* Your assessment is very important for improving the work of artificial intelligence, which forms the content of this project
Download - ISpatula
Survey
Document related concepts
Transcript
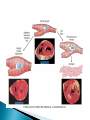
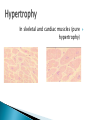
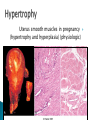
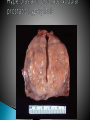
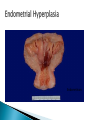
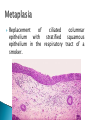
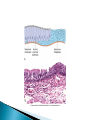
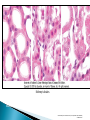
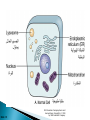
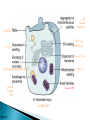
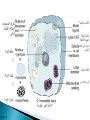
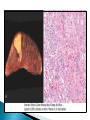
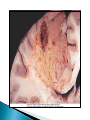
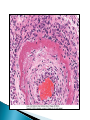
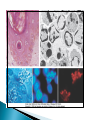
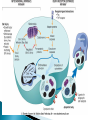
Faculty of Medicine Department of Pathology Khairat Battah,MD Cells are active participants in their environment, constantly adjusting their structure and function to accommodate changing demands and extracellular stresses. Cells tend to maintain their intracellular milieu within a fairly narrow range of physiologic parameters; that is, they maintain normal homeostasis. As cells encounter physiologic stresses or pathologic stimuli, they can undergo adaptation, achieving a new steady state and preserving viability and function. If the adaptive capability is exceeded or if the external stress is inherently harmful, cell injury develops . Within certain limits injury is reversible, and cells return to a stable baseline; however, severe or persistent stress results in irreversible injury and death Adaptive mechanisms to stresses. Causes of cell injury. Reversible and irreversible cell injury. Mechanism of cell injury Patterns of cell death (apoptosis and necrosis). Reversible changes in size, number, phenotype, metabolic activity or function in response to changes in environment. Adaptation can be both physiologic and pathologic. principal adaptive responses: Hypertrophy. Hyperplasia. Atrophy. Metaplasia. Hypertrophy is an increase in cell size resulting in an increase in the size of the organ. Increased amount of structural proteins and organelle without increasing the number of cells. Occurs in cells that have limited capacity to divide. Physiologic vs pathologic. Caused by increased functional demand (workload) or stimulation by hormones or growth factors. Heart: left ventricle hypertrophy(pathologic). In skeletal and cardiac muscles (pure hypertrophy) Uterus smooth muscles in pregnancy (hypertrophy and hyperplasia) (physiologic) There is a limit for hypertrophy, beyond which the muscle is no longer able to compensate for the increased burden. Some times subcellular organelle may undergo selective hypertrophy, Example: drugs cause smooth ER hypertrophy. Increased number of cells resulting in increased mass of the organ or tissue. Takes place in cells capable of dividing. Physiologic vs pathologic. • • Physiological hyperplasia compensatory) Examples: (hormonal – Uterine enlargement during pregnancy – Female breast in puberty & lactation – Compensatory hyperplasia in the liver after partial resection. or Pathological hyperplasia ◦ Hyperplasia of the endometrium (excessive hormone stimulation). ◦ Nodular prostatic hyperplasia ◦ Wound healing (Effects of growth factors). ◦ Infection by papillomavirus (HPV) Pathologic hyperplasia can be a fertile soil for development of neoplasia Prostate Endometrium Reduced size of an organ or tissue as a result from a decrease in cell size . Mechanism of atrophy include combination of decreased protein synthesis and increased protein degradation in cells. reduced metabolic activity and degradation of cellular proteins Autophagy (“self-eating”) is the process in which the starved cell eats its own components in an attempt to survive with resulting increases in the number of autophagic vacuoles. Physiologic : -Embryonic development. -Gravid uterus involution. -Hormonal withdrawal after menopause. Pathologic: -Decreased workload (Disuse atrophy) -Loss of innervation (Denervation atrophy) -Diminished blood supply. -Inadequate nutrition. -Loss of endocrine stimulation. -Aging (senile atrophy Metaplasia is a “reversible” change in which one differentiated cell type is replaced by another cell type. New cell type is better has survival advantage with the current stress or irritation but important protective mechanisms are lost Persistence of factors causing metaplasia may lead to progression into malignant transformation. Vitamin A is essential for normal epithelial differentiation, its deficiency may also induce squamous metaplasia in the respiratory epithelium. Examples: respiratory , esophagus, cervix, muscle. Replacement of ciliated columnar epithelium with stratified squamous epithelium in the respiratory tract of a smoker. Oxygen Deprivation: hypoxia and ischemia Chemical agents & Drugs. Physical agents: trauma, extremes of tempreture, radiation, chemicals. Infectious Agents: viruses to worms. Immunological reactions. Genetic factors: chromosomal to single aminoacid defect Nutritional Imbalances: Deficiency vs excess. Aging. All stresses and noxious influences exert their effects first at the molecular or biochemical level. Cellular function may be lost long before cell death occurs. The morphologic changes of cell injury (or death) occur very late. cellular swelling . and fatty change. Cellular swelling is the result of failure of energy-dependent ion pumps in the plasma membrane, leading to an inability to maintain ionic and fluid homeostasis. Fatty change occurs in hypoxic injury and various forms of toxic or metabolic injury, and is manifested by the appearance of small or large lipid vacuoles (hepatocytes and myocardial cells) 1. Cellular swelling , the first manifestation of almost all forms of injury to cells. Difficult to appreciate with the light microscope, appears as small clear vacuoles within the cytoplasm. (Hydropic change or vacuolar degeneration). 2.Fatty change is manifested by the appearance of lipid vacuoles in the cytoplasm. These changes are seen in cells involved in fat metabolism as hepatocytes and myocardial cells. (1) plasma membrane alterations such as blebbing, blunting or distortion of microvilli, and loosening of intercellular attachments. (2) mitochondrial changes such as swelling and the appearance of phospholipid-rich amorphous densities. (3) dilation of the ER with detachment of ribosomes and dissociation of polysomes. (4) nuclear alterations, with clumping of chromatin. Kidney tubules Downloaded from: StudentConsult (on 24 September 2011 08:38 PM) © 2005 Elsevier ,الجسيم الحال يحلول الشبكة الهيولية الباطنة النواة المتقدرة خلية طبيعية Slide 1.5 W.B. Saunders Company items and derived items Copyright (c) 1999 by W.B. Saunders Company تكدس الجسيمات داخل الغشائية مجلة(فقاعة) تورم الشبكة الهيولية الباطنة تورم عام تبعثر الريباسات تالزن(تراكم)الكروماتين(الصبغين)النووي تورم متقد ري التهام ذاتي بالجسيمات الحالة كثافات صغيرة األذية العكوسة Slide 1.6 The type of cell death that is associated with loss of membrane integrity and leakage of cellular contents Denaturation of intracellular proteins and enzymatic digestion of cells. Digestion enzymes are derived from lysosomes of dying cells and leukocytes. 1. 2. 3. Discontinuities in plasma and organelle membranes. Marked dilation of mitochondria with the appearance of large amorphous densities. Disruption of lysosomes. Intracytoplasmic myelin figures (aggregates of damaged cell membranes (phospholipids)). 1. 2. 3. Profound nuclear changes culminating in nuclear dissolution. Nuclear changes assume one of three patterns: karyolysis: The basophilia of the chromatin may fade), presumably secondary to deoxyribonuclease (DNase) activity. pyknosis, characterized by nuclear shrinkage and increased basophilia; the DNA condenses into a solid shrunken mass. karyorrhexis, the pyknotic nucleus undergoes fragmentation then completely disappears أشكال نخاعينية تمزق الجسيمات الحالة والتحلل الذاتي تحلل الشبكة الهيولية الباطنة عيوب في الغشاء الخلوي تغلظ النواة أو تكثفات كبيرة تحلل النواة تورم متقد ري أو تجزؤ النواة األذية الغير عكوسة Slide 1.7 Patterns of Tissue Necrosis preservation of the architecture of dead tissue for at least some days. Denaturation of structural proteins and enzymes. Eosiniphilic anucleated cells that persist for days. Cells are removed by inflammatory leukocytes. Ischemia in all solid organs except the brain may lead to coagulative necrosis. Infarction: localised area of coagulative necrosis. digestion of the dead cells resulting into a liquid jelly-like mass. In focal bacterial or fungal infections and in hypoxic death in central nervous system. Acute inflammation: Creamy yellow material due to accumulation of dead leukocytes (pus). Not a distinctive pattern of cell death, used in clinical practice. It is usually applied to a limb, generally the lower leg, that has lost its blood supply. When bacterial infection is superimposed, coagulative necrosis is modified by the liquefactive action of the bacteria and the attracted leukocytes ( wet gangrene). White cheeselike friable necrosis. The tissue architecture is completely obliterated and cellular outlines cannot be discerned. Prototype: Tuberculosis Typical finding is granuloma :Collection of fragmented or lysed cells with amorphous granular eosinophilic debris surrounded by macrophages. usually used in clinical terms and it is not a specific type. Necrosis (destruction) of fat. Typical example: pancreatic enzymes (lipases) release in acute pancreatitis. The fatty acids result from the breakdown of fat combine with calcium leading to the formation of white chalky areas (Saponification). Immune reactions involving blood vessels. Complexes of antigens and antibodies deposited in the walls of blood vessels. Immune complexes deposits along with fibrin result in a bright pink material on light microscopy. Example: vasculitis (polyarteritis nodosa) Phagocytosis. Replacement by scar. Regeneration. Calcification. several general principles are relevant to most forms of cell injury: The cellular response to injurious stimuli depends on the type of injury, its duration, and its severity. The consequences of an injurious stimulus depend on the type, status, adaptability, and genetic makeup of the injured cell Cell injury results from functional and biochemical abnormalities in one or more of several essential cellular components Multiple biochemical alterations may be triggered by any one injurious insult. Depletion of ATP Mitochondrial Damage and Dysfunction Influx of Calcium Accumulation of Oxygen-Derived Free Radicals (Oxidative Stress) Defects in Membrane Permeability Damage to DNA and Proteins The activity of plasma membrane ATPdependent sodium pumps is reduced – cellular swelling compensatory increase in anaerobic glycolysis- lactic acid accumulates decreased intracellular pH and decreased activity of many cellular enzymes. Failure of ATP-dependent Ca2+ pumps influx of Ca2+ - with damaging effects on numerous cellular components structural disruption of the protein synthetic apparatus Failure of oxidative phosphorylation leads to progressive depletion of ATP formation of reactive oxygen species The mitochondria also contain several proteins that, when released into the cytoplasm, tell the cell there is internal injury and activate a pathway of apoptosis. Cytosolic free calcium is normally maintained at lower concentration than that of extracellular calcium or of sequestered intracellular mitochondrial and ER calcium. Increased cytosolic Ca2+ activates a number of enzymes, with potentially deleterious cellular effects (phospholipases, proteases , endonucleases) Increased intracellular Ca2+ levels may also induce apoptosis This is seen in the following situations: Ischemia-reperfusion chemical and radiation injury toxicity from oxygen and other gases cellular aging microbial killing by phagocytic cells and tissue injury caused by inflammatory cells. Several biochemical mechanisms may contribute to membrane damage: Decreased phospholipid synthesis Increased phospholipid breakdown Oxygen free radicals Cytoskeletal abnormalities. Lipid breakdown products. Pathway of cell death induced by a tightly regulated suicide program in which cells activate enzymes capable of degrading the cells' own nuclear DNA and nuclear and cytoplasmic proteins. Fragments of the apoptotic cells then break off, giving the appearance that is responsible for the name (apoptosis, "falling off"). The plasma membrane of the apoptotic cell remains intact. Apoptotic bodies (contain portions of the cytoplasm and nucleus) become targets for phagocytosis before their contents leak out and so there would be no inflammatory reaction. Physiologic situations: To eliminate cells that are no longer needed OR to maintain a steady number of various cell populations in tissues. Embryogenesis. involution of hormone-dependent tissues upon hormone withdrawal. ( endometrial and breast after pregnancy) Cell loss in proliferating cell populations. (GIT) Death of host cells after serving their useful function. ( Neutrophils and lymphocytes in inflammation) Elimination of potentially harmful self-reactive lymphocytes. Cell death induced by cytotoxic T lymphocytes (tumor cells and viraly infected cells) Examples: DNA damaged cells. Cells with accumulation of misfolded proteins. Certain infections (viral ones): may be induced by the virus (as in human immunodeficiency virus infections) or by the host immune response (as in viral hepatitis). Pathologic atrophy in parenchymal organs after duct obstruction (pancreas, parotid and kidney) Cell shrinkage: dense cytoplasm, tightly packed organelles. Chromatin condensation: peripherally under the nuclear membrane. Formation of cytoplasmic blebs and apoptotic bodies: blebbing then fragmentation into membrane bound apoptotic bodies composed of cytoplasm and tightly packed organelles with or without nuclear fragments. Phagocytosis of apoptotic cells or cell bodies by macrophages (quickly hence no inflammation). The fundamental event in apoptosis is the activation of enzymes called caspases . Two main pathways: 1- Mitochondrial pathway (intrinsic) 2- Death receptor pathway (extrinsic) 1- mitochondrial pathway (intrinsic) responsible for apoptosis in most situations. Leak of cytochrome c out of mitochondria and activation of caspase 9… 2- death receptor pathway (extrinsic) Involved in elimination of self-reactive lymphocytes and in killing of target cells by some cytotoxic T lymphocytes. Activation of caspase 8. Feature Necrosis Apoptosis Cell size Enlarged (swelling) Reduced (shrinkage) Nucleus Pyknosis → karyorrhexis → karyolysis Fragmentation into nucleosome-size fragments Plasma membrane Disrupted Intact; altered structure, especially orientation of lipids Cellular content Enzymatic digestion; may leak out of cell Intact; altered structure, especially orientation of lipids Adjacent inflammation Frequent No Physiologic or pathologic role Invariably pathologic (culmination of irreversible cell injury) Often physiologic, means of eliminating unwanted cells; may be pathologic after some forms of cell injury, especially DNA damage THANK YOU