* Your assessment is very important for improving the work of artificial intelligence, which forms the content of this project
Download PDF
Aging brain wikipedia , lookup
Neuropsychology wikipedia , lookup
Neuroregeneration wikipedia , lookup
Time perception wikipedia , lookup
Eyeblink conditioning wikipedia , lookup
Neuroeconomics wikipedia , lookup
Brain–computer interface wikipedia , lookup
Human brain wikipedia , lookup
Environmental enrichment wikipedia , lookup
Activity-dependent plasticity wikipedia , lookup
Cortical cooling wikipedia , lookup
Recovery International wikipedia , lookup
Neural correlates of consciousness wikipedia , lookup
Premovement neuronal activity wikipedia , lookup
Cognitive neuroscience of music wikipedia , lookup
Muscle memory wikipedia , lookup
Embodied language processing wikipedia , lookup
Cerebral cortex wikipedia , lookup
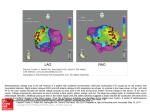
Basic Sciences Effects of Postinfarct Myelin-Associated Glycoprotein Antibody Treatment on Motor Recovery and Motor Map Plasticity in Squirrel Monkeys Scott Barbay, PhD; Erik J. Plautz, PhD; Elena Zoubina, PhD; Shawn B. Frost, PhD; Steven C. Cramer, MD; Randolph J. Nudo, PhD Downloaded from http://stroke.ahajournals.org/ by guest on June 16, 2017 Background and Purpose—New insights into the brain’s ability to reorganize after injury are beginning to suggest novel restorative therapy targets. Potential therapies include pharmacological agents designed to promote axonal growth. The purpose of this study was to test the efficacy of one such drug, GSK249320, a monoclonal antibody that blocks the axon outgrowth inhibition molecule, myelin-associated glycoprotein, to facilitate recovery of motor skills in a nonhuman primate model of ischemic cortical damage. Methods—Using a between-groups repeated-measures design, squirrel monkeys were randomized to 1 of 2 groups: an experimental group received intravenous GSK249320 beginning 24 hours after an ischemic infarct in motor cortex with repeated dosages given at 1-week intervals for 6 weeks and a control group received only the vehicle at matched time periods. The primary end point was a motor performance index based on a distal forelimb reach-and-retrieval task. Neurophysiological mapping techniques were used to determine changes in spared motor representations. Results—All monkeys recovered to baseline motor performance levels by postinfarct day 16. Functional recovery in the experimental group was significantly facilitated on the primary end point, albeit using slower movements. At 7 weeks post infarct, motor maps in the spared ventral premotor cortex in the experimental group decreased in area compared with the control group. Conclusions—GSK249320, initiated 24 hours after a focal cortical ischemic infarct, facilitated functional recovery. Together with the neurophysiological data, these results suggest that GSK249320 has a substantial biological effect on spared cortical tissue. However, its mechanisms of action may be widespread and not strictly limited to peri-infarct cortex and nearby premotor areas. (Stroke. 2015;46:1620-1625. DOI: 10.1161/STROKEAHA.114.008088.) Key Words: FAM168B protein, human ◼ haplorhini ◼ neuronal plasticity ◼ neurophysiology ◼ recovery of function ◼ stroke P harmacological treatments to improve functional outcomes after stroke remain limited beyond the use of thrombolytic agents during the first few hours. As our understanding of regenerative mechanisms that underlie the recovery of function matures, it is expected that approaches to target long-term recovery will receive increasing attention.1 This study focuses on GSK249320, a monoclonal antibody that blocks the axon outgrowth inhibition molecule, myelin-associated glycoprotein (MAG). Brain levels of MAG spontaneously increase after a stroke.2 The putative mechanism of action for this MAG antibody is the disinhibition of neurite sprouting and growth, thus allowing new neuronal connections to be formed.3 Many neurophysiological and neuroanatomical events related to behavioral recovery have been described in the days to weeks after a stroke. After focal ischemic infarct in primary motor cortex (M1) in both rodents and nonhuman primates, motor representations in spared cortical areas undergo functional reorganization in parallel with motor recovery.4,5 Furthermore, growth-promoting and growth-inhibiting genes are upregulated in spared neural tissue both adjacent to the infarct and in more remote areas connected with infarct.2,6,7 Finally, axonal sprouting and the formation of new corticocortical connections occur after focal cortical infarcts.8,9 Thus, it is reasonable to presume that pharmacological agents that Received November 22, 2014; final revision received February 23, 2015; accepted March 16, 2015. From the Department of Molecular and Integrative Physiology, Landon Center on Aging, University of Kansas Medical Center (S.B., E.J.P., E.Z., S.B.F., R.J.N.); and Department of Neurology and Department of Anatomy and Neurobiology, University of California, Irvine (S.C.C.). Current address for E.J.P.: Department of Neurology and Neurotherapeutics, University of Texas Southwestern Medical Center, Dallas. Current address for E.Z.: Department of Neurology, Harvard Medical School, Boston, MA. Guest Editor for this article was Miguel Perez-Pinzon, PhD. The online-only Data Supplement is available with this article at http://stroke.ahajournals.org/lookup/suppl/doi:10.1161/STROKEAHA. 114.008088/-/DC1. Correspondence to Randolph J. Nudo, PhD, University of Kansas Medical Center, Landon Center on Aging, MS 1005, 3901 Rainbow Blvd, Kansas City, KS 66160. E-mail [email protected] © 2015 American Heart Association, Inc. Stroke is available at http://stroke.ahajournals.org DOI: 10.1161/STROKEAHA.114.008088 1620 Barbay et al Effects of Postinfarct MAG Antibody Treatment on Recovery 1621 promote neurite sprouting and growth might amplify these events and thereby increase the extent of behavioral recovery during this period. Previous studies in rodents have demonstrated that a MAG antibody can neutralize MAG-mediated inhibition and promote neurite outgrowth associated with improved behavioral recovery after injury to the cerebral cortex.3,10 A study in 47 healthy human subjects found GSK249320 to be well tolerated in doses ≤25 mg/kg body weight.11 A recent clinical trial enrolled 42 patients 24 to 72 hours after stroke onset and found GSK249320 to be safe.12 The primary goal of this study was to evaluate the efficacy of GSK249320 for enhancing recovery of skilled use of the forelimb in a nonhuman primate model of focal cortical ischemia and to determine whether its effects are evident in the organization of movement representations in spared cortical areas. Downloaded from http://stroke.ahajournals.org/ by guest on June 16, 2017 Methods Nine adult male squirrel monkeys were used in this study. All experiments were conducted in accordance with institutional and federal guidelines for care and use of experimental animals and with approval from the Institutional Animal Care and Use Committee. A procedural timeline is presented in Figure 1. Monkeys were assigned randomly to 1 of 2 groups, differing only with regard to whether they received postinfarct injections of GSK249320 (experimental group) or vehicle (control group). One investigator was wholly responsible for animal behavioral training and postinfarct testing and was blind to the treatment condition. Each monkey underwent preinfarct training on a pellet retrieval task for 10 days, and then probe trials were conducted once per week for 2 weeks.13 Next, monkeys underwent the first of 2 surgical procedures. In the first procedure, the distal forelimb (DFL) representations in primary motor cortex (M1), dorsal premotor cortex (PMd), and ventral premotor cortex (PMv), as well as the surrounding proximal representations, were identified by intracortical microstimulation mapping techniques. Then, a cortical infarct that targeted 80% of the M1 DFL was created by electrocoagulation of surface vasculature (Figure 2). After recovery from anesthesia, monkeys were returned to their home cages. The active drug, identified as GSK249320 and supplied by GSK, United Kingdom, is an antibody to MAG. It binds with human MAG or its monkey ortholog with similar affinity (data on file). GSK249320 (30 mg/kg per dose; experimental group) or vehicle (control group) was administered intravenously every 7 days for 7 weeks, beginning PRE-INFARCT POST-MORTEM POST-INFARCT Motor Motor Exp HP Training Testing Motor Testing Histology Motor Testing Histology Drug/Vehicle Inj. Motor Testing Motor Motor Cont HP Training Testing -6 -5 -4 -3 -2 -1 1 Map 1/Infarct 2 3 4 5 6 7 8 9 Map 2 Weekly Time Line Relative to Infarct Figure 1. Timeline of experimental procedures. In the figure and throughout the text, experimental (Exp) time periods are referenced to the number of days or weeks relative to the infarct; that is, week -6=6 weeks before infarct. Baseline behavioral performance was defined by 2 motor testing sessions during weeks -3 and -2. After an intracortical microstimulation mapping procedure and infarct (time, 0), each monkey received 7 weekly injections of GSK249320 or vehicle beginning 24 hours post infarct. Behavioral testing occurred weekly beginning on day 3; filled circle: drug or vehicle injection and open triangles: behavioral testing session. Cont indicates control; and HP, hand preference testing. 24 hours after infarct. Postinfarct behavioral performance on the retrieval task was assessed weekly on days after the injections. Then, in a second surgical procedure, intracortical microstimulation mapping was repeated to verify the extent of the infarct and to determine whether motor representations were altered. After a 2-week survival period, animals were humanely euthanized and the brains were removed for histological analysis. The primary end point was a function of the number of finger flexions required to retrieve a pellet (flexions per retrieval performance index) measured during probe sessions. Secondary end points included retrieval success rate, time required to perform different phases of the retrieval task, and frequency of aiming errors, again measured during the probe sessions. Statistical tests included repeated-measures ANOVA and Bonferroni post hoc comparisons maintaining α-level at 0.05. Group averages are reported as mean+SEM. Additional details can be found in the online-only Data Supplement. Results Infarct Size and Location The mean extent of the infarct across the cortical surface was 12.03±1.04 mm2 in the experimental group and 10.97±1.07 mm2 in the control group. There was no statistically significant difference between groups in the absolute lesion size: t(5)=0.69; P=0.52 (Figure 3). The M1 DFL damage relative to each monkey’s baseline DFL was 81.5±1.61% for the experimental group and 79.1+0.98% for the control group, closely in line with our 80% target (Figure 2). There was no statistically significant difference between groups in the relative lesion size: t(5)=1.136; P=0.31. As intended, all infarcts spared DFLs at the rostral edge of the M1 DFL, as well as a small portion located medial and lateral to the infarct region. No damage was evident in PMd or PMv. Behavioral Assessment of Skilled Hand Use There was a significant effect of group (F[1,5]=12.732; P=0.001), time (F[8,40]=15.696; P<0.0001), and group X time interaction (F[8,40]=5.899; P<0.0001) in the primary end point, flexions per retrieval performance index. Bonferroni multiple comparison tests revealed significant group differences on days 3 (t[1,40]=6.95; P=0.009) and 9 (t[1,40]=3.86; P=0.018). That is, performance was better in the experimental group on day 3 (experimental group=1.55+0.22; control group=3.44+0.73) and day 9 (experimental group=1.01+0.06; control group=1.92+0.60). On day 3, there was no overlap between the groups. There were no differences between groups before the infarct or on day 16 through 44 (Figure 4A). With respect to secondary end points, there was a significant effect of group (F[1,5]=8.48; P=0.01), time (F[3,15]=5.54; P=0.0092), and group X time interaction (F[3,15]=6.537; P=0.0048) for time in well. Bonferroni multiple comparison test revealed that the experimental group required significantly more time (3.17±0.71) than the control group (0.93±0.22) on day 3 only (t[1,15]=5.70; P<0.0004; Figure 4B). Assessing reach and retrieval times separately, there was a significant effect of time for both reach (F[3,15]=8.217; P=0.0018) and retrieval (F[3,15]=12.904; P=0.0002) but there was no effect of group nor group X time interaction. Bonferroni comparisons indicate that time to reach was significantly greater on day 3 than on preinfarct day 1 (t[1,15]=4.39; P=0.003) and day 2 (t[1,15]=4.03; P=0.0066), as well as day 9 (t[1,15]=3.57; P=0.0168). Retraction time was greater on days 3 1622 Stroke June 2015 and 9 compared with preinfarct days 1 and 2. Other significant differences included day 3 versus preinfarct day 1 (t[1,15]=4.69; P=0.0018) and preinfarct day 2 (t[1,15]=5.16; P=0.0006); day 9 versus preinfarct day 1 (t[1,15]=3.42; P=0.0228) and preinfarct day 2 (t[1,15]=3.90; P=0.0084). Days 3 and 9 were not significantly different: (t[1,15]=1.27; P>0.9999). There was also a significant effect of time for aiming errors (F[3,15]=12.904; P=0.0028). Bonferroni comparisons indicated that the average aiming errors for all monkeys were greater than preinfarct measures on days 3 and 9. There was no significant effect of group nor group X time interaction in aiming errors: day 3 versus preinfarct day 1 (t[1,15]=3.0; P=0.054) and preinfarct day 2 (t[1,15]=3.60; P=0.0156). Day 9 versus preinfarct day 1 (t[1,15]=3.60; P=0.0156) and preinfarct day 2 (t[1,15]=3.626; P=0.0150). Days 3 and 9 were not significantly different: (t[1,15]=0.022; P>0.9999). Postinfarct Changes in Spared Motor Representations 20.0 Exp 17.5 Cont 15.0 12.5 10.0 7.5 5.0 2.5 0.0 Absolute Infarct size Relative Infarct size 100 90 80 70 60 50 40 30 20 10 0 % M1 DFL Area Forelimb movements typically were evoked throughout the peri-infarct M1, PMd DFL, and PMv DFL at low current levels (Figure 2). In addition, forelimb movements could be evoked from sites along the border of the infarcted territory. However, because these sites were typically no >250 µm from the border and current thresholds were relatively high (often 25–30 µA), these sites were eliminated from the analyses because the evoked movements were likely due to current spread into the spared tissue. In 3 of the intracortical microstimulation mapping procedures, proximal but not DFL movements were evoked in PMd (baseline maps: Exp-3, Cont-3; postinfarct maps: Cont-3). This lack of distal movements at threshold current levels is not unusual in PMd of squirrel monkeys, and thus, these data were retained for the statistical analyses. DFL movements were observed at threshold currents in each of the PMv mapping procedures. Area means (±SEM) for the DFLs before and after the infarct are presented in Table. In the peri-infarct M1 DFL, there were no statistically significant differences between groups (group effect: F(1,5=0.0068, P=0.9374; time effect: F(1,5)=2.0365, P=0.2129; group X time interaction: F(1,5)=0.21374, P=0.6633). In the PMd DFL, there were no statistically significant differences between groups (group effect: F[1,5]=2.7294, P=0.1594; time effect: F[1,5]=3.0600, P=0.1407; group X time interaction: F[1,5]=1.0144, P=0.3601). It should be noted though that PMd DFL increased in each of the 4 monkeys in the experimental group but in only one of the 3 monkeys in the control group (χ2=9.561; P=0.002). In PMv, there was no group (F[1,5]=0.5553; P=0.4897) nor time effect (F[1,5]=0.1821; P=0.6873), but there was a significant group X time interaction (F[1,5]=11.0953; P=0.0208). Bonferroni multiple comparison tests revealed a decrease in PMv DFL in the experimental group after the infarct (experimental preversus experimental postinfarct area), but this difference was not significant [t(1,5)=2.87; P=0.07]. There was also no significant difference between the pre- and post control group maps (t[1,5]=1.92; P=0.112; Figure 5). M1 DFL Area mm 2 Downloaded from http://stroke.ahajournals.org/ by guest on June 16, 2017 Figure 2. Sequence of photographs of surface vasculature in frontal cortex before (left), immediately after (middle), and 7 weeks after (right) an ischemic infarct in M1 distal forelimb (DFL; case control [Cont]-1). Left, Vascular pattern with superimposed movement representations in M1, dorsal premotor cortex (PMd), and ventral premotor cortex (PMv). M1 DFL (≈80%) was targeted for the infarct based on intracortical microstimulation (ICMS) maps of evoked movements (movement coding: red=digit; green=wrist/forearm; purple=proximal+wrist/forearm; yellow=digit+wrist; and grey=proximal). Dashed white line indicates intended infarct territory. In the case illustrated here, a large vein that bisected the DFL was intentionally spared but not its branches. White box encloses the enlarged region shown in middle and right images. Middle, Ischemic region in M1 DFL several minutes after the infarct was completed. The ischemic area is readily distinguishable because of blanching of the tissue. Visual glare is because of the presence of saline on the brain surface during the procedure. This infarct technique produces sharply defined borders between ischemic and normally perfused tissue (indicated by blue boundary) and creates an infarct through all 6 layers of cerebral cortex but spares the underlying white matter. Right, Photograph of ischemic region 7 weeks after the infarct in M1 DFL. As intended, the perimeter of the M1 DFL, as well as PMd and PMv (not shown), was spared by the infarct, as evidenced by ICMS-evoked movements. Scale bar, 500 µm. Figure 3. Lesion size in experimental (Exp) and control (Cont) groups. There were no statistical differences in either absolute or relative lesion size between groups. DFL indicates distal forelimb. Barbay et al Effects of Postinfarct MAG Antibody Treatment on Recovery 1623 B 5.0 4.5 4.0 3.5 3.0 2.5 2.0 1.5 1.0 0.5 0.0 Exp * Cont * -14 -7 3 infarct 9 16 23 30 37 5.0 Retrieval Time Index Flexions/Retrieval Index A Exp Cont * 4.0 3.0 2.0 1.0 0.0 44 -14 -7 3 9 infarct Day Day Figure 4. Motor performance. A, Flexions per retrieval performance index. The control (Cont) group was significantly more impaired than the experimental (Exp) group on days 3 and 9. B, Retrieval time index. The Exp group took more time to retrieve food pellets from the Klüver board on postinfarct day 3 than the Cont group. The infarct had no significant effect on flexion time for a successful retrieval for the Cont group. *Indicates significant difference between groups (post hoc test, P<0.05). Small circles (Exp) and triangles (Cont) correspond to individual case data. Individual data are shown only for days 3 and 9 as the variance was small on the other days, and small circles would overlap. Error bars, mean±SEM. Downloaded from http://stroke.ahajournals.org/ by guest on June 16, 2017 Because of the significant interaction effect in PMv and the potential for differential effects between PMv and PMd in experimental versus control groups, we also examined PMv–PMd difference scores. A 2-way ANOVA revealed a significant group X time interaction (F[1,5]=8.75; P=0.0308). Bonferroni adjusted multiple comparisons showed that for the experimental group, the baseline PMv–PMd difference scores were greater than the postinfarct difference scores (t[1,5]=3.678; P=0.0286). There was no prepost difference in PMv–PMd difference scores for the control group (t[1,5]=0.7557; P=0.9678). Discussion Loss of function after stroke is due not only to the local ischemic damage, but also to a large extent, a disconnection of functional neural networks throughout the brain. Therefore, investigations into various therapeutic strategies to improve motor function after stroke have targeted interventions thought to promote neural reorganization, restoring functional connectivity within the spared motor networks. Many recent restorative therapies have targeted myelin-associated axon outgrowth inhibition molecules, inhibitors that promote a nonpermissive growth environment after stroke, reducing neurite outgrowth,14,15 thus constraining neural plasticity and limiting recovery.16 One current putative intervention strategy is to neutralize such inhibition, thereby promoting axonal growth and sprouting to restore movement-related communication in cortical networks. Of the various myelin-associated inhibitors, MAG is of interest as it has been shown to be upregulated in peri-infarct tissue after ischemic damage in the aged brain.2 This study examined the efficacy of a new monoclonal antibody, GSK 249320, to MAG (ie, MAG antibody) in squirrel monkeys for recovery after an ischemic infarct in M1. A pilot experiment conducted before this study demonstrated that after cortical infarcts in M1 of squirrel monkeys, MAG antibody was present within the infarcted tissue within hours after a single administration (Figure I in the online-only Data Supplement). With an infarct targeting of 80% of the M1 DFL, the deficit was transient in both groups. However, GSK249320, initiated intravenously 24 hours after the infarct, facilitated more rapid recovery of DFL motor function, including the primary end point, flexions per retrieval performance index. The experimental group demonstrated superior motor performance on the reach-and-retrieval task on day 3 (2 days after initial treatment) and day 9 (1 day after second treatment). In fact, the deficit was unusually mild in the experimental group even on day 3. This rapid recovery in motor skill is somewhat surprising given the putative effects of MAG antibody on axonal growth promotion. However, evidence from other studies suggests that, in addition to blocking MAG-mediated inhibition of neurite outgrowth, treatment with MAG antibody protects oligodendrocytes from oxidative-stress induced cell death.3 Because oligodendrocytes play a critical role in axonal integrity and are particularly sensitive to ischemic injury,17,18 it is possible that treatment with GSK249320 in this study exerted its therapeutic benefits through protection of oligodendrocytes in the vicinity of the infarct. There is also evidence suggesting that MAG antibody may enhance synaptic plasticity. In the mature central nervous system, MAG receptors, such as NgR1 (part of the Nogo receptor family), maintain synaptic stability by inhibiting dendritic changes in morphology and function, such as long-term potentiation.19,20 Blocking these pre- and postsynaptic inhibitory receptors could account for an immediate activity-dependent modulation of synaptic connections supporting a rapid increase in functional recovery. Comparing neurophysiological map changes revealed unexpected findings. Given that MAG is known to be upregulated in peri-infarct cortex,2 it might be expected that GSK249320 would have its greatest effect in the 20% of the Table. Size of Movement Representations Before and After Infarct (mm2) Peri-Infarct Peri-Infarct M1 M1 PMv PMv PMd PMd Group Preinfarct Postinfarct Preinfarct Postinfarct Preinfarct Postinfarct Exp 2.65±0.25 3.00±0.41 3.49±0.68 2.75±0.44 0.59±0.28 1.38±0.43 Cont 2.75±0.28 2.93±0.39 2.98±0.67 3.55±0.25 0.41±0.31 0.62±0.33 Cont indicates control; Exp, experiment; PMd, dorsal premotor cortex; and PMv, ventral premotor cortex. 1624 Stroke June 2015 Pre-infarct Post-infarct Exp Cont B 1.5 Change in Area (mm2) (map2-map1) A * 1.0 0.5 0.0 Exp -0.5 Cont -1.0 Peri-Infarct M1 -1.5 PMv PMd digit wrist/forearm proximal digit + wrist/forearm proximal + wrist/forearm no response (30 µA) Figure 5. Changes in size of distal forelimb representations. A, Ventral premotor cortex (PMv) distal forelimb (DFL) motor maps before and 7 weeks after infarct in representative experimental (Exp) and control (Cont) cases. Movement coding: red=digit, green=wrist/forearm, purple=proximal+wrist/forearm, yellow=digit+wrist; blue=proximal; and black=no response at 30 µA). B, Comparison of peri-infarct, PMv, and dorsal premotor cortex (PMd) DFL representations before and 7 weeks after infarct. Bar graphs portray difference scores between postinfarct and preinfarct maps. Means for each time point are shown in the Table. There was a differential effect of treatment on map area in PMv (*P<0.05). Error bars, mean±SEM. Downloaded from http://stroke.ahajournals.org/ by guest on June 16, 2017 M1 DFL spared by the infarct. However, although the periinfarct M1 DFL increased slightly in both experimental and control groups (0.35 mm2 in Exp; 0.18 mm2 in Cont), there was no significant difference between groups (P=0.93). Thus, if the behavioral benefit seen with GSK249320 is based on expansion of DFL representation in peri-infarct cortex, then it is likely to be due to some other neuroanatomical or neurophysiological change. Similarly, PMd DFL maps did not change significantly, although a larger sample size may yet reveal a group effect since in the present sample, P=0.16, with larger PMd DFL maps in the experimental group (Figure 5). On the basis of our previous studies demonstrating an increase in PMv DFL after M1 infarct in squirrel monkeys not subjected to postinfarct intervention,5,8 we expected to see postinfarct expansions in both groups but larger increases in the experimental group. We have also shown in a recent study that spared premotor representations expand as a function of rehabilitative training in a rat model of ischemic infarct.4 As expected, in this study, PMv DFL increased in size in each of the 3 control monkeys but decreased in size in each of the 4 experimental monkeys. On the basis of our historical data,5,8 the expected increase in PMv DFL in animals with an 80% infarct in the M1 DFL, but no rehabilitative training, is 33.4% at 3 months post infarct. In this study, the increase in PMv DFL representation in the control group was 17.5% at 7 weeks post infarct. Furthermore, we have shown in other studies that after M1 lesions, spared motor maps first decrease and then increase in size with increasing time after infarct.4,21 Given the longer survival times in the historical controls, the size of the increase in PMv DFL in control monkeys in this study is within the expected range. However, in the experimental group, PMv maps decreased by 19.2%. We have only observed decreases in PMv DFL when infarcts were <50% of the M1 DFL8 or were derived at much earlier time points after the infarct (<3 weeks).4,22 Because there is no evidence that GSK249320 affected lesion size, this result suggests that the drug treatment may have had an unusual effect on cortical motor networks to support recovery of DFL performance compared with what is typically observed during spontaneous recovery alone. One possible explanation for the discrepant map results in PMv is that monkeys in the experimental group used a particularly successful compensatory reaching strategy. Although movement kinematics were not explicitly examined in this study, some support for a successful compensatory strategy in the experimental group can be derived from examination of the secondary behavioral end point, time in well. Although monkeys in the experimental group demonstrated superior scores on the flexions per retrieval performance index, they also spent more time with their digit(s) in the food well to perform retrievals on day 3. It is possible that slower and more deliberate movements were part of a compensatory strategy that facilitated improvement. Furthermore, if monkeys in the experimental group differentially used proximal arm and shoulder muscles for reaching or stabilization, recovery may have been accompanied by an expansion of proximal forelimb representations at the expense of distal representations in PMv. Consistent with this hypothesis, examination of Figure 5 reveals that at many specific sites, intracortical microstimulation resulted in distal movements in the preinfarct map but proximal movements in the postinfarct map. This observation is most evident in the experimental group. Previous studies from this laboratory using a similar infarct model have shown that a variety of compensatory strategies are used soon after the infarct. The kinematic patterns that are eventually used after recovery often are different than those used before the infarct.23 Alternatively, compensatory strategies could involve attentional or motivational mechanisms related to a more focused effort leading to more successful retrievals. In a virtual lesion study in healthy humans using transcranial magnetic stimulation, Davare et al24 provided evidence for a functional dissociation between PMd and PMv. Their results supported the view that PMv is involved more in the grasping component of a grip-lift task, whereas PMd is involved more in the lifting phase, recruiting more proximal muscles. If the trend toward larger distal representations in PMd and smaller distal representations in PMv with GSK249320 treatment is borne out in larger samples, then it is reasonable to suggest that the differential functional specialization of premotor areas in precision grasping is altered by postinfarct GSK249320. We propose that reorganization of spared cortical networks results in a functional rebalancing of premotor cortical areas, so that Barbay et al Effects of Postinfarct MAG Antibody Treatment on Recovery 1625 Downloaded from http://stroke.ahajournals.org/ by guest on June 16, 2017 PMd assumes a greater role in the grasp phase and PMv in the lift-retrieval phase of the task. In the context of the previous literature, the present results narrow the range of possibilities for the mechanisms underlying the functional benefits of GSK249320. Plasticity in peri-infarct M1 DFL is an unlikely candidate, unless a different neurophysiological or neuroanatomical end point is needed to capture the effect of GSK249320. It is likely that compensatory kinematic or attentional strategies, supported by plasticity in premotor cortical areas, contributed to faster recovery in the experimental group. The best case can be made for plasticity in PMv, although PMd is still a potential site. In the future, larger studies should (1) expand on the present results to examine other premotor areas (PMd and the supplementary motor area) and (2) focus on kinematic end points. It would also be interesting to know whether GSK249320 given to normal monkeys would affect the motor map representations in PMv and PMd similarly to what we have shown in the experimental group. As plasticity after neural injury is likely to be a network response, it is also possible that subcortical or contralateral structures are involved. However, it is clear that GSK249320 is safe in a nonhuman primate model of focal cortical ischemia, accelerates functional recovery, and has a significant effect on the neurophysiology of spared motor representations. Acknowledgments We thank Robert Cross, Ann Stowe, and Numa Dancause for technical assistance in the study. Sources of Funding This study was funded by GlaxoSmithKline. Disclosures Dr Nudo has served as a consultant for Gerson Lehrman Group, MicroTransponder, and St. Jude Medical. Dr Cramer has served as a consultant for GlaxoSmithKline, Dart Neuroscience, and MicroTransponder, and is a cofounder of personalRN. The other authors report no conflicts. References 1. Adams HP Jr, Nudo RJ. Management of patients with stroke: is it time to expand treatment options? Ann Neurol. 2013;74:4–10. doi: 10.1002/ ana.23948. 2. Li S, Carmichael ST. Growth-associated gene and protein expression in the region of axonal sprouting in the aged brain after stroke. Neurobiol Dis. 2006;23:362–373. doi: 10.1016/j.nbd.2006.03.011. 3. Irving EA, Vinson M, Rosin C, Roberts JC, Chapman DM, Facci L, et al. Identification of neuroprotective properties of anti-MAG antibody: a novel approach for the treatment of stroke? J Cereb Blood Flow Metab. 2005;25:98–107. doi: 10.1038/sj.jcbfm.9600011. 4. Nishibe M, Urban ET III, Barbay S, Nudo RJ. Rehabilitative training promotes motor recovery but delayed motor map reorganization in a rat cortical ischemic infarct model [published online ahead of print July 22, 2014]. Neurorehabil Neural Repair. http://nnr.sagepub.com/content/ early/recent. Accessed April 3, 2015. 5. Frost SB, Barbay S, Friel KM, Plautz EJ, Nudo RJ. Reorganization of remote cortical regions after ischemic brain injury: a potential substrate for stroke recovery. J Neurophysiol. 2003;89:3205–3214. doi: 10.1152/ jn.01143.2002. 6. Urban ET III, Bury SD, Barbay HS, Guggenmos DJ, Dong Y, Nudo RJ. Gene expression changes of interconnected spared cortical neurons 7 days after ischemic infarct of the primary motor cortex in the rat. Mol Cell Biochem. 2012;369:267–286. doi: 10.1007/s11010-012-1390-z. 7.Overman JJ, Clarkson AN, Wanner IB, Overman WT, Eckstein I, Maguire JL, et al. A role for ephrin-A5 in axonal sprouting, recovery, and activity-dependent plasticity after stroke. Proc Natl Acad Sci U S A. 2012;109:E2230–E2239. doi: 10.1073/pnas.1204386109. 8. Dancause N, Barbay S, Frost SB, Zoubina EV, Plautz EJ, Mahnken JD, et al. Effects of small ischemic lesions in the primary motor cortex on neurophysiological organization in ventral premotor cortex. J Neurophysiol. 2006;96:3506–3511. doi: 10.1152/jn.00792.2006. 9. Hinman JD, Rasband MN, Carmichael ST. Remodeling of the axon initial segment after focal cortical and white matter stroke. Stroke. 2013;44:182–189. doi: 10.1161/STROKEAHA.112.668749. 10. Thompson HJ, Marklund N, LeBold DG, Morales DM, Keck CA, Vinson M, et al. Tissue sparing and functional recovery following experimental traumatic brain injury is provided by treatment with an anti-myelin-associated glycoprotein antibody. Eur J Neurosci. 2006;24:3063–3072. doi: 10.1111/j.1460-9568.2006.05197.x. 11. Abila B, Cunningham E, Simeoni M. First-time-in-human study with GSK249320, a myelin-associated glycoprotein inhibitor, in healthy volunteers. Clin Pharmacol Ther. 2013;93:163–169. doi: 10.1038/ clpt.2012.227. 12. Cramer SC, Abila B, Scott NE, Simeoni M, Enney LA; MAG111539 Study Investigators. Safety, pharmacokinetics, and pharmacodynamics of escalating repeat doses of GSK249320 in patients with stroke. Stroke. 2013;44:1337–1342. doi: 10.1161/STROKEAHA.111.674366. 13.Nudo RJ, Jenkins WM, Merzenich MM, Prejean T, Grenda R. Neurophysiological correlates of hand preference in primary motor cortex of adult squirrel monkeys. J Neurosci. 1992;12:2918–2947. 14. Schwab ME, Strittmatter SM. Nogo limits neural plasticity and recovery from injury. Curr Opin Neurobiol. 2014;27:53–60. doi: 10.1016/j. conb.2014.02.011. 15. Fang PC, Barbay S, Plautz EJ, Hoover E, Strittmatter SM, Nudo RJ. Combination of NEP 1-40 treatment and motor training enhances behavioral recovery after a focal cortical infarct in rats. Stroke. 2010;41:544– 549. doi: 10.1161/STROKEAHA.109.572073. 16. Lee JK, Kim JE, Sivula M, Strittmatter SM. Nogo receptor antagonism promotes stroke recovery by enhancing axonal plasticity. J Neurosci. 2004;24:6209–6217. doi: 10.1523/JNEUROSCI.1643-04.2004. 17.Arai K, Lo EH. Experimental models for analysis of oligodendrocyte pathophysiology in stroke. Exp Transl Stroke Med. 2009;1:6. doi: 10.1186/2040-7378-1-6. 18.Mifsud G, Zammit C, Muscat R, Di Giovanni G, Valentino M. Oligodendrocyte pathophysiology and treatment strategies in cerebral ischemia. CNS Neurosci Ther. 2014;20:603–612. doi: 10.1111/ cns.12263. 19. Akbik F, Cafferty WB, Strittmatter SM. Myelin associated inhibitors: a link between injury-induced and experience-dependent plasticity. Exp Neurol. 2012;235:43–52. doi: 10.1016/j.expneurol.2011.06.006. 20.Raiker SJ, Lee H, Baldwin KT, Duan Y, Shrager P, Giger RJ. Oligodendrocyte-myelin glycoprotein and Nogo negatively regulate activity-dependent synaptic plasticity. J Neurosci. 2010;30:12432– 12445. doi: 10.1523/JNEUROSCI.0895-10.2010. 21. Eisner-Janowicz I, Barbay S, Hoover E, Stowe AM, Frost SB, Plautz EJ, et al. Early and late changes in the distal forelimb representation of the supplementary motor area after injury to frontal motor areas in the squirrel monkey. J Neurophysiol. 2008;100:1498–1512. doi: 10.1152/ jn.90447.2008. 22. Plautz EJ, Barbay S, Frost SB, Dancause N, Stowe AM, Zoubina EV, et al. Injury-Induced Reduction and Recovery of Hand Area Representation in Ventral Premotor Cortex Following Ischemic Infarct in Primary Motor Cortex. “Abstract”. Program No. 878.8, 2004 Abstract Viewer/Itinerary Planner. Washington, DC: Society for Neuroscience; 2004. 23. Friel KM, Nudo RJ. Recovery of motor function after focal cortical injury in primates: compensatory movement patterns used during rehabilitative training. Somatosens Mot Res. 1998;15:173–189. 24.Davare M, Andres M, Cosnard G, Thonnard JL, Olivier E. Dissociating the role of ventral and dorsal premotor cortex in precision grasping. J Neurosci. 2006;26:2260–2268. doi: 10.1523/ JNEUROSCI.3386-05.2006. Effects of Postinfarct Myelin-Associated Glycoprotein Antibody Treatment on Motor Recovery and Motor Map Plasticity in Squirrel Monkeys Scott Barbay, Erik J. Plautz, Elena Zoubina, Shawn B. Frost, Steven C. Cramer and Randolph J. Nudo Downloaded from http://stroke.ahajournals.org/ by guest on June 16, 2017 Stroke. 2015;46:1620-1625; originally published online April 30, 2015; doi: 10.1161/STROKEAHA.114.008088 Stroke is published by the American Heart Association, 7272 Greenville Avenue, Dallas, TX 75231 Copyright © 2015 American Heart Association, Inc. All rights reserved. Print ISSN: 0039-2499. Online ISSN: 1524-4628 The online version of this article, along with updated information and services, is located on the World Wide Web at: http://stroke.ahajournals.org/content/46/6/1620 Data Supplement (unedited) at: http://stroke.ahajournals.org/content/suppl/2015/05/04/STROKEAHA.114.008088.DC1 Permissions: Requests for permissions to reproduce figures, tables, or portions of articles originally published in Stroke can be obtained via RightsLink, a service of the Copyright Clearance Center, not the Editorial Office. Once the online version of the published article for which permission is being requested is located, click Request Permissions in the middle column of the Web page under Services. Further information about this process is available in the Permissions and Rights Question and Answer document. Reprints: Information about reprints can be found online at: http://www.lww.com/reprints Subscriptions: Information about subscribing to Stroke is online at: http://stroke.ahajournals.org//subscriptions/ SUPPLEMENTAL MATERIAL Supplemental Methods Overview Nine adult male squirrel monkeys (Worldwide Primates, Inc., Miami, FL; Genus: Saimiri; >3 years old; weight range: 837 - 1,179 g) were used in the present study. All experiments were conducted in accordance with institutional (Kansas University Medical Center) and federal (National Institutes of Health) guidelines for care and use of experimental animals and with approval from the Institutional Animal Care and Use Committee. Monkeys were housed in a climate-controlled vivarium maintaining ambient temperatures between 240 C and 280 C and relative humidity between 30% and 70%. Food (Purina Lab Diet #5088) was provided twice daily (supplemented with fruit and vegetables) and water was provided ad libitum through an automatic watering system. On days when behavior was assessed, feeding was scheduled after assessment (see behavioral methods). Routine health checks were made daily by Laboratory Animal Resource staff under supervision of institutional veterinary staff. Monkeys were assigned randomly to one of two groups, differing only with regard to whether they received post-infarct injections of GSK249320 (Experimental or Exp group) or vehicle (Control or Cont group). Each monkey underwent pre-infarct training on a pellet retrieval task for 10 days to establish a proficient, but not maximized performance level. After the training period, but before surgical procedures, probe trial sessions were conducted on the retrieval task once/week for two weeks. Next, monkeys underwent the first of two surgical procedures. In the first procedure, the distal forelimb representations (DFLs) in primary motor cortex (M1), dorsal premotor cortex (PMd) and ventral premotor cortex (PMv), as well as the surrounding proximal representations were identified by intracortical microstimulation (ICMS) mapping techniques. Then, a cortical infarct that targeted the M1 DFL was created by electrocoagulation of surface vasculature. After recovery from anesthesia, monkeys were returned to their home cages. GSK249320 (Exp group) or vehicle (Cont group) was administered intravenously every seven days for seven weeks, beginning 24 hr after infarct. Post-infarct behavioral performance on the retrieval task was assessed weekly on days following the injections. Then, in a second surgical procedure, ICMS mapping was repeated to verify the extent of the infarct, and to determine if motor representations were altered. At the conclusion of the ICMS mapping procedures, a neuronal tract-tracer was injected into the DFL in PMv. After a two-week survival period, animals were humanely euthanized and the brains removed for histological analysis. Blinding procedures To minimize experimenter bias, one investigator was wholly responsible for animal behavioral training and post-infarct testing, and was blind to the treatment condition. A second investigator was wholly responsible for random assignment of monkeys to experimental groups and for preparation and injection of the drug or vehicle. During cortical mapping sessions, ICMS-evoked responses were defined by other investigators blind to the treatment condition. Behavioral procedures All behavioral testing and training was conducted using a modified Klüver board apparatus.1 This device consisted of a Plexiglas board attached to the front of the monkey’s home cage, with five food wells drilled into its surface. Each well was 5mm in depth and varied in diameter from 25-9.5 mm (designated wells 1-5 respectively). The behavioral task consisted of reaching through the cage bars, inserting one or more fingers into a well, and retrieving a 1 single, small food pellet (45 mg; BioServe, Flemington NJ). Pellets were placed one at a time into one of the five food wells in a randomized block design (i.e., equal number of trials for each well). Behavioral sessions were videotaped for later analysis. Experimental time periods are referenced to the number of days or weeks relative to the infarct; i.e., Week -6 (minus 6) = six weeks prior to infarct, Day 7 = seven days after infarct. Throughout the behavioral phase of these experiments (Weeks -6 through 7), monkeys were maintained on a controlled food schedule, such that they were provided a total daily amount of food (pellets during behavioral testing/training + laboratory chow after behavioral testing/training) equal to 3% of their ad libitum body weight. This amount of food is adequate to maintain a stable body weight over time. On training and testing days, the number of pellets consumed was tallied, and supplemental food provided as needed to reach the 3% value. Hand (distal forelimb) preference testing (Week -6). Monkeys were required to retrieve 100 food pellets from the Klüver board (20 trials/well). Testing was conducted over a minimum of two days (maximum 50 trials/day). The animal was free to use either hand to perform the task. Based on the number of reaches and retrievals made with each hand, the monkey’s hand preference was defined as the hand used on more than 50% of trials. Pre-infarct training phase (Week -5 to -4). Pre-infarct training was conducted using each monkey’s preferred distal forelimb five days per week for a total of ten days. Each training session consisted of a 25 trial probe session followed immediately by a 30 minute training session. The probe session consisted of randomized presentation of pellets into one of the five wells until a total of five trials per well was performed. The training session consisted of trials conducted in only one or two wells, depending on the training day. Training began on the largest well on Day -35 and progressed to the smallest well according to the following schedule: Day 35, 100% well 1; Day -34, 100% well 2; Day -33, 100% well 3; Day -32, 50% well 3 and 50% well 4; Day -31, 100% well 4; Day -28, 50% well 4 and 50% well 5; Day -27-to-24, 100% well 5. The strategy was to provide exposure to all wells, with an emphasis on increasingly more difficult wells, but avoiding overtraining on any individual well. Pre-infarct baseline testing (Week -3 to -2). Monkeys were given 50 probe trials (10 trials per well) once per week to determine baseline performance. Post-infarct testing (Week 1-7). Monkeys were tested once per week during a 50 trial probe session (10 trials per well). Testing began on Day 3, then weekly on Day 9, 16, 23, 30, 37, and 44. Lack of appetite and motivation associated with surgical recovery precluded reliable testing on Day 1-2. Thus, post-infarct testing occurred on the day following drug/vehicle injections, except for Week 1, when testing occurred two days following drug/vehicle. Surgical procedures Monkeys were sedated with an initial dose of ketamine (20 mg, i.m.) followed by atropine (0.054mg, i.m.). Supplemental doses were given as needed as the monkeys were weighed and examined in preparation for the surgical procedure. The scalp, arm (opposite the hemisphere to be mapped) and leg (for catheterization) were shaved and cleaned. The trachea was treated with a local anesthetic (20% benzocaine spray and 2% lidocaine jelly) and intubated (2.5-3.0 mm i.d. tube). Gas anesthesia was then introduced (1.5% to 3% halothane - 75% nitrous oxide), and the saphenous vein catheterized with a 24g angiocath for delivery of lactated Ringers with 3% dextrose (10ml/kg/hr) and other required fluids. Ophthalmic ointment was liberally applied to the corneas and the monkey placed into a stereotaxic frame. The incision area was 2 infiltrated with 0.5% bupivacaine (1 ml s.c.) and scrubbed with alcohol and iodine. Penicillin-G benzathine + procaine (45K IU, s.c.), dexamethasone (0.50mg i.v.) and mannitol (2g/kg i.v.) were given. Then, under aseptic conditions and 1.5% – 3% halothane- 75% nitrous oxide anesthesia, a craniectomy (~1.5 cm square) was performed over M1, PMd and PMv contralateral to the monkey’s preferred hand, and the dura excised. A sterile plastic chamber was secured over the opening and filled with sterile silicone oil to protect the cortex. Inhalation anesthesia was withdrawn, and ketamine (5mg per dose), supplemented by diazepam (0.01mg per dose), was administered intravenously as needed. Vital signs were monitored and maintained within normal limits throughout the procedure. 2 Heart rate, oxygen saturation, respiration rate and expired CO 2 were monitored with a gas monitoring system and core body temperature was monitored with a homothermic blanket system. Following a neurophysiological mapping procedure (see below), inhalation anesthesia was reinstated, the dura replaced by silicone film, the bone flap cemented into place with dental acrylic, and the incision sutured and treated with 0.5% bupivacaine (1ml s.c.) penicillin-G benzathine + procaine (45K IU, s.c.), topical antibiotic spray (Furazolidone) and acetaminophen (20 mg oral) supplemented as needed determined by veterinary consultation. Monkeys were monitored in a temperature controlled incubator until recovery from anesthesia was complete and the animal was alert and stable (indicated by mobility, eating/drinking, waste elimination), and then returned to their home cages. Prolonged care procedures included wound care as needed, suture removal at ~2 weeks post-surgery and daily monitoring of general health. See Nudo et al.3 and Plautz et al.4 for further details. Cortical mapping procedures Established ICMS mapping techniques were used to derive detailed maps of movement representations in the distal and proximal forelimb areas of M1, PMd and PMv contralateral to the monkey’s preferred hand1,3,4,5. Briefly, a microelectrode (tapered and beveled glass micropipette, 10-25 µm o.d. tip, filled with 3.5M saline) was introduced into cortical layer V on a grid pattern (350-500 µm interpenetration distances) with reference to surface vasculature. At each site, ICMS was used to determine the movement(s) evoked at threshold current levels restricted to ≤30 µA. Pulse trains (thirteen 200-µsec pulses at 350 Hz) were repeated at 1 Hz, and evoked movements were described using conventional terminology. Mapping proceeded until the DFL, composed of finger, thumb, wrist, and forearm movements, was fully surrounded by proximal (e.g., elbow, shoulder, trunk, face) representations or by non-responsive sites. Two ICMS mapping procedures were performed in each monkey: the first, after pre-infarct training and baseline testing was completed (Map1); the second, seven weeks after the infarct was created and experimental treatments were completed (Map2). Cortical infarct procedures Based on ICMS mapping results, the surface vasculature overlying a selected region of the M1 DFL was occluded by bipolar electrocoagulation to create an ischemic injury3,6. The infarct targeted approximately 80% of the M1 DFL while sparing as much of the surrounding proximal representation as possible. The rationale for the scope of the infarct was to produce mild to moderate motor deficits, while sparing a portion of the M1 DFL so that neurophysiological changes could be assessed. Guided by vascular constraints, the infarct was induced principally in the caudal portion of the M1 DFL, intentionally sparing distal representations in the rostral portion of the map. Coagulated vessels included fine arterial and venous capillaries as well as larger vessels but avoided pass-through vessels supplying other cortical areas. This technique does not mimic clinical stroke per se, since it 1) affects the entire vascular bed within a focal cortical territory, 2) produces a minimal penumbra and 3) produces 3 strictly cortical damage without affecting subcortical structures. However, it is advantageous in that it produces a very reliable and selective focal ischemic injury with sharp borders, ideal for assessing chronic neurophysiological changes in functional maps. The infarct was created at the conclusion of the first cortical mapping procedure (Map1), after inhalation anesthesia had been reinstated. Occluded vessels were monitored by visual inspection for several minutes for evidence of reperfusion and re-occluded as needed. A digital image of the infarct was acquired to compare to the intended target and to delineate borders during map analysis. Drug/vehicle injection procedures The active drug, identified as GSK249320 and supplied by GSK UK, is an antibody to MAG. It binds with human MAG or its monkey orthologue with similar affinity (data on file). Based on results of pharmacokinetic analysis in three squirrel monkeys prior to the current study, the drug dose was 30 mg/kg per dose for each of seven weekly doses, delivered by intravenous bolus, using an injection volume that was calculated based on the animal’s weight obtained on the day of infarct. Stock solution of GSK249320 was prepared as 100 mg/ml. The vehicle solution was a sterile, inactive acetate solution. Starting at 24 hr post-infarct and weekly thereafter on Day 8, 15, 22, 29, 36 and 43, monkeys were injected with either 30 mg/kg of GSK249320 or an equivalent volume of vehicle solution. All injections were made through the saphenous vein. A 0.9% saline injection (0.5 ml) was used to clear the catheter and ensure that the experimental injection was fully infused. Injections were made in awake animals to avoid any effects of co-administered anesthetics. Euthanasia and histological processing Monkeys were euthanized by a pre-anesthetic dose of ketamine (25 mg i.m.) followed by a lethal dose of Euthasol (1.5ml i.p.), then perfused trans-cardially with 0.9% saline containing 0.2% heparin and lidocaine, followed by 3% paraformaldehyde in 0.1M phosphate buffered saline (PBS) at pH 7.4. The brain was removed and a portion of the cerebral cortex from the frontal and parietal lobes was separated from the underlying structures.7 The tissue was postfixed and cryoprotected in a series of solutions over two days, then sectioned tangentially to the cortical surface (50µm thickness) on a freezing microtome. This process verified the location of the lesion relative to peri-infarct M1, PMd and PMv. Lesion size estimation The areal extent of the M1 lesion was determined for each monkey based on a comparison of changes in cortical tissue, blood vessel patterns and physiology from the pre-and post-infarct maps.8 Digital photographs of the cortex derived during Map2 were compared with digital photographs derived during Map1 to reveal cortical tissue that was necrotic. Immediately after ischemic cortical injury, the necrotic cortex becomes blanched and thus easily distinguished from non-infarcted tissue. The border around the peri-infarct area was confirmed with ICMS. Relative lesion size was determined by measuring the distal forearm area from Map1 outlined by the actual infarct determined in Map2. For example, Map1 was intended to be 80% of M1 distal forearm, but the actual area infarcted could be greater or less than the intended infarct based on the actual infarct exposed seven weeks later during Map2. Due to the small size of the infarcts relative to the entire squirrel monkey brain, as well as the necrosis of the infarcted tissue and cavitation of the injury site, this technique is considered more reliable in these studies for estimating lesion size compared to histologic analysis.8 4 Analysis of behavioral performance All behavioral performance metrics were analyzed from slow-motion and frame-byframe playback of the time-stamped video records. Because monkeys typically vary in their preinfarct performance levels (at least without substantial overtraining), normalized scores were used to reduce the influence of pre-infarct baseline performance. This is especially important in non-human primate studies in which large sample sizes are not feasible. Therefore, we utilized a normalized performance index as the ratio of the raw performance score for each session to the average of the pre-infarct baseline scores (raw performance score ÷ average baseline performance). The primary endpoint was a function of the number of finger flexions required to retrieve a pellet (flexions per retrieval performance index) measured during probe sessions on all five wells. Secondary endpoints included retrieval success rate, time required to perform different phases of the retrieval task, and frequency of aiming errors, again measured during the probe sessions on all five wells. Flexions per retrieval. Flexions per retrieval were calculated for each probe session by dividing the total number of times that the digits flexed in an attempt to grasp the pellet divided by the total number of successful retrievals. The flexions per retrieval score thus provided a reliable metric of digital dexterity, with lower scores indicating better performance. A flexions per retrieval performance index was calculated by dividing scores on each post-infarct day by the average score from the two baseline testing sessions. Retrieval time. Retrieval time was defined as the amount of time (in seconds) required for the entire reach and retrieval sequence, including time to propel the distal forelimb to the board (reach time), time that the distal forelimb was in contact with the board (time on board), time that the digits were within the well (well time), and time to retract the distal forelimb back into the cage (retraction time). More specifically, reach began when the digits crossed the plane of the cage bars and ended when they either first contacted the surface of the Klüver board or the food well. Time on board began when the digits first contacted the board surface ended when one or more of the digits entered the target well. Time in well began when one or more of the digits entered the target well and ended when the digits were no longer contacting the well and a pellet was successfully retrieved. Retraction time began when the digit emerged from the well and ended when the distal forelimb crossed the plane of the cage bars. For simplicity, times were analyzed only for trials in which the monkey performed a complete movement sequence that included only one finger flexion to successfully remove the pellet from the well. To normalize retrieval times relative to each individual animal’s pre-injury levels, a retrieval time index was calculated by dividing retrieval times on each post-infarct day by the average retrieval time from the two baseline testing sessions. Thus, the reported retrieval time index is unitless. Aiming errors. A failure to insert the fingers directly into the well was defined as an aiming error. For each monkey, the percentage of aiming errors per session was calculated and normalized to the average of baseline performance as described for retrieval data. Analysis of ICMS maps A computer algorithm was used to unambiguously delineate functional boundaries for analysis of representational areas.1 Distal forelimb maps were analyzed in peri-infarct M1, PMd and PMv separately. 5 Statistical analysis Statistical analyses were performed using JMP v10.0 (SAS Institute, Inc. Cary NC). Statistical tests included repeated-measures ANOVA and Bonferroni post-hoc comparisons. Group averages are reported as Mean + SEM. Attrition Two monkeys, one in the Exp group and one in the Cont group, died during the experimental period. Neither of these two monkeys had received GSK249320. One monkey died of acute respiratory and cardiac failure during the first surgical procedure (Exp group), and the other monkey died approximately two weeks after the first procedure (Cont group). This monkey had received two injections of the vehicle (24hrs and 1week post-infarct). Necropsy revealed mild enteritis and pneumonitis, but the cause of death was not conclusive. Of the remaining seven monkeys, four were injected with MAG antibody (Exp group) and three were given Supplemental Figures Supplemental Figure Legend I. Histological verification of infarct and immunohistochemical staining for Anti-MAG at the level of M1 in a squirrel monkey. Results are derived from a pilot experiment prior to the full study. In the case illustrated here, MAG antibody (37mg/kg) was given to a squirrel monkey 24 hours after an ischemic infarct targeting 80% of the distal hand 6 area of M1 (DFL M1). The brain was perfused and post fixed with 4% paraformaldehyde and cryoprotected 6.5 hours after the injection (30.5 hrs. post-infarct). Coronal frozen sections were cut at 20µm thickness. A. Drawing of a coronal section of the brain through the infarct in DFL M1. B. Hemotoxolin and Eosin stained section showing intact white matter underlying the infarct. C. Fluoro-Jade B stained section confirming presence of injured/degenerating neurons within the infarcted hemisphere.9 Insert at lower right is a magnification of the infarcted area. D. Primary/secondary antibody stained section through infarct (1:500 donkey anti-human, 1:200 biotinylated rabbit anti-donkey, Vector ABC, DAB visualization). Labeling for MAG antibody is seen within the infarct and peri-infarct area. No labeling for MAG antibody could be seen in the homotopic contralesional hemisphere or in the peri-infarct region (not shown). Supplementary References 1. 2. 3. 4. 5. 6. 7. 8. 9. Nudo RJ, Jenkins WM, Merzenich MM, Prejean T, Grenda R. Neurophysiological correlates of hand preference in primary motor cortex of adult squirrel monkeys. J Neurosci. 1992;12:2918-2947 Nudo RJ, Larson D, Plautz EJ, Friel KM, Barbay S, Frost SB. A squirrel monkey model of poststroke motor recovery. ILAR J. 2003;44:161-174 Nudo RJ, Milliken GW, Jenkins WM, Merzenich MM. Use-dependent alterations of movement representations in primary motor cortex of adult squirrel monkeys. J Neurosci. 1996;16:785-807 Plautz EJ, Milliken GW, Nudo RJ. Effects of repetitive motor training on movement representations in adult squirrel monkeys: role of use versus learning. Neurobiol Learn Mem. 2000;74:27-55 Gould HJ, 3rd, Cusick CG, Pons TP, Kaas JH. The relationship of corpus callosum connections to electrical stimulation maps of motor, supplementary motor, and the frontal eye fields in owl monkeys. J Comp Neurol. 1986;247:297-325 Jenkins WM, Merzenich MM. Reorganization of neocortical representations after brain injury: a neurophysiological model of the bases of recovery from stroke. Prog Brain Res. 1987;71:249-266 Gould HJ, 3rd, Kaas JH. The distribution of commissural terminations in somatosensory areas I and II of the grey squirrel. J Comp Neurol. 1981;196:489-504 Friel KM, Nudo RJ. Recovery of motor function after focal cortical injury in primates: compensatory movement patterns used during rehabilitative training. Somatosens Mot Res. 1998;15:173-189 Schmued LC, Hopkins KJ. Fluoro-Jade B: a high affinity fluorescent marker for the localization of neuronal degeneration. Brain Res. 2000;874:123-130 7