasm. The mitochondria contained far less label than
would have been expected if the distribution were uniform, whereas both vesicles and plasma membrane
contained far more label. The study of both paraformaldehyde- and glutaraldehyde-fixed material in this and
the subsequent analysis (see below) led to the same
conclusion regarding the localization of radioactive
sources. Although we are unable to rule out a low level
of nonspecific association of radioactivity with tissue
due to glutaraldehyde fixation,27 our data shows this
had little if any effect on the localization.
In order to estimate the relative amount of radioactivity contained in identifiable organelles rather than
in grain compartments, we utilized the hypothetical
grain distribution method.36"38 We obtained estimates
of relative source densities within organelles which
would lead to a given pattern of real grains over grain
compartments. 38 The analysis took three stages. First,
a hypothetical source:grain compartment matrix was
generated using overlays designed by Salpeter38 based
on knowledge of the resolution of the autoradiographic
technique. This matrix provided estimates of the silver
grains to be expected over defined grain compartments
if each organelle contributed to the distribution in proportion to its fractional area in the collection of micrographs (see definitions of source and grain compartments in Fig. 4 and Table 1). Second, real silver
grains were tabulated over grain compartments using
the same definitions that were used in generating the
hypothetical matrix. Comparison of the expected totals
in each grain compartment with the observed totals,
although formally analogous to the method used by
Williams,39 differs in its definition of compartments.
The hypothetical matrix and expected distribution under the assumption of uniform distribution of sources
are compared to the observed distribution of silver
grains in Table 1. Expected and observed distributions
differed from each other significantly (P < 0.001). As
in our preliminary analyses using the probability circle
method, the major deviations from uniformity were
attributable to the low level of mitochondrial and high
level of membrane compartment labeling compared to
an expected distribution if sources of radiolabel were
Downloaded From: http://iovs.arvojournals.org/pdfaccess.ashx?url=/data/journals/iovs/933115/ on 06/18/2017
OS
M
e
m »-
N
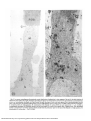
Fig. 2. Low power autoradiograms illustrating the overall distribution of radioactivity in inner segments 2 hr and 15 min after injection of
radioactive amino acids (2 hr in light). This figure and subsequent figures were obtained from retinas fixed in a glutaraldehyde-paraformaldehyde
mixture, a, Autoradiogram developed with Agfa-Gevaert fine grain developer of entire rod inner segment from a postmetamorphic juvenile
retina. The silver grains are localized predominantly over the Golgi apparatus and to a lesser extent over RER at this period of incorporation.
C: connecting cilium; G: Golgi apparatus; M: mitochondria of the ellipsoid; N: nucleus; ROS: rod outer segment (bar = 1 ^m; X6,500). b,
Autoradiogram developed with Phenidon-ascorbic acid of myoid region of a similar inner segment from a tadpole retina. The radiolabeled
protein is concentrated in the Golgi apparatus which extends from the perinuclear region to the base of the ellipsoid. G: Golgi apparatus; M:
mitochondria; N: nucleus (bar = 1 (im; X 16,000).
Downloaded From: http://iovs.arvojournals.org/pdfaccess.ashx?url=/data/journals/iovs/933115/ on 06/18/2017
No. 10
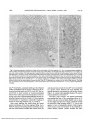
Fig. 3. High power autoradiograms illustrating the
distribution of radioactivity
in the rod periciliary region,
2 hr and 15 min after injection of radioactive amino acids into a postmetamorphic
X. laevis juvenile. Developed
with Agfa-Gevaert fine grain
developer, a, Section through
the connecting cilium showing association of silver grains
with vesicular membrane
profiles and basal rod outer
segment (ROS) disks and a
lack of grains over mitochondria (M). Some vesicles
are juxtaposed to the grooves
(G) of the periciliary ridge
complex. The connecting
cilium (C) joins the ROS to
the inner segment. Vesicles
(V) are clustered beneath the
basal body and are scattered
and compressed among the
densely packed mitochondria. Radiolabeled protein is
associated with the vesicles
and some has passed to the
ROS along the basal disks at
this time—2 hr (see Table 1)
(bar = I M m; X27,000). b,
Section through the periciliary region as evidenced by
presence of an associated
centriole of the basal body
complex (B) and the ridge (R)
and scalloped grooves (G) of
the periciliary ridge complex.
At this time of incorporation
(2 hr), silver grains are associated with vesicles (V) and
periciliary grooves. A vesicle
is captured during apparent
fusion with the base of a
groove (arrow) and may represent either delivery of opsin
by exocytosis or endocytosis
of plasma membrane (bar
- 1 (jm; X30,500).
1393
VESICULAR TRANSPORT OF OPSIN / Papermasrer er al.
ROS
G .R
M
uniform. The major contributor to the high x 2 value
was the vesicle-cytoplasm compartment which contained more than 10 times the number expected on
the basis of a uniform distribution (Table 1).
Finally, in our third stage of analysis, a computer
program designed to minimize x 2 by varying the source
density values was used to fit the hypothetical distribution in grain compartments so that the calculated
compartment totals corresponded to the observed grain
totals (Table 2). The optimized source density values
ROS
R
0*
V
B
are listed in Table 3 in units related to the numbers of
hypothetical grains in the matrix and as a percent of
total radioactivity. For comparison, the area fraction
of each source and relative specific activity (% activity/
% area) are also presented. Table 2 also includes the
minimized x 2 values which correspond to the optimized source densities.
The results in Tables 2 and 3 were derived by simulation but provide our best estimate of the radioactivity contained in each source. The data suggest that
Downloaded From: http://iovs.arvojournals.org/pdfaccess.ashx?url=/data/journals/iovs/933115/ on 06/18/2017
1394
INVESTIGATIVE OPHTHALMOLOGY b VISUAL SCIENCE / Ocrober 1985
Vol. 26
•**TT
Fig. 4. Autoradiogram of the ellipsoid region of a tadpole retina after 2 hr and 15 min of incorporation of radiolabeled amino acids. Developed
with Kodak D-19. This figure illustrates some of the grain compartments used for quantitative analysis. The dark line outlines the region defined
as ellipsoid. The dotted line defines a RIM region 5 HD units wide around the ellipsoid. This region could contain silver grains which originate
from sources of decay in the ellipsoid. It constitutes both the potential source compartment and a grain compartment. This RIM region is also
heterogeneous in that it contains myoid, ROS, and extracellular components. In Table 1, it constitutes the source compartments named myoid,
extracellular, and ROS and the grain compartments named extracellular-RIM and ROS-RIM. Other source and grain compartments are
denned in Table I. Open arrows indicate some of the intermitochondrial vesicles in the ellipsoid. G: groove of the periciliary ridge complex;
R: ridge (bar = I \im\ X20,000).
Downloaded From: http://iovs.arvojournals.org/pdfaccess.ashx?url=/data/journals/iovs/933115/ on 06/18/2017
No. 10
VESICULAR TRANSPORT OF OPSIN / Popermosrer er ol.
vesicle membranes contained about 30% of the radioactivity, and had a specific activity 17 times higher than
surrounding cytoplasm. In contrast, we estimated that
mitochondria, which occupy 64% of the area,f contained only 12% of the radioactivity and had a specific
activity of 0.23. The source compartment labeled
"plasma membrane" also exhibited a high specific activity. Although not considered as a separate source,
much of this radioactivity appeared to be associated
with the apical plasma membrane of the inner segment.
However, close apposition of apical plasma membrane
and lightly labeled basal ROS disks in most micrographs made it virtually impossible to distinguish the
relative contributions of the two adjacent membranes
as sources.
EM Immunocytochemistry
The other eye from each tadpole was embedded in
glutaraldehyde cross-linked bovine serum albumin to
localize immunoreactive opsin in the inner segment.
Because of the low contrast in BSA-embedded retinas,
we continued this investigation using Lowicryl K4M
(Polysciences) because of its superior tissue contrast.31'32
The enhanced contrast readily demonstrated opsin
bearing sites in Golgi and on vesicles in the ellipsoid
region.
Myoid region: The Golgi of X. laevis rod photoreceptors is axially oriented and may be semicylindrical
or wedge-shaped since longitudinal sections occasionally demonstrated paired sets of Golgi membranes on
each side of the myoid while oblique sections generated
crescentic or V-shaped profiles (Figs. 5a-c). Antiopsin
binding to the Golgi zone was easily appreciated even
at low magnification as a result of the enhancement of
labeling density by the three-stage technique (Fig. 5a).
Labeling of Golgi membranes was nearly confluent, an
indication that opsin may be highly concentrated at
this site prior to its transport toward the ROS. Variation
of labeling within the Golgi apparatus was considerable,
however (Fig. 5b). Although a mean labeling density
could be obtained by point counting (353 ± 29), the
labeling densities spanned a much larger range than
the ROS. Some domains of the Golgi apparatus were
as low as 200 ferritins/Mm2 while other areas approached ROS in labeling density (Fig. 5a; Table 4).
Surrounding the Golgi membranes was an ill-defined
zone from which ribosomes were excluded (Figs. 5ac). This zone was invariably unlabeled by antiopsin so
that the Golgi membrane profiles were well demarcated
f This stereometric estimate is obtained from Table 1 by omitting
area contributions from ROS and the extracellular compartments
and computing the percent area of mitochondria in the ellipsoid
proper.
1395
from the adjacent RER. Control sections labeled with
biotinyl nonimmune IgG, biotinyl antilgG, and avidinferritin were negligibly labeled (Fig. 5 c). We have previously observed dense labeling of Golgi zones of frog
photoreceptors with these antibodies applied to BSAembedded tissues.29
Between the Golgi and the mitochondria, smooth
endoplasmic reticulum membranes are closely packed.
Antiopsin binding was negligible over these membranes
and approached background levels (Fig. 5d; Table 4).
These unlabeled membranes were also shown to be
inactive in incorporation of radiolabeled amino acids
into protein and of radiolabeled choline and glycerol
into glycerolipids by Mercurio and Holtzman.43 Profiles
of closely packed membranes of unlabeled smooth endoplasmic reticulum were also found, occasionally, in
a cytoplasmic channel that extended between the mitochondria of the ellipsoid from the myoid toward the
periciliary ridge complex (Fig. 4).
Ellipsoid region: Vesicular profiles labeled by antiopsin were observed not only within the channel that
spanned the ellipsoid but also between the closely
packed mitochondria (Fig. 6). Vesicles were not more
commonly seen in the channel than outside it between
mitochondria. These vesicular profiles usually corresponded in size to those seen in epon sections and may
also represent cross-sections of serpentine cisternae.
There appeared to be some artifactual expansion of
some of the vesicles during Lowicryl embedding, however. Many of the vesicles were highly labeled but some
were unlabeled or were labeled by antiopsin heterogeneously. Some were slightly labeled, others were eccentrically labeled, and a few were confluently labeled
about the circumference of the vesicle in a pattern consistent with the binding of antibody on the cut edge of
the vesicle membrane. Some labeling appeared within
the vesicle interior, probably as a consequence of a
tangential section of the vesicle's cytoplasmic surface
or of labeling of the interior of the vesicle membrane.
Inspection of the figures revealed that vesicle membrane labeling density varies from sparse (ca 7/^m) to
confluent (ca 55//um) on labeled vesicles. Because of
this heterogeneity and for reasons based on the unique
geometry of vesicles (see Discussion), we were unable
to compare the vesicle membrane labeling density directly to the labeling density of the adjacent RIS and
ROS plasma membranes that are detailed in Table 4.
Stereo images of the Golgi and ellipsoid regions do not
indicate significant penetration of the surface of the
Lowicryl section. Thus labeling densities are not confounded by superposition of ferritins from within the
depth of the section.
Poor tissue contrast in BSA-embedded retinas and
compression caused by dehydration obscured potential
distinction of collapsed vesicles between mitochon-
Downloaded From: http://iovs.arvojournals.org/pdfaccess.ashx?url=/data/journals/iovs/933115/ on 06/18/2017
1396
INVESTIGATIVE OPHTHALMOLOGY G VISUAL.SCIENCE / October 1985
Vol. 26
RER
N
RER
MC
Fig, 5. Immunocytochemical localization of opsin in the myoid region of the inner segment of A', laevis rod photoreceptors embedded in
Lowicryl K4M. These figures and Figures 6-8 are of Xenopus laevis adults killed I hr after light onset after entrainment to a 12 hr light: 12 hr
dark cycle of light exposure. The three-stage label consisted of affinity purified biotinyl-rabbit IgG of antifrog opsin, biotinyl-sheep F(ab')2 of
antirabbit F(ab') 2 , and avidin-ferritin. The three-stage label enhances the density of bound ferritins without increasing background. An average
of 5-7 ferritins are clustered on each bound antiopsin. a, The closely stacked Golgi membranes (G) near the nucleus (N) are obscured by the
dense label of antiopsin-ferritin complexes bound on the section surface. The perinuclear plasma membrane (arrows) and adjacent Miiller cell
(MC) with its processes (P) are unlabeled (bar = 1 /im; original magnification, X20,000). b, This axially arrayed Golgi is labeled at a density
(449/Vm2) that is near the mean density of several rods (see Table 4). The labeling is not uniform over the entire Golgi, however. The Golgi
zone is separated from the surrounding RER by a low-contrast border which is relatively unlabeled (bar = 0.5 ftm; original magnification,
X 39,000).
dria.40 Nonetheless, antiopsin labeling in the ellipsoid
of BSA embedded rods was largely confined to the intermitochondrial space (see Fig. 9 in ref. 40). The improvement in tissue contrast of Lowicryl-embedded
tissues more readily permitted interpretation of the labeling in this area. Mitochondria] labeling density approached background levels (Table 4). Control sections
demonstrated no significant nonspecific binding of
second or third stage reagents (Fig. 7c; Table 4).
Only scant labeling was noted along the lateral
plasma membrane of the inner segment (Figs. 5a, 6;
Table 4). Sections including plasma membrane alongside the actin filament bundles that extend from the
calycal processes toward the myoid45 were not labeled
to a greater extent. Between the actin bundles, the
plasma membrane was labeled by rare ferritin clusters
(Figs. 5-7), a result which parallels studies of R. pipiens
retinas labeled by immersion.46
Periciliary ridge complex: The plasma membrane
surrounding the base of the connecting cilium of R.
pipiens rods and cones is highly folded into an array
of nine ridges and grooves to form a domain termed
the periciliary ridge complex (PRC).47 X. laevis rods
appear to have a comparable domain. Both longitudinal sections and cross-sections of the ellipsoid revealed densely labeled vesicles beneath the PRC
Downloaded From: http://iovs.arvojournals.org/pdfaccess.ashx?url=/data/journals/iovs/933115/ on 06/18/2017
No. 10
VESICULAR, TRANSPORT OF OPSIN / Papermosrer er ol.
1397
M
SER
RER
Fig. 5c. Control section labeled with biotinyl-nonimmune IgG, biotinyl-sheep F(ab')2 of antirabbit F(ab')2, and avidin-ferritin. The Golgi
membranes (G) are unlabeled. A rare antibody-ferritin complex is circled (bar = 0.5 fim; original magnification, X32,000). d, Near the junction
of the myoid and ellipsoid, smooth endoplasmic reticulum (SER) membranes form close-packed arrays. SER membranes and the adjacent
mitochondria (M) are virtually unlabeled. The very tow level of labeling of the lateral plasma membrane (arrow) indicates that this membrane
apparently does not participate as a major pathway for transport of opsin to the outer segment (see Table 4) (bar = 1 fim; original magnification,
X28,000). Inset, Epon-embedded rod cell illustrating the conventional appearance of the subellipsoid SER (bracket) (bar = I jim; original
magnification, X 14,000).
grooves (Figs. 7-9). Vesicular profiles were observed
in all planes of section which indicated that their shape
was predominantly spherical in this region and probably did not arise from an elaborately folded cisternal
array (compare, for example, the appearance of the
subellipsoid SER cisternae, Fig. 5d, inset). In an occasional section, a vesicle was captured in apparent
fusion with the base of the groove of the PRC (Fig. 8).
Microtubules radiated from the basal body region into
the ellipsoid but vesicles were not obviously aligned
along them. Despite their close proximity to the plasma
membrane of the grooves and ridges of the PRC, the
vesicles were excluded from the volume immediately
surrounding the accessory centriole and basal body of
the connecting cilium (Fig. 7a-c). The area of vesiclefree cytoplasm measured 0.1-0.2 jum in diameter. The
high labeling density of these periciliary vesicles was
difficult to quantify because the diameter of the antibody-ferritin clusters precluded assignment of the
bound ferritins to one vesicle or its neighbor (see Discussion). The labeling density clearly exceeded the labeling of the adjacent RIS plasma membrane which
could be easily quantitated (Fig. 7; Table 4). On some
vesicles, the labeling density approached the density of
the rod inner segment plasma membrane.
The basal plasma membrane of the connecting cilium usually was unlabeled. In cross-sections, the cilium
was occasionally labeled on its plasma membrane (Fig.
9). This may be an indication of transport along the
plasma membrane of the cilium to the outer segment
in axial lanes47'48 and is the subject of further study.
Beyond the basal cilium, the plasma membrane of the
distal cilium and rod outer segment was confluently
labeled at levels 10-fold above the adjacent inner segment plasma membrane (Fig. 7a; Table 4). The inner
segment plasma membrane was labeled at levels which
approached background (Figs. 5-7; Table 4). This emphasizes the extraordinary polarity of opsin distribution
in the rod photoreceptor plasma membrane despite its
continuity across the connecting cilium.
Discussion
Our study provides direct evidence that specific vesicular membranes vectorially transport newly synthesized opsin across the large intracellular space from the
Golgi zone to the periciliary ridge complex. Quantitative analysis of sources of radiolabeled protein decay
indicated that the most heavily labeled structures in
the ellipsoid were the membranous vesicles. Despite
Downloaded From: http://iovs.arvojournals.org/pdfaccess.ashx?url=/data/journals/iovs/933115/ on 06/18/2017
1398
INVESTIGATIVE OPHTHALMOLOGY G VISUAL SCIENCE / Ocrober 1985
Vol. 26
M
Fig. 6. Longitudinal section of the ellipsoid region (Lowicryl K.4M embeddment) labeled with antiopsin-ferritin complexes as in Figure 5.
An oblique channel passing between the mitochondria (M) to the base of the connecting cilium is often observed in sections through the center
of the cell. Free ribosomes, rough endoplasmic reticulum cisternae, microtubules, stacked smooth endoplasmic reticulum, short tubular cisternae,
and vesicles are often seen in this channel. This micrograph illustrates an unusual degree of clustering of heavily labeled vesicles in the channel
(V). Most vesicles are packed between the closely apposed mitochondria (arrows). Occasionally, labeled vesicles are found beneath the lateral
plasma membrane (open arrow). The labeling density of the lateral plasma membrane varies (arrowheads) but does not approach the level of
vesicle or Golgi (G) labeling (bar = 1 ^m; original magnification, X31,000). Inset, Higher magnification image of ellipsoidal vesicles illustrating
the labeling of the vesicle margins (original magnification, X74,000).
Downloaded From: http://iovs.arvojournals.org/pdfaccess.ashx?url=/data/journals/iovs/933115/ on 06/18/2017
No. 10
1399
VESICULAR TRANSPORT OF OPSIN / Popermosrer er ol.
M
M
M
Fig. 7. Longitudinal section of the connecting cilium joining the inner and outer segments of X. laevis rod photoreceptors. a, Section of
Lowicryl-embedded retina labeled with antiopsin-ferritin complexes as in Figure 5. The tightly clustered vesicles (V) beneath the cilium (C) as
well as the rod outer segment (ROS) disks and ROS plasma membrane (arrowhead) are confluently labeled. Mitochondria (M) and the lateral
plasma membrane of the inner segment (arrow) are unlabeled (bar = I ^m; X48,000). b, Section of epon-embedded retina. Vesicles clustered
beneath the cilium are comparable in size and distribution to those seen after Lowicryl embeddment (bar = I nm; x35,000). c, Control section
labeled as in Figure 5a inset. Ferritin density is insignificant (bar = 1 fim; X30,000).
the small tissue volume they occupied (Tables 1-3),
they exhibited a relative specific activity far greater than
any other organelle. Two points should be considered
regarding the reliability of our source density estimates.
First, similar results were obtained in three additional
analyses (data not shown). Second, error estimates for
the source density values using the procedure of SaU
peter et al38 indicated that the individual density values
in the analyses were reliable (see Table 3, column 1).
We found that a substantial proportion (41.7%) of
the total radioactivity was associated with cytoplasm.
In the myoid region, a higher proportion of the total
radioactivity was associated with rough endoplasmic
reticulum and Golgi apparatus (Fig. 1). The silver
Downloaded From: http://iovs.arvojournals.org/pdfaccess.ashx?url=/data/journals/iovs/933115/ on 06/18/2017
1400
INVESTIGATIVE OPHTHALMOLOGY & VISUAL SCIENCE / October 1985
Vol. 26
pected. Previous studies21 have shown that most of incorporated label in the intermediate and microsomal
membrane fractions (fractions 2 + 3) from whole retina
was contained in opsin but that cytosol fractions (fraction 4) contained nonopsin radiolabeled proteins.
Overall, the high relative specific activity of the vesicle
compartment (Table 3, column 4) highlights the preponderant role of this set of membranes in transport
of newly synthesized opsin.
V
B
M
Fig. 8. Oblique section passing through the periciliary ridge complex
(PRC). A vesicle (arrow) is captured while in apparent fusion with
the groove of the PRC. Some labeled vesicles between mitochondria
(arrowhead) may be as confluently labeled as the juxtaciliary vesicles,
others are eccentrically labeled. Vesicles are excluded from the cytoplasm surrounding the accessory centriole of the basal body. B:
accessory centriole of the basal body; G: PRC groove; R: PRC ridge;
M; mitochondria; ROS: rod outer segment (bar = 0.5 fim; original
magnification, X45,000).
grains in the "myoid-RIM" region (see Fig. 4 for definitions) were included largely in the "cytoplasm" grain
compartment. Because many grains probably originated from disintegrations in adjacent heavily labeled
RER and Golgi compartments, their inclusion in "cytoplasm" would cause us to overestimate the labeling
of this compartment in the ellipsoid. The assignment
of radioactive sources to cytoplasm may also reflect an
underestimate of the vesicle-related radioactivity that
would occur because the large filamentous silver grains
obscure underlying ultrastructure. The small size of
the vesicles would lead to an erroneous association of
grains with cytoplasm and an overestimation of the
cytoplasmic grain density. Despite these uncertainties,
a significant level of cytoplasmic labeling would be ex-
To determine if the radiolabeled vesicle population
also contained opsin, we conducted parallel immunocytochemical localization studies with antiopsin antibodies. Binding of these antibodies was restricted to
vesicular and cisternal profiles in intermitochondrial
spaces and the vesicle-rich region adjacent to the base
of the connecting cilium (Figs. 6-9; Table 4). In a more
recent study, combined freeze-fracture and immunocytochemical labeling demonstrated binding of sheep
antibovine opsin to the interiors of ellipsoidal vesicles.48
The Golgi apparatus was labeled prominantly by
antiopsin. The.labeling density was less than the density
over ROS but much greater than the adjacent RER
(Figs. 5a-c). To the extent that immunocytochemical
labeling density reflects antigen density—and not just
antigen exposure—the greater labeling of Golgi apparatus embedded in both Lowicryl and albumin suggests that newly synthesized opsin is concentrated in
Golgi membranes prior to its vectorial transport to the
periciliary ridge complex. This result with Xenopus
retinas parallels our earlier localization of opsin in the
Golgi of Rana rod photoreceptors.29
The Golgi apparatus also became heavily labeled
with radioactive proteins within 1 hr of incorporation.
The time-course of passage of newly synthesized protein from RER to Golgi evaluated by EM autoradiography was comparable in these X. laevis retinas and
the Rana retinas studied by Young and Droz18 and
Hall et al.17 The structure of bovine opsin's oligosaccharides and autoradiographic studies of radiolabeled
sugar incorporation in frog retinas suggested that the
Golgi probably completes the processing of the oligosaccharides by addition of N-acetyl-glucosamine to the
nonreducing terminal.49"51 Amphibian opsins are also
glycosylated and comparable synthetic steps may occur
in their photoreceptors. The high density of opsin in
the Golgi zone revealed by immunocytochemistry also
suggests that it may serve as a center for concentration
of opsin prior to its distribution to the outer segment.
Comparable functions for the Golgi apparatus have
been proposed in studies of virus biosynthesis.7'8
The absence of opsin in soluble cytosol fractions21
and the similar size of vesicular intramembranous particles (IMP) and of ROS IMP in freeze fracture studies23
suggested that the vesicles contained opsin. The IMP
density of the vesicles was only half that of the ROS
Downloaded From: http://iovs.arvojournals.org/pdfaccess.ashx?url=/data/journals/iovs/933115/ on 06/18/2017
No. 10
VESICULAR TRANSPORT OF OPSIN / Popermosrer er ol.
*
*
•
<
1401
.
V
Fig. 9. Cross-section of the basal portion of a rod photoreceptor connecting cilium (C) as it arises from the periciliary ridge complex (embedded
in Lowicryl K4M and labeled as in Figure 5 with antiopsin serum 1). The animal was killed 3 hx after light onset. The ridges (R) and grooves
(G) form a deep invagination about the base of the cilium. Vesicles (V) are tightly clustered in this region and are highly labeled by antibodyferritin complexes on their margins. Occasional vesicles are labeled on the vesicle's interior (open arrow). Antibodies are also bound on the
plasma membranes of the ridges and grooves. A few complexes label the plasma membrane of the cross-sectioned basal cilium alongside the
microtubules. This may be an indication of a final pathway for transport of opsin from the inner to the outer segment along the ciliary plasma
membrane (bar = 1 fim; original magnification, X51,000).
disks which may be an indication that a two-fold concentration of opsin and destruction or retrieval of an
equivalent amount of IMP-free membrane occurs.
Preliminary evidence for retrieval of membrane has
been demonstrated in short-term retina cultures that
were exposed to horseradish peroxidase. Some of the
vesicles in the ellipsoid region became labeled with endocytosed peroxidase. 5253 Some of the unlabeled vesicles of the ellipsoid region revealed in both the autoradiographic and immunocytochemical portions of this
study may represent this population of endocytic vesicles (Figs. 4, 6-9). It should be noted that we did not
identify separate subpopulations of vesicles. In the autoradiographic analysis, the inclusion of an unlabeled
subset of vesicles would cause us to underestimate the
true specific activity of the vesicles transporting newly
synthesized opsin.
In the immunocytochemical studies, many of the
intermitochondrial vesicles in the ellipsoid region and
the vesicles clustered beneath the periciliary ridge
complex appeared as poorly demarcated pale oval
structures covered partially by an area of clustered label
(Fig. 9, open arrow). Other vesicles were labeled on
their circular margins (Figs. 7-9). This variation in labeling distribution and vesicle appearance would be
expected because the vesicle's small diameter approached the thickness of the thin sections. If some of
the vesicles were tangentially sectioned so that their
membranes were barely exposed on the thin section's
surface, the membrane-bound label would appear to
fall over the vesicle's interior. For this reason, we cannot
expect and did not observe label exclusively associated
with the margins of circular or elongated membrane
vesicles and cisternae cut in cross section.
Use of thin-sectioned retinas embedded in hydrophilic media eliminates the impermeable plasma
membranes as a barrier to labeling by antibody-ferritin
complexes by exposing intracellular antigenic sites di-
Downloaded From: http://iovs.arvojournals.org/pdfaccess.ashx?url=/data/journals/iovs/933115/ on 06/18/2017
1402
INVESTIGATIVE OPHTHALMOLOGY G VISUAL SCIENCE / Ocrober 1985
rectly on the section surface. Quantitative comparison
of labeling densities of well-ordered intracellular membranes exposed on the surface of such sections may be
informative. Such comparisons of labeling densities of
rod and cone photoreceptor outer segments readily
demonstrated different degrees of immunocytochemical cross-reactivity among these cells.29 We have applied this quantitative approach confidently to certain
domains of the rod inner segment (Table 4).
In the less homogeneous areas of the inner segment,
however, the geometric factors just discussed, as well
as the actual density of an antigen, may contribute
significantly to the observed labeling density. Interpretation of labeling densities on these domains is therefore
of much more limited value so that we could not determine an apparent labeling density of the periciliary
vesicles with the same degree of confidence. The vesicles in the ellipsoid and beneath the periciliary ridge
complex were labeled to a variable extent. Some were
unlabeled, others were eccentrically or lightly labeled,
while many were confluently labeled (Figures 6 and
9). If only labeled vesicles are considered, the densities
ranged approximately from 7 to 60 ferritins//*m of vesicle membrane length. The most confluently labeled
vesicles clearly exceeded the labeling densities of the
adjacent RIS plasma membranes and approached the
appearance of the labeling of the ROS plasma membrane. Since some of the vesicles are closely packed,
however, the apposed membranes may contribute to
this appearance. This technique is clearly unable to
establish directly the absolute antigen density of the
vesicles.
Identification of opsin as a major vesicle component,
however, increases the likelihood that the IMP observed
in the vesicles by freeze fracture are opsin clusters.
Freeze fracture readily permitted an evaluation of particle density in different domains of the cell and indicated that the opsin density of vesicles was half that of
the ROS disks.23 Comparisons of labeling density over
other domains of the inner segment are offered as an
indication of the variation of labeling in repeated experiments in order to document the reproducibility of
the observations and to highlight the high labeling density in the Golgi apparatus and the clear demarcation
of the boundaries of opsin insertion in the plasma
membrane (Table 4).
The high labeling density of the Golgi apparatus and
the periciliary vesicles and the large numbers of these
vesicles clustered beneath the PRC suggests that opsin
transport from sites of synthesis in the rough endoplasmic reticulum to the outer segment is associated
with high steady-state concentration of the protein at
these two sites. To what extent the absolute opsin density in these sites is subject to physiologic stimuli is not
yet known. We attempted to evaluate the role of light
Vol. 26
to determine if the periciliary vesicle population might
become depleted by light exposure. Previously, it had
been observed that most of the new disks formed during
a day were assembled in the first 8 hr.15 We did not
observe any large-scale depletion of the vesicle population at any time in the circadian cycle (cf Figs. 7-9),
even after prolonged exposure to darkness and short
exposure to light, a condition which favors a major
increase in disk assembly.54
Stimulated by these observations, we have begun intensive study of the periciliary region of the cell tc
identify the cellular components involved in the last
stages of opsin delivery to the outer segment. We have
observed that the plasma membrane of proximal portions of both amphibian and rat connecting cilia were
nearly unlabeled by antiopsin when compared to the
dense label of membranes of the distal portions of the
cilium or the adjacent vesicles beneath the cilium.40
High resolution scanning electron micrographs of the
apical plasma membrane of the frog rod and cone inner
segment revealed an extraordinary array of nine ridges
and grooves surrounding the connecting cilium which
was named the periciliary ridge complex.47 It may be
a site for insertion of opsin-bearing vesicles as they terminate their passage through the inner segment. Both
the autoradiographic and immunocytochemical results
indicate that the vesicles may fuse with the base of the
groove (Figs. 3b, 8). Low density labeling of the base
of the cilium contrasts sharply with the nearly confluent
labeling of the distal ciliary plasma membrane (Fig.
7a). Consequently, we have proposed that the base of
the cilium may be part of a one-way gate forming the
boundary between the outer and inner segment—permitting passage of opsin to the ROS but restricting
back diffusion on to the lateral plasma membrane of
the inner segment.23'40'46
The photoreceptor cell apparently has separated,
both spatially and kinetically, the processes of membrane protein biosynthesis, transport and insertion. Our
evidence that opsin laden vesicles transport opsin in
rod photoreceptors raises several fundamental issues
concerning the cell's mechanisms for intracellular vectorial transport of membrane proteins. Since the vesicle
membranes are often confluently labeled and have high
10 nm IMP density, it is likely that the opsin content
of these specific vesicles is quite high, perhaps as high
as 50% of the density of opsin in the ROS disk. Does
opsin contain, in some domain of the molecule, the
specific information perceived by the inner segment as
an "address" to direct it uniquely to one pole of the
cell or does the vesicle contain other molecules with
this function? What provides the "address" for the
membrane proteins destined for the lateral and synaptic
membrane proteins? If the Golgi apparatus is a center
for sorting membrane molecules to their specific des-
Downloaded From: http://iovs.arvojournals.org/pdfaccess.ashx?url=/data/journals/iovs/933115/ on 06/18/2017
No. 10
VESICULAR TRANSPORT OF OPSIN / Popermosrer er ol.
tination, is the same Golgi apparatus used for biosynthesis and sorting of other photoreceptor membrane
proteins as appears to be the case for cells infected simultaneously with two viruses?7 While these same
questions can be asked of any polarized cell, the photoreceptor continues to provide an especially valuable
object to probe them without perturbing its normal
functions and renewal of its membranes.
12.
13.
Key words: antibody, autoradiography, Golgi, immunocytochemistry, membrane biosynthesis, rhodopsin, vesicle,
Xenopus
14.
Acknowledgments
15.
The authors are grateful to Dr. Beth Burnside for pointing
out the possible role of inner segment vesicles from her early
unpublished studies of monkey retinas. The authors thank
Drs. Ewald Weibel and Jean Pierre Kraehenbuhl for helpful
discussions of morphometry and immunocytochemistry, Dr.
Frederick Schmidt for his efforts in writing the computer
program for autoradiographic analysis, and Dr. Miriam Salpeter for advice on autoradiography and for running sample
autoradiographic data sets from our study on her computer
program to confirm the accuracy of our derived program.
16.
17.
18.
19.
References
20.
1. Abrahamson DR and Rodewald R: Evidence for the sorting of
endocytic vesicle contents during the receptor-mediated transport
of IgG across the newborn rat intestine. J Cell Biol 91:270, 1981.
2. Kuhn LC and Kraehenbuhl JP: The membrane receptor for
polymeric immunoglobulin is structurally related to secretory
component. J Biol Chem 256:12490, 1981.
3. Steinman RM, Mellman IS, Muller WA, and Cohn ZA: Endocytosis and the recycling of plasma membrane. J Cell Biol 96:1,
1983.
4. Willingham MC and Pastan I: The receptosome: an intermediate
organelle of receptor-mediated endocytosis in cultured fibroblasts.
Cell 21:67, 1980.
5. Matlin K, Bainton DF, Pesonen M, Louvard D, Genty N, and
Simons K: Transepithelial transport of a viral membrane glycoprotein implanted into the apical plasma membrane of MadinDarby canine kidney cells. I. Morphological evidence. J Cell Biol
97:627, 1983.
6. Pesonen M and Simons K: Transepithelial transport of a viral
membrane glycoprotein implanted into the apical plasma membrane of Madin-Darby canine kidney cells. II. Immunological
Quantitation. J Cell Biol 97:638, 1983.
7. Rindler MJ, Ivanov IE, Pleskin H, Rodriguez-Boulan E, and
Sabatini D: Viral glycoproteins destined for apical or basolateral
plasma membrane domains traverse the same Golgi apparatus
during their intracellular transport in doubly infected MadinDarby canine kidney cells. J Cell Biol 98:1304, 1984.
8. Rothman JE, Fries E, Dunphy WG, and Urbani LJ: The Golgi
apparatus, coated vesicles, and the sorting problem. Cold Spring
Harbor Symp Quant Biol 46(Pt. 2):797, 1982.
9. Rodriguez-Boulan E and Sabatini.DD: Asymmetric budding of
viruses in epithelial monolayers: a model system for study of
epithelial polarity. Proc Natl Acad Sci USA 75:5071, 1978.
10. Rodriguez-Boulan EJ and Pendergast M: Polarized distribution
of viral envelope glycoproteins in the plasma membrane of infected epithelial cells. Cell 20:45, 1980.
11. Rodriguez-Boulan EJ: Membrane biogenesis, enveloped RNA
21.
22.
23.
24.
25.
26.
27.
28.
29.
30.
31.
1403
viruses and epithelial polarity. In Modern Cell Biology, Vol 1,
Satir BH, editor. New York, Alan R Liss, 1982, pp. 119-170.
Rodriguez-Boulan E, Green RF, Meiss HK, and Sabatini DD:
Enveloped viruses as tools for the study of epithelial polarity. In
Perspectives in Differentiation and Hypertrophy, Anderson W
and Sadler W, editors, Elsevier Science Publishing, 1982, pp.
51-64.
Rodriguez-Boulan E, Paskiet KT, Salas PJI, and Bard E: Intracellular transport of influenza virus hemagglutinin to the apical
surface of Madin-Darby canine kidney cells. J Cell Biol 98:308,
1984.
Besharse JC: The daily light-dark cycle and rhythmic metabolism
in the photoreceptor-pigment epithelium complex. In Progress
in Retinal Research, Vol 1, Osborne N and Chader G, editors.
New York, Pergamon Press, 1982, pp. 81-124.
Besharse JC, Hollyfield JG, and Rayborn ME: Turnover of rod
photoreceptor outer segments. II. Membrane addition and loss
in relationship to light. J Cell Biol 75:507, 1977.
Pfenninger KH and Maylie-Pfenninger MF: Lectin labeling of
sprouting neurons. II. Relative movement and appearance of
glycoconjugates during plasmalemmal expansion. J Cell Biol 89:
547, 1981.
Hall MO, Bok D, and Bacharach ADE: Biosynthesis and assembly
of the rod outer segment membrane system: formation and fate
of visual pigment in the frog retina. J Mol Biol 45:397, 1969.
Young RW and Droz B: The renewal of protein in retinal rods
and cones. J Cell Biol 39:169, 1968.
Young RW: Visual cells and the concept of renewal. Invest
Ophthalmol 15:700, 1976.
Young RW: Passage of newly formed protein through the connecting cilium of retinal rods in the frog. J Ultrastruct Res 23:
462, 1968.
Papermaster DS, Converse CA, and Siu J: Membrane biosynthesis
in the frog retina: opsin transport in the photoreceptor cell. Biochemistry 14:1343, 1975.
Papermaster DS, Converse CA, and Zorn M: Biosynthetic and
immunochemical characterization of a large protein in frog and
cattle rod outer segment membranes. Exp Eye Res 23:105, 1976.
Besharse JC and Pfenninger KH: Membrane assembly in retinal
photoreceptors. I. Freeze-fracture analysis of cytoplasmic vesicles
in relationship to disc assembly. J Cell Biol 87:451, 1980.
Holtzman E, Schacher S, Evans J, and Teichberg S: Origin and
fate of membranes of secretion granules and synaptic vesicles:
membrane circulation in neurons, gland cells and retinal photoreceptors. In The Synthesis, Assembly and Turnover of Cell
Surface Components, Poste G and Nicholson GL, editors. New
York, Elsevier-North Holland, 1977, pp. 165-246.
Kinney MS and Fisher SK: The photoreceptors and pigment
epithelium of the larval Xenopus retina: morphogenesis and outer
segment renewal. Proc R Soc Lond [Biol] 201:149, 1978.
Nieuwkoop PD and Faber J: Normal Table of Xenopus laevis
(Dandin). Amsterdam, North Holland Publishing, 1967, p. 245.
Peters T Jr and Ashley CA: An artefact in radioautography due
to binding of free amino acids to tissues by fixatives. J Cell Biol
33:53, 1967.
Spurr AR: A low-viscosity epoxy resin embedding medium for
electron microscopy. J Ultrastruct Res 26:31, 1969.
Papermaster DS, Schneider BG, Zorn MA, and Kraehenbuhl
JP: Immunocytochemical localization of opsin in the outer segments and Golgi zones of frog photoreceptor cells. J Cell Biol
77:196, 1978.
Schneider BG and Papermaster DS: Immunocytochemistry of
retinal membrane protein biosynthesis at the electron microscopic
level by the albumin embedding technique. Methods Enzymol
96:485, 1983.
Altman LA, Schneider BG, and Papermaster DS: Rapid embed-
Downloaded From: http://iovs.arvojournals.org/pdfaccess.ashx?url=/data/journals/iovs/933115/ on 06/18/2017
1404
32.
33.
34.
35.
36.
37.
38.
39.
40.
41.
42.
43.
INVESTIGATIVE OPHTHALMOLOGY & VISUAL SCIENCE / Ocrober 1985
ding of tissues in Lowicryl K.4M for immunoelectron microscopy.
J Histochem Cytochem 32:1217, 1984.
Carlemalm E, Garvito M, and Villiger W: Resin development
for electron microscopy and an analysis of embedding at low
temperature. J Microsc 126:123, 1982.
Salpeter MM and Bachmann L: Autoradiography. In Principles
and Techniques of Electron Microscopy: Biological Applications,
Vol 2, Hayat MA, editor. New York, Van Nostrand Reinhold,
1972, pp. 219-278.
Kopriwa BM: A comparison of various procedures for fine grain
development in electron microscopic radioautography. Histochemistry 44:201, 1975.
Salpeter MM, Bachmann L, and Salpeter EE: Resolution in electron microscope radioautography. J Cell Biol 41:1, 1969.
Blackett NM and Parry DM: A new method for analyzing electron
microscope autoradiographs using hypothetical grain distributions. J Cell Biol 57:9, 1973.
Blackett NM and Parry DM: A simplified method of "hypothetical grain" analysis of electron microscope autoradiographs.
J Histochem Cytochem 25:206, 1977.
Salpeter MM, McHenry FA, and Salpeter EE: Resolution of
electron microscope autoradiography. IV. Application to analysis
of autoradiographs. J Cell Biol 76:127, 1978.
Williams MA: The assessment of electron microscope autoradiographs. Adv Opt Elect Microsc 3:219, 1969.
Papermaster DS and Schneider BG: Biosynthesis and morphogenesis of outer segment membranes in vertebrate photoreceptor
cells. In Cell Biology of the Eye, McDevitt D, editor. New York,
Academic Press, 1982, pp. 475-531.
Papermaster DS, Reilly P, and Schneider BG: Cone lamellae
and red and green rod outer segment disks contain a large intrinsic
membrane protein on their margins: an ultrastructural immunocytochemical study of frog retinas. Vision Res 22:1417, 1982.
Ainsworth SK and Karnovsky MJ: An ultrastructural staining
method for enhancing the size and electron opacity of ferritin
in thin sections. J Histochem Cytochem 20:225, 1972.
Mercurio AM and Holtzman E: Ultrastructural localization of
glycerolipid synthesis in rod cells of the isolated frog retina. J
Neurocytol 11:295, 1982.
Vol. 26
44. Young RW: The renewal of photoreceptor outer segments. J
Cell Biol 33:61, 1967.
45. Chaitin MH, Schneider BG, Hall MO, and Papermaster DS:
Actin in the photoreceptor connecting cilium: immunocytochemical localization to the site of outer segment disk formation.
J Cell Biol 99:239, 1984.
46. Nir I and Papermaster DS: Differential distribution of opsin in
the plasma membrane of frog photoreceptors: an immunocytochemical study. Invest Ophthalmol Vis Sci 24:868, 1983.
47. Peters KR, Palade GE, Schneider BG, and Papermaster DS: Fine
structure of a periciliary ridge complex of frog retinal rod cells
revealed by ultrahigh resolution scanning electron microscopy.
J Cell Biol 96:265, 1983.
48. Defoe DM and Besharse JC: Membrane assembly in retinal photoreceptors. II. Immunocytochemical analysis of freeze-fractured
rod photoreceptor membranes using anti-opsin antibodies. J
Neurosci 1985, 5:1023.
49. Bok D, Hall MO, and O'Brien P: The biosynthesis of rhodopsin
as studied by membrane renewal in rod outer segments. In International Cell Biology (1976-1977), Brinkley BR and Porter
KR, editors. New York, Rockefeller University Press, 1977, pp.
608-617.
50. Fukuda MN, Papermaster DS, and Hargrave PA: Rhodopsin
carbohydrate: structure of small oligosaccharides attached at two
sites near the NH 2 -terminus. J Biol Chem 254:8201, 1979.
51. Liang CJ, Yamashita K, Muellenberg CG, Shichi H, and Kobata
A: Structure of the carbohydrate moieties of bovine rhodopsin.
J Biol Chem 254:6414, 1979.
52. Besharse JC and Forestner DM: Horseradish peroxidase uptake
by rod photoreceptor inner segments accompanies outer segment
disc assembly. 39th Ann Proc Electron Microsc Soc Am 486,
1981.
53. Holtzman E and Mercurio AM: Membrane circulation in neurons
and photoreceptors: some unresolved issues. Int Rev Cytol 67:
1, 1980.
54. Besharse JC, Hollyfield JG, and Rayborn ME: Turnover of rod
photoreceptor outer segments: accelerated membrane renewal
in rods after exposure to light. Science 196:536, 1977.
Downloaded From: http://iovs.arvojournals.org/pdfaccess.ashx?url=/data/journals/iovs/933115/ on 06/18/2017