* Your assessment is very important for improving the workof artificial intelligence, which forms the content of this project
Download Studies of focal adhesion assembly
SNARE (protein) wikipedia , lookup
Endomembrane system wikipedia , lookup
Magnesium transporter wikipedia , lookup
Protein moonlighting wikipedia , lookup
Cytokinesis wikipedia , lookup
Histone acetylation and deacetylation wikipedia , lookup
Phosphorylation wikipedia , lookup
G protein–coupled receptor wikipedia , lookup
Cooperative binding wikipedia , lookup
Intrinsically disordered proteins wikipedia , lookup
Protein phosphorylation wikipedia , lookup
P-type ATPase wikipedia , lookup
List of types of proteins wikipedia , lookup
Extracellular matrix wikipedia , lookup
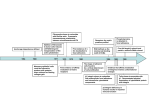
Iain D. Campbell Department of Biochemistry, University of Oxford, South Parks Road, Oxford OX1 3QU, U.K. Abstract Recent studies of some proteins involved in the formation of focal adhesions are described. These include fibronectin, integrins, talin, Dok1 and filamin. Emphasis is placed on features that facilitate regulated assembly of complexes; these include a modular construction and flexible regions that provide interaction sites whose affinity can be adjusted by conformational masking and phosphorylation. Introduction Cell migration depends on the formation of transient self-assembling complexes called focal adhesions. Integrin adhesion receptors are central components of these complexes since they span the cell membrane to link the ECM (extracellular matrix) with numerous intracellular molecules, including the cytoskeleton [1,2]. A schematic view of some of these components is shown in Figure 1. A more complete description of the interaction network involved can be found in Zaidel-Bar et al. [3]. Focal adhesion complexes are formed by proteins with a wide range of functions, including adhesion receptors for the ECM, such as integrins, signalling molecules, such as Src and FAK (focal adhesion kinase), and adaptors that facilitate intracellular complex formation, such as paxillin. Focal adhesions are dynamic, but are formed by multiple specific protein–protein interactions. Some general structural features of the component proteins can be discerned: (i) they are usually modular, constructed from a relatively limited repertoire of folded domains of similar structure [4]; (ii) the domains are often connected by intrinsically unfolded linker regions that provide binding sites for other proteins; and (iii) protein–protein interactions can be modulated by phosphorylation and by changes in site exposure caused, for example, by cell-mediated tension. A multidomain structure with flexible joining regions appears to be very well suited for providing a variety of binding sites in different conditions. I will briefly illustrate some of these general features with examples from our own recent work. Fibronectin Fibronectin (Fn) is a large dimeric glycoprotein found in relatively high concentrations in plasma as well as the ECM [5]. Fn performs a wide range of functions, including provision of binding sites for collagen and integrins. It is Key words: Dok1, fibronectin, filamin, focal adhesion, integrin, talin. Abbreviations used: Dok1, downstream of kinase 1; ECM, extracellular matrix; FAK, focal adhesion kinase; FERM, 4.1/ezrin/radixin/moesin; Fn, fibronectin; Fn30, Fn N-terminal 30 kDa fragment; PTB, phosphotyrosine-binding. 1 email [email protected] Biochem. Soc. Trans. (2008) 36, 263–266; doi:10.1042/BST0360263 composed of three different domain types: Fn1, Fn2 and Fn3. High-resolution structures of many of these component domains and domain combinations have been available for some time [6]; the main remaining uncertainties are associated with how Fn forms complexes with its various partners. Fn has conformational flexibility that exposes different binding sites in different conditions; for example, it is much more active in binding integrins when it is in the ECM than when in its soluble plasma form. The process by which Fn forms insoluble fibrillar networks in the ECM is still not well understood. Hidden or ‘cryptic’ sites found in Fn3 domains have been hypothesized to function as nucleation points, thereby initiating fibrillogenesis [7]. The requirement for integrin function has led to the suggestion that exposure of these sites arises from tension-mediated mechanical rearrangement of Fn3 domains. We showed recently that an interaction complex is formed between the first two Fn3 domains, 1 Fn3 and 2 Fn3 [8], although the two domains are connected covalently by a long flexible linker. Tension-induced separation of the two domains could thus induce a considerable lengthening of this domain pair without any required domain unfolding. For recent papers on the controversial topic of the role of domain unfolding, see, for example, [9,10]. The weak, but specific, interdomain interaction maintains 1 Fn2 and 2 Fn3 in a closed conformation, a form that associates only weakly with the Fn N-terminal 30 kDa fragment (Fn30). It was observed, however, that disruption of this interdomain interaction by amino acid substitutions, to make an ‘open’ form of 1 Fn3–2 Fn3, dramatically enhances association with Fn30. Truncation analysis of 1 Fn3–2 Fn3 reveals that the interdomain linker is also necessary for this interaction between 1 Fn3–2 Fn3 and Fn30 [8]. This example illustrates how cryptic sites could be exposed by mechanical tension, followed by fibril formation arising from multiple interactions between different regions of Fn. As well as having important structural properties, Fn signals to intracellular spaces via adhesion receptors. The best-characterized signalling sequence in Fn is the RGD (Arg-Gly-Asp) sequence in 10 Fn3 [11]. Formation of an RGD–integrin complex results in a range of downstream effects, including phosphorylation of FAK. We have shown C The Authors Journal compilation C 2008 Biochemical Society Biochemical Society Annual Symposium No. 75 Studies of focal adhesion assembly 263 264 Biochemical Society Transactions (2008) Volume 36, part 2 Figure 1 Schematic view of a focal adhesion complex The proteins are drawn so as to indicate their modular nature and some of the flexible linker regions joining them. The ECM at the top of the diagram is made up of molecules such as Fn (a multidomain protein constructed from three main module types: Fn1, Fn2 and Fn3; the segment shown only contains Fn3 domains). Integrins, membrane-spanning αβ heterodimers [2], bind to ECM components using their extracellular regions. The short flexible intracellular tail regions of integrins are linked to the actin cytoskeleton by talin, which relationships of various recombinant IGD-containing Fn fragments showed that even conservative mutations in the IGD motif have severely reduced potency, although the structure of the fragments remains essentially the same [15]. The wide range of potency observed in different contexts (for example, longer Fn fragments are found to have less IGD activity than shorter ones) can be partly attributed to exposure of cryptic sites in some Fn conformations [15]. has a cloverleaf-like FERM head domain and a rod-like actin-binding domain [17]. Filamin A, a large two-armed dimer, each arm of which has an actin-binding domain and 24 Ig-like domains, cross-links actin filaments as well as binding to integrin tails [22]. Important other associated molecules include kinases [e.g. Src, PIPKIγ (phosphatidylinositol phosphate kinase type Iγ ) and FAK] and phosphatases. Paxillin and vinculin facilitate the formation of the dynamic complexes by cross-linking several different proteins. previously some examples of structure-induced modulation of integrin–Fn-binding affinity: 8 Fn3 promotes integrin α5β1 binding via stabilization of the 9 Fn3 domain [12], and the interdomain tilt angle determines the integrin-dependent function of 9 Fn3 and 10 Fn3 [13]. Regions other than 9 Fn3–10 Fn3 have also been implicated in signalling. A truncated form of Fn was found to promote cell migration via IGD (Ile-Gly-Asp) motifs present in Fn1 modules [14]. The receptor for this induced effect is still not known, but recent investigations of the structure–function C The C 2008 Biochemical Society Authors Journal compilation Integrin cytoplasmic tails and their role in affinity modulation Integrin cytoplasmic domains (tails) are relatively short; ∼50 amino acids compared with ∼1000 in integrin extracellular domains. In the absence of protein ligands, these tails are largely unstructured [16]; this means that, although relatively few amino acids are involved, they expose a relatively large accessible binding surface. Regulation of integrin affinity (activation) is essential in normal and pathological cell migration. Talin, a large intracellular molecule that binds integrin tails and actin [17], has been shown to be a key intracellular regulator of integrin activation [18]. Binding of the PTB (phosphotyrosine-binding) subdomain from the talin FERM (4.1/ezrin/radixin/moesin) head domain is sufficient to activate β3 integrins [18]. The structure of a complex between the talin PTB domain and the integrin β3 tail was determined recently [19]; the membrane-proximal region of the β3 tail forms a well-defined helix that makes specific contacts with the talin PTB domain. Structurebased mutagenesis also showed that these specific membrane proximal contacts with the β tail are required for integrin activation and that they are unique to the talin PTB domain [19]. Integrin tails also bind other PTB domains, but these do not usually activate integrins. Indeed, binding of some PTB domains appears to reduce integrin activation [19]. We have shown recently how the PTB domain of Dok1, a downstream of kinase signalling protein [20], might provide further regulation of integrin activation by competition with talin. The structure and binding affinities of Dok1–β3 complexes were analysed using crystallography and NMR and compared with those of talin [21]. The unphosphorylated integrin tail was shown to have a 3-fold preference for the talin PTB domain over that of Dok1. Phosphorylation of Tyr747 , however, enhanced binding to Dok1 ∼400-fold (from 14.3 mM to 37 μM), while the binding affinity to the talin PTB domain decreased ∼2-fold (from 3.5 mM to 6.5 mM). Phosphorylation of Tyr747 thus switches the binding preference of the integrin tail from talin to the Dok1. Since Dok1 interacts exclusively with the canonical NPXY motif in the β3 integrin tail, and not the membrane proximal region, its binding does not activate integrins. Phosphorylation of Tyr747 thus provides a switch that promotes the inactive state of integrins by increasing the binding of a non-activating PTB domain. Biochemical Society Annual Symposium No. 75: Structure and Function in Cell Adhesion Filamin References Filamins are large actin-cross-linking proteins that connect membrane-spanning receptors to the cytoskeleton [22]. The ability of integrins to transmit biochemical signals and mechanical force across cell membranes depends on interactions with the actin cytoskeleton. In a high-resolution structure, we showed that the integrin β tail forms an extended β-strand that interacts with β-strands C and D of the 21st immunoglobulin-like domain from filamin A (21 IgFln) [23]. This filamin-binding site on β tails partially overlaps the talin-binding site; filamin and talin also compete for binding to integrin tails. Such competition is again a possible mechanism for modulating the degree of talindependent integrin activation. Phosphothreonine-mimicking mutations of tails inhibited binding of filamin, but not talin, indicating that phosphorylation provides an additional way of controlling integrin function [23]. In a follow-up structural study of a filamin fragment containing three Ig-like domains (19−21 IgFln), we found that they had an unexpected domain arrangement, with 20 IgFln partially unfolded and 21 IgFln in close proximity to 19 IgFln [24]. The N-terminus of 20 IgFln formed a β-strand that associated with the CD face of 21 IgFln, the same binding site as that occupied by integrin tails. Disruption of this 20 IgFln–21 IgFlnmasking interaction enhanced filamin binding to integrin β tails. Structural and functional analysis of other IgFln domains suggests that this kind of auto-inhibition by adjacent IgFln domains may also occur with 18 IgFln–19 IgFln. This masking effect could explain the observed increased integrin binding in filamin splice variants [25] and the affinity could be modulated by tension-induced removal of the masking strand. 1 Campbell, I.D. and Ginsberg, M.H. (2004) The talin-tail interaction places integrin activation on FERM ground. Trends Biochem. Sci. 29, 429–435 2 Luo, B.H. and Springer, T.A. (2006) Integrin structures and conformational signaling. Curr. Opin. Cell Biol. 18, 579–586 3 Zaidel-Bar, R., Itzkovitz, S., Ma’ayan, A., Iyengar, R. and Geiger, B. (2007) Functional atlas of the integrin adhesome. Nat. Cell Biol. 9, 858–867 4 Campbell, I.D. (2003) Modular proteins at the cell surface. Biochem. Soc. Trans. 31, 1107–1114 5 Vakonakis, I. and Campbell, I.D. (2007) Extracellular matrix: from atomic resolution to ultrastructure. Curr. Opin. Cell Biol. 19, 578–583 6 Potts, J.R. and Campbell, I.D. (1996) Structure and function of fibronectin modules. Matrix Biol. 15, 313–320 7 Mao, Y. and Schwarzbauer, J.E. (2005) Fibronectin fibrillogenesis, a cell-mediated matrix assembly process. Matrix Biol. 24, 389–399 8 Vakonakis, I., Staunton, D., Rooney, L.M. and Campbell, I.D. (2007) Interdomain association in fibronectin: insight into cryptic sites and fibrillogenesis. EMBO J. 26, 2575–2583 9 Abu-Lail, N.I., Ohashi, T., Clark, R.L., Erickson, H.P. and Zauscher, S. (2006) Understanding the elasticity of fibronectin fibrils: unfolding strengths of FN-III and GFP domains measured by single molecule force spectroscopy. Matrix Biol. 25, 175–184 10 Smith, M.L., Gourdon, D., Little, W.C., Kubow, K.E., Eguiluz, R.A., Luna-Morris, S. and Vogel, V. (2007) Force-induced unfolding of fibronectin in the extracellular matrix of living cells. PLoS Biol. 5, e268 11 Ruoslahti, E. and Pierschbacher, M.D. (1987) New perspectives in cell adhesion: RGD and integrins. Science 238, 491–497 12 Altroff, H., van der Walle, C.F., Asselin, J., Fairless, R., Campbell, I.D. and Mardon, H.J. (2001) The eighth FIII domain of human fibronectin promotes integrin α5β1 binding via stabilization of the ninth FIII domain. J. Biol. Chem. 276, 38885–38892 13 Altroff, H., Schlinkert, R., van der Walle, C.F., Bernini, A., Campbell, I.D., Werner, J.M. and Mardon, H.J. (2004) Interdomain tilt angle determines integrin-dependent function of the ninth and tenth FIII domains of human fibronectin. J. Biol. Chem. 279, 55995–56003 14 Schor, S.L., Ellis, I.R., Jones, S.J., Baillie, R., Seneviratne, K., Clausen, J., Motegi, K., Vojtesek, B., Kankova, K., Furrie, E. et al. (2003) Migration-stimulating factor: a genetically truncated onco-fetal fibronectin isoform expressed by carcinoma and tumor-associated stromal cells. Cancer Res. 63, 8827–8836 15 Millard, C.J., Ellis, I.R., Pickford, A.R., Schor, A.M., Schor, S.L. and Campbell, I.D. (2007) The role of the fibronectin IGD motif in stimulating fibroblast migration. J. Biol. Chem. 282, 35530–35535 16 Ulmer, T.S., Calderwood, D.A., Ginsberg, M.H. and Campbell, I.D. (2003) Domain-specific interactions of talin with the membrane-proximal region of the integrin β3 subunit. Biochemistry 42, 8307–8312 17 Critchley, D.R. (2005) Genetic, biochemical and structural approaches to talin function. Biochem. Soc. Trans. 33, 1308–1312 18 Tadokoro, S., Shattil, S.J., Eto, K., Tai, V., Liddington, R.C., de Pereda, J.M., Ginsberg, M.H. and Calderwood, D.A. (2003) Talin binding to integrin β tails: a final common step in integrin activation. Science 302, 103–106 19 Wegener, K.L., Partridge, A.W., Han, J., Pickford, A.R., Liddington, R.C., Ginsberg, M.H. and Campbell, I.D. (2007) Structural basis of integrin activation by talin. Cell 128, 171–182 20 Yamanashi, Y. and Baltimore, D. (1997) Identification of the Abl- and rasGAP-associated 62 kDa protein as a docking protein, Dok. Cell 88, 205–211 21 Oxley, C.L., Anthis, N.J., Lowe, E.D., Vakonakis, I., Campbell, I.D. and Wegener, K.L. (2007) An integrin phosphorylation switch: the effect of β3 integrin tail phosphorylation on DOK1 and talin binding. J. Biol. Chem. 283, 5420–5426 22 Nakamura, F., Osborn, T.M., Hartemink, C.A., Hartwig, J.H. and Stossel, T.P. (2007) Structural basis of filamin A functions. J. Cell Biol. 179, 1011–1025 23 Kiema, T., Lad, Y., Jiang, P., Oxley, C.L., Baldassarre, M., Wegener, K.L., Campbell, I.D., Ylanne, J. and Calderwood, D.A. (2006) The molecular basis of filamin binding to integrins and competition with talin. Mol. Cell 21, 337–347 24 Lad, Y., Kiema, T., Jiang, P., Pentikainen, O.T., Coles, C.H., Campbell, I.D., Calderwood, D.A. and Ylanne, J. (2007) Structure of three tandem filamin domains reveals auto-inhibition of ligand binding. EMBO J. 26, 3993–4004 Conclusions I have illustrated briefly how various proteins in focal adhesions form complexes, using examples from our own recent work. Most of the observed interaction affinities are relatively weak (1–100 μM), but they are specifically tuned to perform their various functions; the various protein– protein interactions are often regulated, for example, by phosphorylation and induced conformational changes. Some emphasis has been placed on possible tension-induced exposure of cryptic sites, but proteolysis is another possible mechanism [26]. Intrinsically disordered regions in proteins seem to play an especially important role in regulated networks of protein–protein interactions [27]. I thank the Wellcome Trust, BBSRC (Biotechnology and Biological Sciences Research Council) and the NIH (National Institutes of Health) Cell Migration consortium for financial support. I also acknowledge the enormous contributions made by my co-workers and collaborators whose names appear in the given references. C The C 2008 Biochemical Society Authors Journal compilation 265 266 Biochemical Society Transactions (2008) Volume 36, part 2 25 Travis, M.A., van der Flier, A., Kammerer, R.A., Mould, A.P., Sonnenberg, A. and Humphries, M.J. (2004) Interaction of filamin A with the integrin β7 cytoplasmic domain: role of alternative splicing and phosphorylation. FEBS Lett. 569, 185–190 26 Schenk, S. and Quaranta, V. (2003) Tales from the crypt[ic] sites of the extracellular matrix. Trends Cell Biol. 13, 366–375 C The C 2008 Biochemical Society Authors Journal compilation 27 Dunker, A.K., Cortese, M.S., Romero, P., Iakoucheva, L.M. and Uversky, V.N. (2005) Flexible nets: the roles of intrinsic disorder in protein interaction networks. FEBS J. 272, 5129–5148 Received 27 December 2007 doi:10.1042/BST0360263