* Your assessment is very important for improving the workof artificial intelligence, which forms the content of this project
Download Cell injury, death and adaptation yemen
Survey
Document related concepts
Cell nucleus wikipedia , lookup
Tissue engineering wikipedia , lookup
Cell membrane wikipedia , lookup
Cell encapsulation wikipedia , lookup
Extracellular matrix wikipedia , lookup
Cell growth wikipedia , lookup
Cell culture wikipedia , lookup
Signal transduction wikipedia , lookup
Cellular differentiation wikipedia , lookup
Cytokinesis wikipedia , lookup
Organ-on-a-chip wikipedia , lookup
Endomembrane system wikipedia , lookup
Transcript
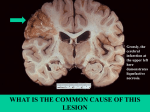
Cell injury, death and adaptation Yemen University Lectures 1 and 2 Dr Heyam Awad FRCPath Coordinator : Dr Heyam Awad • Email: [email protected] • Lectures will be available on my university website www.ju.edu.jo • Office hours: Monday and Wednesday 10-12 .. Office in hospital 3rd floor. Lecture distribution • • • • • • Cell injury 4+lab Inflammation 6 +2labs Repair 3 +lab Haemodynamic diseases 5+lab Neoplasia 8 +2 labs Genetic diseases 8 + 2 labs EVALUATION • 1 EXAM • THEORY AND PRACTICAL. • mock What is pathology? • Patho… disease • Logy… study • Pathology = study of disease involves: causes of disease.. Etiology : mechanisms.. pathogenesis :morphological changes. • Etiology: Origin of disease , underlying causes. • Pathogenesis: steps in the development of disease…… cellular and molecular changes . • Morphology: macroscopic and microscopic changes. Why to study pathology??? Cellular adaptations and cell injury • Cells maintain a steady state.. Homeostasis. • Stresses .. Adaptation….. New homeostatic state with preservation of function. • Stress beyond capability of adaptation.. Cell injury. • Cell injury… reversible within certain limits • Then .. Irreversible…. Ends in cell death. • Two types of cell death: necrosis and apoptosis. Adaptation • • • • Hyertrophy Hyperplasia Atrophy metaplasia Adaptation • Adaptive changes are reversible. • Can be physiologic or pathologic. • Hypertrophy: Increased cell size. • Hyperplasia: increased number of cells.. Cell division. • Metaplasia: change from one adult cell type to another • Atrophy: decreased size. Hypertrophy versus hyperplasia Hypertrophy • Increased cell size. • Due to increased organelles and proteins. • Increased intracellular synthesis.. Caused by: increased demands, hormones or growth factors. Physiologic hypertrophy • Uterus during pregnancy… due to estrogen • Skeletal muscle… due to increased demand Physiologic hypertrophy Pathologic hypertrophy • Cardiac.. Hypertensive heart disease • Pathogenesis.. Two types of signals: mechanical: stretch and trophic: growth factors and androgenic hormones Pathologic hypertrophy Pathologic hypertrophy hyperplasia • Only in tissues that can replicate. • Can be physiologic or pathologic. Physiologic hyperplasia • Hormonal: uterus, breast. • Compensatory: after removal or loss of part of tissue. Pathologic hyperplasia • Due to excess in hormones or growth factors. • E:g endometrial hyperplasia. • Controlled.. Responds to decreased stimulation. This differentiates it from cancer Normal endometrium Endometrial hyperplasia atrophy • Shrinkage in cell size due to loss of cell substance. Causes • decreased work load. • Loss of innervation • Loss of endocrine stimulation. • Aging Atrophy • Physiologic: endometrial atrophy during menopause • Pathologic: loss of innervation. atrophy Mechanisms: • Decreased protein synthesis. • Degradation of cellular proteins. • Autophagy…. Literally means self eating. Brain atrophy Muscle atrophy metaplasia • Adult cell type replaced by another adult cell type. • Arise in reprogrammed stem cells to differentiate along a new pathway. Epithelial metaplasia • Respiratory epithelium to squamous. • Barrett's mucosa.. Esophegeal squamous to columnar Barrett’s mucosa Normal esophegeal mucosa Metaplastic, Barrett’s mucosa Metaplasia in mesenchymal tissue • Usually pathologic • Ossification of soft tissue due to injury. Cell injury Causes of cell injury/ 1 • • • • • • • Chemical agents Infections. Immunologic Genetic factors Nutritional imbalances Physical agents Aging. Causes of cell injury/ 2 Oxygen deprivation.. Hypoxia and ischemia. Hypoxia= oxygen deficiency Ischemia = loss of blood supply due to impaired arterial flow or reduced venous return. -Ischemia is the most common cause of hypoxia. -Other causes of hypoxia: *reduced oxygen carrying capacity in anemia or carbon monoxide deficiency. *inadequate oxygenation of the blood as in pnumonia. Rules and principles/ 1 • Cell response to injurious stimuli depend on type, duration and severity of the injury. • Example: low dose of a toxin can cause reversible injury whereas larger dosed can cause cell death. • Short-lived ischemia.. Reversible • Ischemia of long duration… death Rules and principles/ 2 • Response to injury also depends on type, status, adaptability and genetic makeup of the injured cell. • Example: skeletal muscle cells can stand 2-3 hours of ischemia without irreversible injury but cardiac muscles die in 20-30 minutes . • Glycogen content in hepatocytes can determine their response to injury.. How? • Genetic polymorphism in cytochrome P-450 influences response to toxins. Rules and principles/ 3 Cell injury results from functional and biochemical changes in essential cellular components, mainly: • Mitochondrial function • Calcium homeostasis • Cell and organelle membranes • DNA • Protein synthesis and folding. Rules and principles/ 4 • All injurious stimuli first affect the molecular or biochemical level. • Cellular functions lost before cell death occurs. • The morphologic changes of cell injury (or cell death) occur very late. Rules/4 example • Ischemia of the heart… coronary artery occlusion. • Myocardial cells loose function ( become noncontractile) after 1-2 minutes of ischemia. • They die 20-30 minutes after ischemia. • It takes 2-3 hours to recognise ultrastructural changes of death (EM) • 6-12 hours by light microscope to appear dead. Morphology of reversible cell injury • Cellular swelling : due to failure of energydependent ion pumps in the plasma membrane causing inability to maintain ion and fluid homeostasis. • Fatty change : small or large lipid vacuoles (hepatocytes and myocardial cells) Cell swelling • The first manifestation of almost all forms of cell injury. • Reversible. • Grossly: organ affected becomes pale and gains weight. • Micro: small clear cytoplasmic vacuoles … which are distended endoplasmic reticulum. Cell swelling Fatty change • In cells participating in fat metabolism: hepatocytes and myocardial cells) • Reversible • Fat droplets. Ultrastructural changes of reversible injury (EM) • (1) plasma membrane changes such as blebbing, blunting or distortion of microvilli, and loosening of intercellular attachments. • (2) mitochondrial changes such as swelling and the appearance of phospholipid-rich amorphous densities. • (3) dilation of the ER with detachment of ribosomes and dissociation of polysomes. • (4) nuclear alterations, with clumping of chromatin. EM changes Morphology of irreversible injury Necrosis • Necrosis = Morphologic changes that follow cell death in living tissues. NECROSIS • Denaturation of intracellular proteins. • Digestion of cells by lysosomal enzymes of dying cells ( autolysis) and leukocytes (heterolysis). Protein denaturation denaturation LM changes 1) Increased cytoplasmic eosinophilia. Cause? 2) Vacoulation of cytoplasm, moth eaten. Cause? 3) Nuclear changes 4) Calcification Nuclear changes one of three patterns 1. karyolysis: decreased chromatin basophilia secondary to deoxyribonuclease (DNAase) activity. 2. pyknosis: nuclear shrinkage and increased basophilia (DNA condenses into a solid shrunken mass. 3. karyorrhexis, fragmentation then disappearance of nucleus. EM changes 1)Discontinuities in plasma and organelle membranes. 2) Marked dilation of mitochondria and large amorphous densities. 3)Disruption of lysosomes. 4) Intracytoplasmic myelin figures Myelin figures • Myelin figures: aggregates of damaged cell membranes (phospholipids). Fate of Myelin figures: • phagocytosed by other cells or further degraded • into fatty acids and calcify Patterns of necrosis • Denaturation of protein predominates…. Coagulative necrosis. • Enzymatic digestion predominates… liquefactive necrosis. • Special circumstances: caseous necrosis and fat necrosis. Coagulative necrosis • preserved architecture of dead tissue . • Denaturation of structural proteins and enzymes… so no cellular proteolysis. • Eosiniphilic anucleated cells • Cells are removed by inflammatory cells. • Ischemia in all solid organs except the brain may lead to coagulative necrosis. Coagulative necrosis Liquifactive necrosis • digestion of the dead cells resulting into a liquid jelly-like mass. • In focal bacterial or fungal infections and in hypoxic death in central nervous system. Caseous necrosis • White cheese like friable necrosis. • Prototype: Tuberculosis • Typical finding is granuloma :Collection of fragmented or lysed cells with amorphous granular eosinophilic debris surrounded by macrophages. Caseous necrosis • White cheese like friable necrosis. • Prototype: Tuberculosis • Typical finding is granuloma :Collection of fragmented or lysed cells with amorphous granular eosinophilic debris surrounded by macrophages. Fat necrosis • used as a clinical terms and not a specific type. • Necrosis of fat. • Typical example: pancreatic enzymes (lipases) release in acute pancreatitis. Fate of necrotic tissue • • • • Phagocytosis. Replacement by scar. Regeneration. Calcification. Mechanisms of cell injury • • • • • • ATP depletion Mitochondrial damage Calcium influx Oxygen derived free radicals membrane defects Damage to DNA and protein ATP depletion • • • • ATP.. Importance? Sources of ATP? Causes of depletion? Effects of ATP depletion? Effects of ATP depletion • Membrane permeability affected due to effects on Na- K pump. • Increased acidity due to increased non oxidative phosphorylation. • Failure of calcium pump.. Increased intracellular calcium. • Structural disruption in protein synthesis apparatus.. Detacment of ribosomes and dissociation of polysomes Mitochondrial damage • Failure of oxidative phosphrylation.. ATP depletion. • Abnormal oxidative phosphorylation.. ROS • Mitochondrial permeability transition pores.. Loss of membrane potentional and pH change • Release of proteins that initiate apoptosis Calcium influx • Activates enzymes… phospholipases, proteases, endonucleases, ATPases… so cell destruction. • Calcium can directly activate caspases… apoptosis Oxygen free radicals • Lipid peroxidation • Cross linking of proteins • DNA damage Membrane permeability defects Caused by: Decreased phospholipid synthesis due to ATP depletion. Increased phospholipid breakdown .. Due to increased calcium ROS. . Lipid breakdown products DNA damage • Initiates apoptosis mechanisms