* Your assessment is very important for improving the workof artificial intelligence, which forms the content of this project
Download The organization of the central control of micturition in cats and
Neuroeconomics wikipedia , lookup
Neuromuscular junction wikipedia , lookup
Development of the nervous system wikipedia , lookup
Clinical neurochemistry wikipedia , lookup
Feature detection (nervous system) wikipedia , lookup
Neuropsychopharmacology wikipedia , lookup
Synaptic gating wikipedia , lookup
Embodied language processing wikipedia , lookup
Optogenetics wikipedia , lookup
Channelrhodopsin wikipedia , lookup
Neuroanatomy wikipedia , lookup
Synaptogenesis wikipedia , lookup
Caridoid escape reaction wikipedia , lookup
Premovement neuronal activity wikipedia , lookup
Central pattern generator wikipedia , lookup
Evoked potential wikipedia , lookup
Circumventricular organs wikipedia , lookup
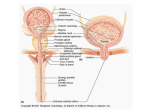
University of Groningen The organization of the central control of micturition in cats and humans Blok, Bertil Feddo Maarten IMPORTANT NOTE: You are advised to consult the publisher's version (publisher's PDF) if you wish to cite from it. Please check the document version below. Document Version Publisher's PDF, also known as Version of record Publication date: 1998 Link to publication in University of Groningen/UMCG research database Citation for published version (APA): Blok, B. F. M. (1998). The organization of the central control of micturition in cats and humans: anatomical and physiological investigations s.n. Copyright Other than for strictly personal use, it is not permitted to download or to forward/distribute the text or part of it without the consent of the author(s) and/or copyright holder(s), unless the work is under an open content license (like Creative Commons). Take-down policy If you believe that this document breaches copyright please contact us providing details, and we will remove access to the work immediately and investigate your claim. Downloaded from the University of Groningen/UMCG research database (Pure): http://www.rug.nl/research/portal. For technical reasons the number of authors shown on this cover page is limited to 10 maximum. Download date: 17-06-2017 The Organization of the Central Control of Micturition in Cats and Humans Anatomical and Physiological Investigations © 1998, B.F.M. Blok All rights reserved. No part of this book may be reproduced or transmitted in any form or by any means, without permission from the author. ISBN: 90-367-0888-5 Cover design: P.O. Gerrits Printed by: Joh. Enschedé en Zonen, Amsterdam This thesis was supported by: Johan Vermeij Stichting Medical Measurements Systems Medtronic Interstim Remmert Adriaan Laan fonds Van Leersumfonds KNAW RIJKSUNIVERSITEIT GRONINGEN The Organization of the Central Control of Micturition in Cats and Humans Anatomical and Physiological Investigations Proefschrift ter verkrijging van het doctoraat in de Medische Wetenschappen aan de Rijksuniversiteit Groningen op gezag van de Rector Magnificus, dr. F. van der Woude in het openbaar te verdedigen op woensdag 20 mei 1998 des namiddags te 2.45 uur door Bertil Feddo Maarten Blok geboren op 15 december 1962 te Noordwijk Promotor: Prof. dr. G. Holstege Promotion Committee: Prof. dr. W.C. de Groat University of Pittsburgh, Pittsburgh Prof. dr. D. Griffiths University of Alberta, Edmonton Prof. dr. R.A. Janknegt University of Maastricht, Maastricht Prof. dr. E.A. Tanagho University of California, San Francisco Paranymphs: Drs. L.J. Mouton Dr. P.O. Gerrits Voor Wessel en Willemijn Contents General Introduction 9 Chapter 1 Ultrastructural Evidence for a Paucity of Projections from the Lumbosacral Cord to the Pontine Micturition Center or M-Region in the Cat: A New Concept for the Organization of the Micturition Reflex with the Periaqueductal Gray as Central Relay Bertil F.M. Blok, Henk de Weerd, and Gert Holstege J. Comp. Neurol. 359:300-309 (1995) 19 Chapter 2 Ultrastructural Evidence for a Direct Pathway from the Pontine Micturition Center to the Parasympathetic Preganglionic Motoneurons of the Bladder of the Cat Bertil F.M. Blok, and Gert Holstege Neurosci. Lett. 222:195-198 (1997) 33 Chapter 3 The Pontine Micturition Center Projects to Sacral Cord GABA Immunoreactive Neurons in the Cat Bertil F.M. Blok, Henk de Weerd, and Gert Holstege Neurosci. Lett. 233:109-112 (1997) 39 Chapter 4 Electrical Stimulation of the Sacral Dorsal Gray Commissure evokes Relaxation of the External Urethral Sphincter in the Cat Bertil F.M. Blok, Jos T.P.W. van Maarseveen, and Gert Holstege Neurosci. Lett., in press 45 Chapter 5 Location of External Anal Sphincter Motoneurons in the Sacral Cord of the Female Domestic Pig Bertil F.M. Blok, Gert Roukema, Bas Geerdes, and Gert Holstege Neurosci. Lett. 216:203-206 (1996) 49 Chapter 6 The Two Pontine Micturition Centers in the Cat are not Interconnected; Implications for the Central Organization of Micturition Bertil F.M. Blok, and Gert Holstege J. Comp. Neurol., submitted 53 Chapter 7 Direct Projections from the Periaqueductal Gray to the Pontine Micturition Center (M-region). An Anterograde and Retrograde Tracing Study in the Cat Bertil F.M. Blok, and Gert Holstege Neurosci. Lett. 166:93-96 (1994) 63 Chapter 8 A PET Study on Brain Control of Micturition in Humans Bertil F.M. Blok, Antoon T.M. Willemsen, and Gert Holstege Brain 120:111-121 (1997) 69 Chapter 9 A PET Study on Cortical and Subcortical Control of Pelvic Floor Musculature in Women Bertil F.M. Blok, Leontien M. Sturms, and Gert Holstege J. Comp. Neurol. 389:535-544 (1997) 83 Chapter 10 Brain Activation during Micturition in Women Bertil F.M. Blok, Leontien M. Sturms, and Gert Holstege Brain, in press 95 General Discussion 105 References 111 Abbreviations 118 Summary 119 Samenvatting 121 Dankwoord 123 List of Publications 125 Curriculum Vitae 127 General Introduction General Introduction The kidneys continuously produce urine, which, for practical reasons, is first collected in the bladder where it is stored until disposal or micturition is possible. Since the individual animal is relatively vulnerable during the release of urine, micturition only takes place when the environment is relatively safe. Furthermore, in many animals urine is used as a marker for territorial demarcation or sexual attraction (a female lets the males know that she is in estrus by leaving a scent trace). Thus, micturition does not take place at random, but is part of a rather complicated behavior, directly related to the survival of the individual or species. In order to investigate this rather complicated behavior it is necessary to identify and define the nature of the muscular and neural structures involved in micturition. This chapter gives a general description of the central organization of motor control. The structures involved in micturition will be introduced, and the aim of the work, described in this thesis, will be explained. Motor system Motoneurons All motor behavior, including micturition, is the result of the activation of specific sets of striated and smooth muscles. Striated or skeletal muscles are innervated by somatic motoneurons, whereas smooth musculature is innervated by sympathetic or parasympathetic postganglionic motoneurons, which, in turn, are innervated by preganglionic motoneurons. The sympathetic and parasympathetic motoneurons and their fibers form the autonomic nervous system. Each muscle is innervated by its own group of motoneurons. Somatic motoneurons innervating muscles of the head are located in various cell groups in the brainstem, those of the remaining parts of the body in the ventral horn of the spinal cord. The axial muscles of the neck and back are innervated by motoneurons in the medial part of the ventral horn throughout the length of the spinal cord, whereas those innervating the muscles of the extremities are located in the lateral part of the ventral horn of the cervical and lumbosacral enlargements. Sympathetic preganglionic motoneurons are present in the lateral horn of the thoracic and upper lumbar cord, whereas parasympathetic preganglionics are located in certain brain stem nuclei, and in the sacral cord (see Holstege, 1996 for review). All central somatic and autonomic motoneuronal cell groups are controlled by other structures in the central nervous system (CNS). The motoneurons together with their control structures form the “motor system” as defined by Holstege (1991; Fig. 1). The basic premotor interneuronal system Motoneurons receive afferents from several sources. Most are excitatory in nature, but normal motor behavior is not possible without inhibitory afferent input to antagonist muscles. In the spinal cord most of the premotor interneurons are located in the intermediate zone of the spinal cord (laminae V to VIII; Rexed, 1952). Premotor interneurons for the somatic motoneurons of the brainstem are located in the pontine and medullary reticular formation, which can be seen as the rostral extent of the spinal intermediate zone (Holstege et al., 1977). Most of these interneurons project to motoneurons at the same level or at levels rostral or caudal to where interneurons are located. The majority of the premotor interneurons terminate on motoneurons nearby, but in some cases they project to motoneurons much further away, for example C2 interneurons projecting to C8 (see Holstege, 1988), the respiratory interneurons in the medulla projecting to the phrenic, intercos- 9 General Introduction Motor system Emotional motor system Voluntary motor system Lateral Medial Lateral eye, neck, axial and proximal body movements Medial specific emotional behaviors independent movements of the extremities gain setting systems including triggering mechanisms of rhythmical and other spinal reflexes Basic system (premotor interneurons) Motoneurons Fig. 1. Schematic overview of the three subdivisions of the motor system (from Holstege, 1996). tal or abdominal motoneurons, and the nucleus retroambiguus (NRA) interneurons in the caudal medulla projecting to motoneurons in the lumbosacral cord involved in mating behavior (see VanderHorst and Holstege, 1996). The “basic motor system” consists of all the premotor interneuronal cell groups in the spinal cord and caudal brainstem (Fig. 1). The voluntary or somatic motor system The influence of the voluntary motor system is evident in hemiplegic patients who are unable to lift their arm or leg voluntarily on one side of their body. The somatic motor system is first described by Kuijpers (for review, see Kuijpers, 1981; Holstege 1991; 1996) and involves structures controlling voluntary non-emotionally directed movements. It consists of a medial and a lateral component. The medial component controls the voluntary control of proximal musculature of the trunk and back, and is important for postural control against grav- 10 ity and the coordination of the head-body movements. This component consists of the ventral cortico-, interstitio-, tecto-, vestibulo-, and reticulospinal tracts, which are located at the level of the spinal cord in the ventral funiculus. These tracts terminate bilaterally on premotor interneurons located medially in the ventral horn, which in turn project to the motoneurons of back and trunk muscles. The lateral component concerns the voluntary control of distal musculature of arms and legs, and is important for behavior like grasping objects and typing on a computer. In most mammals the lateral component consists of the lateral corticobulbospinal tract and the rubrospinal tract. The lateral corticospinal tract originates in the motor and premotor cortex, passes through the internal capsule, cerebral peduncle and pyramidal tract to cross via its decussation at the transition between brainstem and spinal cord. The fibers terminate on premotor interneurons and motoneurons in the lateral General Introduction part of the ventral horn. Interruption of the lateral corticospinal tract by a cerebral stroke in the internal capsule results in a paralysis of one or more contralateral limbs. The rubrospinal tract, originating in the nucleus ruber in the mesencephalon, also plays an important role in the lateral component, but not in humans, where it seems to be “overgrown” by the corticospinal tract. The emotional motor system Many studies demonstrate that certain parts of the limbic system give rise to a descending system, which is completely separate from the somatic motor system. Holstege (1992; 1996) used the term “emotional motor system” for the other motor system, originating in parts of the limbic system or in structures strongly related to it. All emotionally related activities together are crucial for the survival of the individual or its species. Also the emotional motor system can be subdivided into a medial and a lateral component (Fig. 1). The medial component, via its projections to the neurons of origin of the diffuse (sub-)coeruleo-, ventral medullary reticulospinal and raphespinal pathways, has a global effect on the level of activity of all the somatosensory and motoneurons by changing their membrane excitability. The medial component has its origin in the medial hypothalamus and mesencephalic periaqueductal gray (PAG). The lateral component represents several distinct pathways underlying specific emotional behaviors, such as vocalization (Jürgens, 1979; Holstege, 1989), blood pressure control (Lovick, 1993; 1996), sexual behavior (Pfaff et al., 1994; VanderHorst and Holstege, 1995), and micturition (Blok and Holstege, 1996). The lateral component has its origin in the central nucleus of the amygdala, the bed nucleus of the stria terminalis, the lateral hypothalamus, but also the mesencephalic PAG. The PAG controls several relatively uncomplicated primary reactions important for the survival of the individual, like ag- gression and defensive behavior, and important for the survival of the species, like maternal and reproductive behavior. Each of these reactions consists of a specific array of motor activities and level setting mechanisms. In order to control this specific array specific parts of the PAG project to specific premotor interneurons (Fig. 2). Micturition related motoneurons and premotor interneurons Micturition is a coordinated action between the bladder muscle and the striated external urethral sphincter (EUS), which closes the bladder outlet. The EUS is part of the pelvic floor musculature. During the storage of urine, the detrusor muscle is relaxed and the EUS is tonically contracted. When micturition takes place, this activation pattern is reversed: the EUS is relaxed and the bladder contracts, resulting in expulsion of urine. Although the preganglionic motoneurons of the bladder and the somatic motoneurons of the EUS are located in the sacral cord (Fig. 3), their premotor interneurons are located in the brainstem. Motoneurons innervating the urinary bladder and external urethral sphincter The parasympathetic preganglionic motoneurons of the smooth muscle of the bladder (musculus detrusor) are located in the sacral intermediolateral cell group (IML). In the cat these neurons are located at the spinal segments S2-S3 and their axons reach the bladder via the pelvic nerve (Morgan et al., 1979). In the rat the parasympathetic preganglionic motoneurons are located in the spinal segments L6-S1 (Hancock and Peveto, 1979; Nadelhaft and Booth, 1984) and in humans in S2-S4 (Pick, 1970). The preganglionic fibers terminate on ganglion cells in the bladder wall. These postganglionic neurons innervate the smooth bladder muscle. The majority of the sympathetic preganglionic motoneurons innervating the bladder 11 General Introduction 12 Limbic system Periaqueductal gray (PAG) Pedunculopontine and cuneiform nuclei Ventral 1/3 of caudal pontine and medullary medial tegmentum sympathetic sensory neurons preganglionics in the in the dorsal horn intermediolateral cell column general level of sympathetic activity nociception control motoneurons and premotor interneurons in the ventral horn and intermediate zone locomotion Barrington's nucleus subretrofacial nucleus nucleus retroambiguus parasympathetic preganglionics in the sacral cord sympathetic preganglionics in the intermediolateral cell column motoneurons of the larynx, pharynx, soft palate, and expiratory muscles motoneurons of the iliopsoas, adductor longus, hamstring, pelvic floor, and axial muscles spinal cord laminae VIII and medial VII T1-T2 intermediolateral cell column C4-T8 lamina X micturition (this thesis) cardiovascular changes vocalization receptive behavior defensive posture ? pupil dilatation ? ? Fig. 2. Schematic overview of the descending projections from the PAG to different regions in the caudal brainstem and spinal cord, and their possible functions. General Introduction (Onufrowicz, 1899). (+) Parasympathetic motoneurons Onuf's nucleus S2 (+) Bladder (+) External urethral sphincter Fig. 3. Schematic overview of the sacral motoneurons involved in micturition. are located in the intermediolateral cell group of the caudal thoracic and rostral lumbar cord and send their axons via the splanchnic nerves to sympathetic ganglion cells in the lumbosacral sympathetic chain, inferior mesenteric ganglia and the major pelvic ganglia (Applebaum et al., 1980; Andersson and Sjögren, 1982; Vera and Nadelhaft, 1992). Postganglionic sympathetic fibers run via the pelvic and hypogastric nerves before innervating the bladder. The sympathetic outflow innervates the smooth muscle fibers of the bladder neck, and is believed to play a role during the urine storage phase (de Groat and Saum, 1972; Vaughan and Satchell, 1992) and during sexual behavior (Kimura et al., 1975). The EUS is innervated by the pudendal nerve. Motoneurons of the EUS in the cat are located in the ventrolateral part of the so-called nucleus of Onuf of the ventral horn at the level of S1 and S2 (Sato et al., 1978; Kuzuhara et al., 1983). The motoneurons of the external anal sphincter are located in the dorsomedial part of the nucleus of Onuf (Kuzuhara et al., 1983). In humans Onuf’s nucleus is located in the segments S1-S3 Premotor interneurons involved in micturition Since the work of Barrington (1925) it is known that the coordinating component of the micturition reflex is not located in the sacral cord, but in the dorsolateral portion of the pontine tegmental field. Bilateral lesions in this area in the cat result in urinary retention. The same is true when the fiber pathways originating in Barrington’s area are interrupted e.g. at the level of the spinal cord. Also in humans interruption of the descending fibers from the pons to the sacral cord, for example in patients with a transection of the spinal cord, results in retention of urine and finally in dyssynergic micturition. In such patients the contraction of the bladder is accompanied by simultaneous contraction of the sphincter. Several studies in various animals have attempted to identify the area originally described by Barrington. Pontine micturition center Barrington’s area or nucleus is also called the pontine micturition center (PMC; Loewy et al., 1979), or M (medial)-region (Holstege et al., 1986). The latter term was chosen because another group of neurons in more lateral parts of the same dorsolateral pontine tegmental field was found to influence micturition and urinary continence. These lateral cells are referred to as L (lateral)-region (Holstege et al., 1986). Anterograde tracing studies in the rat (Loewy et al., 1979), opossum (Martin et al, 1979), cat (Holstege et al., 1979, 1986), and monkey (Westlund and Coulter, 1980) have shown that neurons in the PMC project, via fibers in the spinal lateral and dorsolateral funiculi, directly to the sacral intermediolateral cell group, which contain the parasympathetic preganglionic motoneurons of the urinary bladder. Neurons in the PMC in the cat project also to the sacral dorsal gray com- 13 General Introduction Fig. 4. Brightfield photomicrographs of autoradiographs showing [3H] leucine injection areas and darkfield photomicrographs showing the spinal distribution of labeled fibers after injections in the M-region (on the left) and in the L-region (on the right) in the cat. Note the dense distribution of labeled fibers to the sacral intermediolateral (parasympathetic motoneurons) and intermediomedial cell groups from the M-region (S2 segment on the left). Note also the pronounced projection to the nucleus of Onuf (S1 segment on the right) from the L-region. Note further the contralateral pathway in the dorsolateral funiculus, terminating in lamina I, the outer part of II, and laminae V and VI throughout the length of the spinal cord (from Holstege et al., 1986). 14 General Introduction missure (DGC) or intermedio-medial cell group (IMM) , but do not project to the nucleus of Onuf (Holstege et al.,1979, 1986; Fig. 4, on the left). Electrical stimulation in the PMC in the cat produces an immediate and sharp decrease in the urethral pressure and pelvic floor electromyogram (EMG), followed in about 2 seconds by a steep rise in the intravesical pressure (Holstege et al. 1986), mimicking normal micturition. L-region The L-region projects, via fibers in the lateral funiculus, bilaterally to the nucleus of Onuf (Holstege et al., 1979; 1986; Fig. 4, on the right). Stimulation in the L-region results in strong excitation of the pelvic floor musculature and an increase in the urethral pressure (Holstege et al., 1986). Bilateral lesions in the L-region give rise to an inability to store urine; bladder capacity is reduced and urine is expelled prematurely by excessive detrusor activity accompanied by urethral relaxation (Griffiths et al., 1990). Apart from the afferents from the PMC- and L-regions, the parasympathetic preganglionic bladder motoneurons and Onuf’s nucleus motoneurons receive also afferents from other sources, like the NRA (VanderHorst and Holstege, 1995) and the paraventricular nucleus of the hypothalamus (Holstege, 1987). However, their descending systems are thought to play a role in other functions as mating behavior, but not in micturition. Ascending pathways involved in micturition Peripheral afferent nerves Most afferent fibers from the bladder enter the sacral cord via the pelvic nerve. The peripheral fibers of the dorsal root ganglia neurons of the pelvic nerve contact the bladder wall mechanoreceptors. The proximal fibers enter Lissauer’s tract and terminate mainly in Rexed’s (1954) laminae I, V, VII, and X of the lumbosacral spinal cord at seg- ments L4-S2 (Morgan et al., 1979 in the cat). The majority of these afferents are thin myelinated and unmyelinated axons, and their conduction velocities are in the A∂ and Cfiber range, respectively (Hulsebosch and Coggeshall, 1982). Most A∂ fibers originate from slowly adapting mechanoreceptors in the bladder wall, and excitation of these fibers results in activation of the micturition reflex. In all likelihood, the A∂ fibers are the peripheral afferent fibers for this reflex (De Groat et al., 1982; Mallory et al., 1989), because the unmyelinated C-fibers in the pelvic nerve do not respond to distention and contraction of the urinary bladder (Jänig and Morrison, 1982). Spinal cord-brainstem pathways involved in the micturition reflex In order to function properly the PMC must be informed about the amount of bladder filling, since micturition can take place only when the bladder contains a certain amount of urine. The current view is that the micturition reflex is a spinobulbospinal reflex. De Groat (1975), on the basis of physiological recording studies in the dorsolateral pons of the rat and cat, suggested that the lumbosacral neurons receiving bladder afferents relay bladder filling information directly to the PMC, which project to the preganglionic bladder motoneurons. This would imply a micturition circuit in which lumbosacral interneurons convey information concerning bladder filling directly to the pontine micturition centers. In a later paper the same author found evidence that the PAG receives bladder information before the PMC (Noto et al., 1989). Aim of the thesis The investigations presented in this thesis are focussed on two main issues. First, which neurons are involved in the micturition reflex, and, second, which structures, although they are not part of the micturition reflex pathway itself, still influence the re- 15 General Introduction flex. Clarification of these issues is very important for the understanding of the control mechanisms in normal individuals, and, moreover, in incontinent patients. It is highly probable that dysfunction of certain brain areas cause urinary incontinence in many elderly (Andrew and Nathan, 1964; Blaivas, 1982). Urge incontinence occurs when patients sense the urge to void, but are unable to delay it long enough to reach the toilet. In healthy individuals this “urge” is not immediately followed by micturition and usually disappears when micturition is not appropriate at that particular time and place. Urge incontinence is also frequently found in patients with stroke (Khan et al., 1981) or with neurodegenerative diseases, as multiple sclerosis (Blaivas et al., 1979). Urge incontinence should not be confused with genuine stress incontinence, which is not the result of lesions in the central nervous system and will not be discussed in this thesis. Anterograde and retrograde tracers were used to identify pathways and neurons involved in micturition. Most of the tracer experiments were done in cats. The tracers were visualized using histo- and immunocytochemical techniques, and the results were analyzed with light- and electron microscopy. Electrical stimulation was used in order to study the effects on the function of the bladder and EUS. In order to localize micturition related neurons in humans positron emission tomography (PET) was used. PET is a non-invasive technique to study changes in regional cerebral blood flow (rCBF) in humans performing specific tasks (Fox and Mintun, 1989). The first part of the study is focussed on the neuronal components of the micturition reflex itself. The micturition reflex consists of sensory (ascending) and motor (descending) components. Afferents from the bladder to sensory neurons in lamina I and V of the lumbosacral cord were thoroughly described in the cat (Morgan et al., 1981). 16 Since it was anatomically unclear whether these lumbosacral cells projected directly to the PMC, ultrastructural experiments were done which resulted in the data presented in the first chapter. It was demonstrated that the PMC of the cat received only a very small projection from the lumbosacral cord, and that none of these afferents contacted retrogradely labeled cells in the PMC. This means that the bladder information reaches the PMC indirectly. The main target of lumbosacral projections to the caudal brainstem of the cat appeared to be the ventrolateral PAG. An important feature of micturition is the synergic action between the bladder muscle and the EUS. Stimulation of the PMC results in the same effect (Holstege et al., 1986). The study presented in chapter two demonstrates that from the PMC terminals in the IML more than 75% are directly in contact with parasympathetic preganglionic bladder motoneurons, and all are excitatory in nature. Chapter three investigated ultrastructurally the nature of the PMC fiber terminations in the dorsal gray commissure (DGC). From the PMC terminals in the DGC 55% made contact with inhibitory GABA-ergic interneurons. This investigation provided evidence that the GABA-ergic interneurons in the DGC play a role in the relaxation of the EUS during micturition, which is substantiated by the observation that electrical stimulation of the sacral DGC results in a relaxation of the EUS. The sacral DGC of the domestic pig contains the motoneurons of the external anal sphincter. Chapter 6 demonstrates that the PMC and L-regions are not interconnected, which makes it probable that the PMC controls micturition only via its sacral projections. Stimulation of the PAG elicits micturition, which indicates a possible role in the motor control of micturition. Since the majority of the afferents from the lumbosacral cord terminate in the PAG, and not in the PMC, the existence of PAG projections to the PMC General Introduction was investigated in chapter 7. The presence of this projection completes the concept of the normal micturition reflex. The chapters 8, 9 and 10 present the findings of the PET scanning experiments in humans. Since nothing was known about the central control of micturition in humans the study was done in male and female volunteers. The results demonstrate that the brainstem structures, which control micturition and continence, seem to be the same in cats and humans. Additionally, emotional related cortical areas play a role in the onset of micturition, but are not part of the micturition reflex itself. Chapter 9 reports the results of a study on the voluntary motor control of the pelvic floor and abdominal musculature. The general discussion puts the results of all chapters into perspective, presenting a scheme for the sensory and motor pathways in the spinal cord and brainstem involved in micturition and continence. 17