* Your assessment is very important for improving the work of artificial intelligence, which forms the content of this project
Download Functional Neuronal Processing of Body Odors
Human brain wikipedia , lookup
Neuroscience in space wikipedia , lookup
Emotion perception wikipedia , lookup
Sensory cue wikipedia , lookup
Metastability in the brain wikipedia , lookup
Psychophysics wikipedia , lookup
Cortical cooling wikipedia , lookup
Aging brain wikipedia , lookup
Neuroeconomics wikipedia , lookup
Cognitive neuroscience of music wikipedia , lookup
Feature detection (nervous system) wikipedia , lookup
Affective neuroscience wikipedia , lookup
Time perception wikipedia , lookup
C1 and P1 (neuroscience) wikipedia , lookup
Embodied language processing wikipedia , lookup
Neuroesthetics wikipedia , lookup
Stimulus (physiology) wikipedia , lookup
Embodied cognitive science wikipedia , lookup
Olfactory bulb wikipedia , lookup
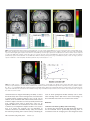
Cerebral Cortex June 2008;18:1466--1474 doi:10.1093/cercor/bhm178 Advance Access publication October 12, 2007 Functional Neuronal Processing of Body Odors Differs from that of Similar Common Odors Johan N. Lundström1,2, Julie A. Boyle1,2, Robert J. Zatorre1,2 and Marilyn Jones-Gotman1,2 Visual and auditory stimuli of high social and ecological importance are processed in the brain by specialized neuronal networks. To date, this has not been demonstrated for olfactory stimuli. By means of positron emission tomography, we sought to elucidate the neuronal substrates behind body odor perception to answer the question of whether the central processing of body odors differs from perceptually similar nonbody odors. Body odors were processed by a network that was distinctly separate from common odors, indicating a separation in the processing of odors based on their source. Smelling a friend’s body odor activated regions previously seen for familiar stimuli, whereas smelling a stranger activated amygdala and insular regions akin to what has previously been demonstrated for fearful stimuli. The results provide evidence that social olfactory stimuli of high ecological relevance are processed by specialized neuronal networks similar to what has previously been demonstrated for auditory and visual stimuli. (Wallace 1977). In fact, humans are able to use signals conveyed in body odor to make accurate kin--nonkin judgments (Weisfeld et al. 2003) and to detect minute differences in genetic composition of unknown individuals (Jacob et al. 2002). It has even been suggested that signals communicating emotions are held within body odors (Chen and Haviland-Jones 1999). Together, these studies suggest that the complex mixture constituting human body odors is a stimulus of high ecological importance for humans. Moreover, due to their complex signal contents, it seems likely that body odors are processed differently than common odors. Although a substantial number of studies have investigated the underlying mechanisms of olfactory processing of human body odor behaviorally, only a few have tried to assess how the brain processes body odors. Recent studies based on overall cortical activation by electroencephalography have demonstrated that our brains treat individual body odors differently depending on their origin (Pause et al. 1999, 2006). Body odors originating from oneself receive preferential processing compared with those from others (Pause et al. 1999). Our brains seem so finetuned to body odors that even minute differences in immunochemistry between individuals influence the processing of them (Pause et al. 2006). However, no study to date has tried to map the neuronal substrates of body odor processing. We sought to determine the neuronal substrates of body odor processing using H2O15 positron emission tomography imaging while women smelled body odors originating from different individuals. Each subject and one of her close longtime friends slept alone for 7 consecutive nights in a t-shirt that had cotton nursing pads sewn into the armpits, which collected and preserved their body odors while shielding them from external odor sources. The 15 participating women were scanned while they smelled the body odors from themselves, their friends, or a stranger; all women. In addition, they smelled a mixture consisting of common odors not found in human underarm sweat, but with similar perceptual characteristics, and, in a baseline condition, a clean odorless nursing pad. This constellation of stimuli allowed us to determine the neuronal substrates of body odor processing and the influence that body odors originating from specific individuals might have on this processing. Further, it allowed us to determine whether odors with high ecological and social importance are processed differently than odors of lower importance. Keywords: body odor, imaging, neural networks, odor identification Introduction Ever since early anatomists labeled humans as microsmatic animals (Broca 1888; Zwaardemaker 1895), there has been a common misconception among scientists and laymen alike that humans have a poorly developed sense of smell. Comparisons among species demonstrate that humans are more sensitive than new world monkeys to an odorant derived from the human body (Laska et al. 2006). This lends support to the theory that the behavioral relevance of an odor may be an important determinant of a species’ olfactory ability (Laska et al. 2005). In other sensory modalities the behavioral relevance of the stimulus in question has been demonstrated to be an important determinant of specialized neuronal processing. Stimuli that have high significance for the individual, by signaling recurrent threats or other important behavioral factors, seem to be ‘‘tagged’’ for priority processing (Ohman et al. 2001). Stimuli such as faces (Morris et al. 1998) and the cries of infants (Seifritz et al. 2003) are processed differently from perceptually comparable stimuli that are considered by the individual to be of limited behavioral relevance. These so-called evolutionary relevant stimuli recruit additional cortical and subcortical areas that are not commonly active in response to stimuli with low evolutionary relevance. In line with this notion, behaviorally relevant olfactory stimuli, such as our body odor, could be hypothesized to recruit nonolfactory structures to a higher degree than comparable and perceptually similar common odors. That humans are highly accurate at identifying individuals based solely on their body odors has been known for some time Ó The Author 2007. Published by Oxford University Press. All rights reserved. For permissions, please e-mail: [email protected] 1 Department of Psychology, McGill University, Montreal, QC, H3A1B1, Canada and 2Montreal Neurological Institute and Hospital, McGill University, Montreal, Quebec H3A 2B4 Canada Materials and Methods Participants Fifteen healthy, right-handed, nulliparous, nonsmoking women (mean age 23 years; standard deviation [SD] ± 2.9; range 18--28 years), recruited from the university’s student body, with an absence of nasal congestion, sinus infection, allergies, or decreased olfactory function, underwent positron emission tomography (PET) imaging (see Supplementary Material for additional information). Only women who described themselves as exclusively heterosexual participated in the study, either as ‘‘full participants’’ or odor donors. This exclusion criterion was used because preference for body odors is influenced by both gender and sexual orientation (Martins et al. 2005), and including men in the study would require a large number of participants. Detailed written informed consent was obtained from all participants prior to enrollment, and all aspects of the study were performed in accordance with the Declaration of Helsinki for experimentation with human subjects. Participants and odor donors were rewarded upon completion of the study with a total of $130 and $30, respectively, and the experimental protocol was approved by the Montreal Neurological Institute (MNI) and Hospital’s Research Ethics Board. Odor Stimuli Body odors were collected from each participant and her long-time close friend (mean length of friendship, 60 months). To collect the body odors, each participant and odor donor slept for 7 consecutive nights in a tight cotton t-shirt with cotton nursing pads sewn into the underarm area. The t-shirts were used to prevent the cotton pads from being contaminated by external odor sources and to ensure a close fit of the pads in the armpits. The t-shirts were stored in a closed ziplocked bag at all times when not worn in bed. All odor donors followed strict instructions that regulated their personal hygiene and diet to ensure that the pads were not contaminated (see Supplementary Material for a detailed description). After behavioral testing, as described below, the pads were sealed in individual odor-free freezer bags and deep frozen (–80 °C) until the day of scanning to prevent degradation of the stimuli. The odor control mixture consisted of cumin oil, anise oil, and indole (see Supplementary Material for a detailed description). A 10% v/v concentration of this mixture was applied to identical nursing pads and subjected to the same storage procedure as the pads containing body odors. Behavioral Testing Session Participants returned the t-shirts on the morning of the eighth day, and the pads were removed from the shirts and used for behavioral testing before being deep frozen. That all participants possessed a functional olfactory sense was determined using a 5-item olfactory identification test (Hummel et al. 2001). All subjects had 4 or more correct identifications. The ability to identify individual body odors was assessed in a counterbalanced order using a psychophysical testing paradigm. A 3-alternative, no feedback forced-choice task with 9 repetitions for each body odor category, with body odors from strangers as foils, was administered. In addition, the agreement between participants’ conscious experience and behavioral performance in the aforementioned task was tested using a confidence assessment task. On each individual trial, participants rated their confidence that they had selected the correct response, using a visual analog scale ranging from 30% (guessing) to 100% (totally sure). Olfactory sensitivity for phenyl ethyl alcohol was assessed using the Sniffin’ Sticks threshold test (Hummel et al. 1997) at the end of the behavioral session. PET Scanning Session The PET session consisted of 5 odor conditions (odor-free Baseline, Odor control, Self [BO], Friend [BO], and Stranger [BO]), each presented twice in a pseudo-randomized order, yielding a total of 10, 60-s scans. Participants were informed that all body odors within each scan would originate from the same identity category. Special care was taken to assert that body odors in the category Stranger had not been presented to the participant during the aforementioned behavioral testing to prevent familiarization with the odor. The cotton pads were positioned inside large-mouth glass bottles and presented during scanning by placing the bottle approximately 10 mm under the participant’s nose. Stimulus onset was 10 s prior to bolus onset and ended approximately 10 s after scanning termination. Stimuli were presented for 3 s with 5 s interstimulus intervals, yielding a total of 10 stimulus presentations for each condition. Scanning was performed with eyes open so that the participants could synchronize their breathing with the onset of the stimulus presentation. Participants were instructed to focus on a mark located inside the scanner directly above their heads, and to breathe normally and consistently throughout all scans regardless of the valence or intensity of the stimuli. To ensure that they focused on the presented stimuli, participants indicated with a mouse click after each presentation whether the stimulus was perceived as stronger or weaker than the previous one. To prevent sniffing behavior that might activate cortical areas associated with olfactory search (Zelano et al. 2005), participants were informed when a blank stimulus would be presented (odor-free Baseline) and were instructed to respond with a random click during each stimulus presentation in that condition. Breathing was monitored with breast and abdominal respiratory belts. No significant differences in breathing were observed between scans. Following each scan, subjects were asked to rate the stimulus for perceived pleasantness and intensity using a 10-point visual analog scale. A minimum of 10 min occurred between each scan to allow for the preparation of the isotope by the cyclotron and to prevent olfactory adaptation. Acquiring of Images and Data Analyses PET scans were obtained with a Siemens Exact HR+ tomograph operating in 3-dimensional acquisition mode using H2O15 water bolus. T1-weighted structural magnetic resonance imaging (MRI) scans (160, 1-mm slices) were obtained for each subject with a 1.5-T Siemens Sonata scanner to provide anatomical detail. PET images were reconstructed using a 14-mm Hanning filter and processed using normal methods (Zatorre et al. 1999). Statistical imaging analyses were done using the in-house program ‘‘DOT,’’ described in detail elsewhere (Worsley et al. 1992). The presence of significant changes in cerebral blood flow (CBF) was established on the basis of an exploratory search for which the t value criterion was set at >3.5. This value corresponds to an uncorrected P value of <0.0002 for a whole-brain search volume. Due to the conservative nature of conjunction analyses (Nichols et al. 2005), we established the presence of significant changes of CBF in the conjunction analysis based on a t value criterion of >3.0, corresponding to an uncorrected P value of <0.001 for a whole-brain search volume. Results Behavioral Results All participating women had a normal olfactory threshold, as indicated by the Sniffin’ Sticks normative values. The mean olfactory threshold was 11.52; higher numbers in this test (16 being the maximum value) indicate higher sensitivity. To investigate whether the subjects were able to correctly identify the individual body odors that would be used during scanning, identification performance and individual sensitivity were recorded prior to the scanning session. All participants who were selected to proceed to the scanning experiment were able to identify correctly both their friend’s (Friend), t(14) = 4.94, P < 0.01, and their own (Self), body odor, t(14) = 16.00, P < 0.01, above chance in a 3-alternative, no feedback forcedchoice task with 9 repetitions for each category using body odors from strangers as foils (Fig. 1A). Anecdotal evidence has indicated that conscious awareness of identification performance of individual body odor is low, which has been taken as indicative of nonconscious processing. To explore this issue, our participants were asked to assess their confidence in the accuracy of their identifications after each trial. To evaluate the agreement between subjects’ conscious judgment and their behavioral performance on the identification task, an Over or Underconfidence score (O/U) was calculated. Confidence is commonly measured as the relationship between the mean subjective probability of being correct, P, to that of the actual Cerebral Cortex June 2008, V 18 N 6 1467 Figure 1. Identification of individual body odors. (a) Average identification performance in each body odor category. The dotted line represents chance performance. The mean correct identification of the Self and Friend body odors were 8.33 (SD 1.29) and 6.80 (SD 2.98), respectively, out of a maximum score of 9. Error bars represent standard error of the mean (SEM). (b) Confidence judgments plotted against actual proportion of correct answers for the 2 body odor categories. The dotted line represents perfect calibration, indicating that subjective experience is well matched to actual performance. Values above the line indicate underconfidence in performance, whereas values under the line indicate overconfidence. Note the relationship between excellent identification performance and large underconfidence when subjects were identifying body odors from Self. proportion of correct discriminations, C (Bjorkman et al. 1993). This difference, P – C, is expressed as an O/U score. Responses are said to be calibrated to the extent that the proportion of correct choices matches the subjective probability. In this experiment, there was a dissociation between participants’ confidence in the 2 body odor categories. They were largely underconfident in their ability to discriminate the Self body odor correctly, O/U = –0.21, whereas they were well calibrated in their ability to identify the Friend body odor, O/U = –0.01 (Fig. 1B). There were no significant differences between the grouped body odors and the Odor control in either perceived pleasantness (4.8, SD ± 0.30 and 4.4, SD ± 0.43, respectively), t(14) = 0.73, P ns (nonsignificant), or intensity (5.0, SD ± 0.33 and 5.7, SD ± 0.45, respectively), t(14) = 1.48, P ns, indicating that the 2 odor categories were perceptually similar. This was further supported by the fact that only 5 participants were able to correctly label the Odor control as a nonbody odor during scanning. The body odors forming the category Strangers originated from the body odors that were presented as Friend or Self to other participants, that is, the Strangers odors were chemically identical to the Friend and Self odors. This unique design allowed us to infer whether subjective ratings of the individual body odor categories could be influenced by category origin of the odor alone rather than the chemical structure. Interestingly, there were significant differences in how the individual body odors were perceived. Repeatedmeasures analysis of variance with Bonferroni post hoc tests indicated that the body odor from the Stranger (intensity 5.9, SD ± 0.43; pleasantness 4.03, SD ± 0.34) was perceived as both more intense (all ts > 2.84, all Ps < 0.01) and less pleasant (all ts > 1.97, all Ps < 0.05) than the other 2 categories. There were, however, no significant differences in either perceived intensity or pleasantness between Self (intensity 4.6, SD ± 0.40; pleasantness 5.1, SD ± 0.39) and Friend (intensity 4.5, SD ± 0.44; pleasantness 5.3, SD ± 0.44) body odors. We did not statistically assess participants’ performance in identifying each type of body odor inside the scanner due to the small number of stimulus repetitions during scanning. 1468 Neuronal Processing of Body Odors d Lundström et al. Body Odor Processing To evaluate the neuronal substrates of body odor processing, we contrasted all body odor conditions (Stranger, Friend, and Self) with the Baseline condition (clean nursing pad). Increased regional CBF (rCBF) was observed in posterior cingulate cortex, posterior occipital gyrus, dorsal postcentral gyrus, and bilaterally in the angular gyrus (Supplementary Table 1a). Interestingly, although these stimuli had a clear olfactory percept, no activation above threshold was observed in regions commonly associated with olfactory processing, such as the piriform or the orbitofrontal cortex (Zatorre et al. 1992; Savic et al. 2000). In fact, Body odors deactivated (Baseline vs. Body odors) anterior parts of the orbitofrontal cortex (Fig. 2). However, when assessing rCBF activation of the perceptually similar Odor control compared with the Baseline condition, activations above threshold were observed in dorsal postcentral gyrus, the middle orbitofrontal cortex and a just below threshold activation (P = 0.002 by global contrast) in the piriform cortex (Supplementary Table 1b). The latter 2 regions are often labeled as secondary and primary olfactory cortex, respectively (Zatorre et al. 1992; Savic et al. 2000). A common activation in the 2 previous contrasts was the postcentral gyrus, an area that has been implicated in the neuronal processing of odors that activate the trigeminal nerve (Boyle et al. 2007). Because both odor categories were perceived as slightly irritating by the pilot group (Supplementary Material) who initially helped us choose a suitable concentration of the Odor control, one might postulate that Body odors and the Odor control both activated the somatosensory system by virtue of their pungent percept. To control for this and to investigate more thoroughly the processing differences between body odors and common odors with a similar percept, 2 additional computations were performed. First, we contrasted body odors against the odor control, revealing that body odors activated the posterior cingulate cortex, occipital gyrus, cuneus, angular gyrus, and the presupplementary motor area (pre-SMA) to a greater extent than did the odor mixture (Supplementary Table 1c). We then Table 1 Significant peaks of increased rCBF Area MNI coordinates x y z t value (A) Conjunction analyses Posterior cingulate cortex Occipital gyrus Angular gyrus Dorsal medial anterior cingulate cortexa Angular gyrusa 1 24 31 9 31 37 98 64 17 54 30 15 37 36 40 4.24 4.09 3.72 2.97 2.71 (B) Body odor from Stranger versus Body odor from friend Precuneus Ventral Insula Inferior frontal gyrus, orbital part Substantia innominata/amygdalaa 8 37 42 13 67 0 37 3 40 16 9 13 3.74 3.55 3.50 3.11 (C) Body odor from Friend versus Body odor from stranger Postcentral gyrus Precentral gyrus Occipital gyrus Paraoccipital transition zone/retrosplenial cortex pre-SMA 5 7 11 21 7 37 25 92 76 15 63 64 6 33 57 4.12 3.97 3.73 3.67 3.52 Note: Anatomical labels follow the nomenclature of the Mai atlas. Positive x values denote a right-sided activation, whereas negative values denote a left-sided activation. Denotes peak activations that just missed significance. a Figure 2. A statistical parametric map (t statistics as represented by the color scale) showing group averaged rCBF response showed to the processing of body odors (contrast Baseline vs. all Body odors) superimposed on group averaged anatomical MRI. Yellow circles mark decreased rCBF in bilateral anterior orbitofrontal cortex (x, y, z MNI coordinates: 27, 58, 11; t 5 4.1 and 25, 58, 13; t 5 3.9). Coordinates denote slice expressed according to the MNI world coordinates system. Left in figure represents left side (L). performed a conjunction analysis to explore regions that were commonly activated in the 2 body odor contrasts (Body odors vs. Baseline + Body odors vs. Odor control). Conjunction analyses yield activations that are consistently found in all individually included images within contrasts, whereas subtraction analyses rely on the average contrast only (Nichols et al. 2005); hence, a conjunction analysis is a more conservative measure of the variable of interest. Increased rCBF above threshold was observed in the posterior cingulate cortex extending into the retrosplenial cortex, occipital gyrus, and angular gyrus. In addition, activation just below threshold was present in the dorsal medial anterior cingulate cortex and the contralateral angular gyrus (Fig. 3 and Table 1). To control for the possibility that the activation in the occipital cortex was mediated by visualization following correct identification of the body odor, we examined specifically scans where the participants were exposed to the body odor from their friends and themselves. This analysis was performed under the assumption that subjects would not try to visualize an individual when smelling either a believed stranger or a believed common odor. The normalized rCBF values from the peak activation in the aforementioned occipital region were extracted, and scans in which subjects had correctly identified their friends and/or themselves were compared with scans in which the odors were incorrectly identified as strangers or nonbody odors. There was no significant difference in rCBF (Supplementary Fig. 1) when subjects correctly identified the body odor compared with when they did not, as assessed by a 2-tailed independent samples Student’s t-test, t(28) = 1.11, P ns. Further, to investigate the consistency of this activation, we explored how many participants demonstrated an increase at the occipital gyrus coordinate in the contrast Body odors versus Baseline using a 5 mm volume of interest (VOI) search sphere. The location of the activation in the occipital cortex was very consistent across individuals, and only 4 participants did not demonstrate a marked increase in rCBF when smelling body odors. These 4 individuals did, however, show a marked increase in rCBF at a location just anterior to the group averaged coordinate. Processing of Individual Body Odors We also explored whether neuronal processing differed according to the relationship categories of the individual body odors. Contrasting each body odor category against Baseline did not produce different results than the combined Body odor analyses presented above (Supplementary Table 2a--c). We then contrasted individual body odor categories with each other. Contrasting the condition of smelling an unknown body odor (Stranger) to that of smelling a known body odor (Friend) yielded significant rCBF increases in the precuneus, ventral insula, and the inferior frontal gyrus. Interestingly, there were also subthreshold activations in the substantia innominata/ amygdala (Supplementary Fig. 2, Table 1b). Reversing the contrast, that is, smelling Friend versus Stranger, yielded a different pattern. A significant increase in rCBF was detected in the postcentral gyrus and extended into the precentral gyrus, occipital gyrus, transverse occipital sulcus extending into the retrosplenial cortex, and the pre-SMA (Table 1c). These latter regions have previously been implicated in studies investigating imagery (Djordjevic et al. 2005). Thus, to explore whether this activation pattern was mediated by degree of familiarity, we regressed the length of friendship with the activation of smelling Friend versus Baseline using a voxel-wise whole-brain regression analyses. Duration of friendship was correlated only with increased rCBF in the right anterior occipitotemporal cortex (Fig. 4) in close proximity to the extrastriate body area (EBA) (Downing et al. 2001). The EBA has previously been implicated in processing information related to the human body (Downing et al. 2001). One might speculate that the longer the participant had known her friend, the more likely she would be to recognize the friend’s body odor, thus recruiting this area more. However, there was no Cerebral Cortex June 2008, V 18 N 6 1469 Figure 3. Statistical parametric maps (t statistics as represented by the color scale) of group averaged rCBF responses to processing of body odors (contrast all Body odors vs. Odor control) superimposed on group averaged anatomical MRI. Blue circles mark increased rCBF in the posterior cingulate cortex (PCC), green circles mark increased rCBF response in the left angular gyrus, and yellow circles mark an increased rCBF response in the right occipital cortex. Coordinates denote center of activation and slice expressed according to the MNI world coordinates system. Left in upper row of pictures represents posterior and middle figures represent left side (L). Graphs under each statistical parametric map represent extracted baseline-corrected rCBF values within the activation peak in question, using a 5 mm VOI search sphere, in each odor category. Error bars represent standard error of the mean (SEM). All statistical parametric maps are thresholded at t 5 2.5. Figure 4. (a) rCBF responses correlating with duration of friendship, assessed by the contrast Friend versus Baseline using voxel-wise whole-brain regression analyses (t statistics as represented by the color scale). Duration of friendship was correlated only with increased rCBF in the right anterior occipitotemporal cortex (x, y, z MNI coordinates: 40, 66, 3; t 5 3.3). (b) Individual rCBF values in relationship duration of friendship (months). Equation was obtained with a bivariate correlation analysis, and solid line represents regression line. rCBF values were extracted from the normalized raw images using a 5 mm VOI search sphere from the center of activation. Please note that cluster on regression line contains more than one value. correlation between length of friendship and ability to detect the friend’s body odor in the screening session, as assessed with a 2-tailed Pearson correlation analyses, r(15) = 0.28, P ns. In addition, when normalized rCBF values extracted from scans in which the subjects were able to correctly identify their friends were compared with scans in which they were not, no significant difference was found between the levels of rCBF, as shown by an independent samples Student’s t-test, t(28) = 0.35, P ns. Hence, the response was not dependent on conscious awareness of stimulus identity. Lastly, we assessed the neuronal processing of the odor of ‘‘Self’’ by contrasting the 1470 Neuronal Processing of Body Odors d Lundström et al. scans in which participants smelled Self body odor to those when smelling Friend—both known odors. Interestingly, no above-threshold activations were found in this contrast. Discussion Neuronal Correlates of Body Odor Processing It is known that visual stimuli with high behavioral relevance for the individual receive preferential processing. Here, in concordance with the emerging view that endogenous odors are of high ecological relevance, we have demonstrated for the very first time that body odors are processed differently than perceptually similar common odors. Body odors activated a network consisting of the posterior cingulate cortex, occipital gyrus, angular gyrus, and the anterior cingulate cortex, none of which is believed to be related to olfactory processing. However, together they form an interesting pattern. Posterior cingulate cortex is known to be active in response to emotional stimuli (Maddock 1999; Cato et al. 2004), whereas the anterior cingulate cortex is believed to regulate attentional efforts (Botvinick et al. 1999). It is thus possible that the processing of body odors is similar to what previously has been demonstrated for highly emotional stimuli, such as visual images of snakes (Fredrikson et al. 1995), where the posterior cingulate cortex works in concert with the anterior cingulate cortex to determine and process these emotional stimuli. Although we are not able to infer a causal direction between these 2 emotions, it is possible that by virtue of their emotional load (Chen and Haviland-Jones 1999), body odors receive not only differential treatment compared with common odors, but also heightened attention. Seen from an evolutionary perspective, stimuli signaling important information or related to recurrent survival threats might have been selected by evolutionary pressure to receive preferential processing in a direct access to emotional and attentional centers of the brain. The angular gyrus is strongly linked to the creation of a visual body construct. Disruption or enhancement of the neuronal signal in the angular gyrus is known to either abolish or alter the percept of a body (Blanke et al. 2002, 2004; Arzy et al. 2006). We are not able to make inferences to other sensory modalities based on this experiment alone; however, based on the strong dissociation in activation of the angular gyrus between the body odors and the perceptually similar common odors, especially in comparison with what has previously been demonstrated for visual stimuli (Blanke et al. 2002, 2004; Arzy et al. 2006), it is tempting to suggest that the angular gyrus serves as an important node in a modalityindependent network responsible for forming the basic percept of a human body in the present—the body construct. Interestingly, there were no above-baseline activations of areas traditionally viewed as olfactory regions (piriform cortex and orbitofrontal cortex) when participants smelled body odors. Although the orbitofrontal cortex was activated when smelling the Odor control, the perceptually similar Body odors strongly deactivated large areas of the anterior orbitofrontal cortex, an area reported to process common odors (Sobel et al. 2000; Poellinger et al. 2001; De Araujo et al. 2003; Gottfried and Dolan 2004; Sabri et al. 2005), without activating the middle orbitofrontal cortex. Similarly, no activation above baseline was seen in the piriform cortex, an area commonly viewed as the primary olfactory cortex, when smelling body odors, although this area was activated for the Odor control. It is not possible to elucidate whether this latter result may be due to the negative control (a clean cotton pad smelling weakly of cotton). As previously postulated by Boehm et al. (2005), our data seem to indicate that endogenous odors are not processed by the secondary olfactory cortex. This is indicative of an early separation of odor processing dependent on the odor’s signaling value, similar to what has been demonstrated for the visual system. It is important to note that in this study, we are only able to demonstrate the neuronal substrates of women smelling women’s body odors. Others have reported that women smelling the male endogenous compound androstadienone activated only the hypothalamus and not the primary or secondary olfactory areas (Savic et al. 2001). Although one might assume that the basic neuronal processing of body odors would not exhibit a vast sex difference, we are not inferring that the results reported here are generalizable to men’s processing of body odors. Whether endogenous odors are processed primarily by other, nonolfactory, cortical areas in humans, as indicated by the lack of activation, or whether there are sex differences in these processes remains to be determined. The Odor control condition activated a region associated with higher odor processing, the middle orbitofrontal cortex. A recent study demonstrated that changing the labeling of a binary mixture of common odors from ‘‘body odor’’ to ‘‘cheddar cheese’’ led to an increased activation due to the labeling, rather than the individual odorants, primarily in the middle orbitofrontal cortex (De Araujo et al. 2005). The authors suggested that this activation was formed by the judgment of perceived pleasantness, a conclusion that is supported by previous studies (Royet et al. 1999). To determine whether the activation in this region was mediated by a hedonic perception in the present study, we regressed pleasantness ratings against the rCBF values in the global contrast Odor control versus Baseline. There was, however, no above threshold correlation between pleasantness ratings and rCBF values in the orbitofrontal cortex. An alternate explanation to this activation might be mixture processing. A recent study from our lab indicates that an area of the orbitofrontal cortex in close proximity to what we report here is specifically involved in the processing of binary odor mixtures (Boyle et al. 2006). This region was activated while subjects smelled an odor mixture, but not while they smelled a single odorant, indicating a mixture identification mechanism. Because the Odor control in that study was a mixture consisting of 3 individual odors, one might speculate that parts of the orbitofrontal cortex process mixtures per se, rather than responding uniquely to binary mixtures. Although this remains to be elucidated, the lack of correlation between pleasantness and rCBF values lends support to the notion that the activation in this area occurred as a response to the mixture that was used as an odor control. The occipital cortex, an area commonly associated with visual processing, was activated in all body odor contrasts. Subjects were scanned with their eyes open in all conditions, and there were no visual differences in how the stimuli were presented. In other words, the visual input for each trial should have been essentially identical, which is supported by the consistency of the finding across participants. The activation of the occipital cortex is a common finding in olfactory neuroimaging studies (Small et al. 1997; Royet et al. 1999, 2001; Qureshy et al. 2000; Zatorre et al. 2000; Cerf-Ducastel and Murphy 2001, 2006; Gottfried et al. 2004; Djordjevic et al. 2005; Plailly et al. 2005, 2006). This indicates that the activation of the occipital cortex cannot be attributed to the processing of body odors per se (or an attempt to visualize the stimuli, as demonstrated in this experiment); rather, the activity probably represents some additional processes elicited by the odorous stimuli. A review of the aforementioned articles reporting visual activation demonstrates no obvious common variable other than the use of olfactory stimuli. Determining whether Cerebral Cortex June 2008, V 18 N 6 1471 the recruitment of cortical areas commonly associated with visual processing is mediated by the odor processing itself or is dependent on higher cognitive processes should be the focus of future studies. Neuronal Correlates of Smelling a Stranger Smelling a stranger’s body odor evoked cortical activations similar to what previously has been reported when viewing masked fearful faces (Whalen et al. 1998). Inferior frontal gyrus and the amygdala have both been linked in several studies to the processing of negative emotional stimuli (Morris et al. 1998; Whalen et al. 1998; Yamasaki et al. 2002). The percept of a novel body odor that has not been encountered before thus seems to activate similar emotional processes that have been previously demonstrated for visual stimuli. This finding supports the view that amygdalar responses to emotional stimuli are not modality specific (Calder et al. 2001). Further, this lends additional support to the view that endogenous odors, which have high social and ecological importance, are processed differently than perceptually comparable common odors. That body odors from strangers seem to evoke more negative emotions than those from friends was further supported by the perceptual ratings. When functioning as a Stranger, body odors were consistently rated as more intense and less pleasant than when functioning as a Friend, that is, a body odor was rated differently depending on its relationship to the individual doing the rating. This is a particularly nice feature of the design and indicates that the neuronal responses reported above are not due to the chemical composition per se; rather, the difference in ratings is most probably mediated by the implicit threat produced by the presence of a stranger in the immediate vicinity. Moreover, this difference in perceptual ratings might explain the activation of the precuneus and the insular regions. Although the insula has been implicated in recognition of fearful stimuli (Morris et al. 1999), a recent study demonstrated a dissociation between these 2 regions. In the study by Morris et al., the amygdala responded selectively to fearful visual stimuli, whereas the insula and the precuneus responded selectively to stimuli evoking disgust (see also Phillips et al. 2004). More importantly, the insula has been reported to respond to disgusting, but not pleasant, odors (Wicker et al. 2003). In light of this, we suggest that the act of smelling a stranger’s body odor activates the separate, yet interlinked, feelings of both fear and disgust, or possibly here, loathing (Calder et al. 2001). This is a sensation that most of us have experienced for sudden visual or auditory stimuli. When exposed to a sudden and novel sound or sight, we experience not only the increase in arousal induced by fear, but also the familiar negative feeling in the gut, a sensation possibly evoked by the extensive anatomical connections between the amygdala and the insula (Amaral et al. 1992). Neuronal Correlates of Familiar Body Odors Smelling a friend activated a network consisting of areas surrounding the central sulcus, occipital cortex including posterior parts of the retrosplenial cortex, and the pre-SMA. These areas are not analogous to those commonly reported when viewing familiar visual stimuli. However, Shah et al. (2001) recently reported that the posterior retrosplenial cortex was uniquely involved in coding modality-independent 1472 Neuronal Processing of Body Odors d Lundström et al. processing of familiar voices and faces. An activation just below significance was also observed in the premotor cortex. In light of this, we suggest that the activations observed when participants were stimulated with a long-time friend’s body odor represent a network coding for person familiarity akin to the network previously suggested by Shah et al. (2001). Interestingly, duration of friendship was significantly correlated with increased rCBF in the EBA, an area that has been implicated in the processing of information related to the human body (Downing et al. 2001). To the best of our knowledge, this is the first nonvisual study demonstrating that the percept of a body activates this area. This indicates that the EBA is responding to body-related stimuli in a modality-independent manner. Participants were able to identify correctly the body odors of themselves and their friends outside the scanner. However, the independence between rCBF values and the ability to identify the body odor, the subjects’ large underconfidence in their ability to identify their own body odor, and the independence between identification of friend’s body odor and duration of friendship supports the notion that part of this process is nonconsciously mediated. It is important to clarify that we are not of the opinion that body odor identification is entirely driven by nonconscious processes, nor do we wish to suggest that explicit learning does not play a role. The correspondence between the length of exposure to a body odor, defined by length of the relationship, and the degree of rCBF activation in the EBA clearly indicates that learning does play a role. However, the lack of correspondence between correct identification and rCBF activation in the EBA supports the notion that this is mediated, on some level, by nonconscious processes. Interestingly, the contrast Self versus Friend did not produce any activations above threshold. The lack of activation for Self in contrast to another individual’s body odor might originate from the underlying mechanism of body odor recognition. It has been demonstrated that rodents use self-referent phenotype matching, the so-called armpit effect, when identifying individual body odors (Mateo and Johnston 2000). In other words, the animal uses its own body odor as a template to assess the identity of an unknown individual. Vogt et al. (2006) recently proposed that the posterior and anterior cingulate cortices are functionally connected. The main responsibility of the posterior cingulate cortex seems to be the processing of emotional stimuli via interaction with the anterior cingulate cortex using ‘‘self’’ as reference frame. If this mechanism of body odor identification is also at play in humans, it would explain both the lack of activation in the aforementioned contrast and would put the angular gyrus activation into an interesting context. As previously stated, disruption of activity in the angular gyrus is associated with a distorted selfreferential space. Patients with an epileptic focus in the angular gyrus often report out-of-body experiences or that their percept of other people’s bodies is distorted (Blanke et al. 2002, 2004). The demonstration by Pause et al. (1999) that body odors originating from oneself receive preferential treatment lends further supports to the notion of self-referent phenotype matching. Future studies are needed to determine whether humans identify body odors using self-referent phenotype matching akin to the mechanism demonstrated in other animals. In conclusion, we demonstrate that body odors are processed differently from perceptually similar common odors by activating regions commonly associated with emotions and with the creation of a basic percept of a human body in the present. In addition to providing the neuronal substrates of processing body odors, a class of olfactory stimuli of high occurrence and ecological interest, this finding is important for several reasons. First, we demonstrate that body odors are indeed processed differently than perceptually similar common odors. These 2 classes of odors are treated differently by the olfactory system. We demonstrate for the first time that body odors are not primarily processed by what are commonly regarded as olfactory cortices, indicating a separation in the processing of odor signals. Second, it seems that the processing of body odors is mostly nonconscious in nature, with limited involvement of explicit learning. This lends support to the emerging view that identification of body odors is an ongoing automatic process that uses self-referent matching with little conscious involvement. Third, we demonstrate for the first time that the amygdala responds to the body odors of a stranger, akin to what has previously been demonstrated for visually masked faces, and that this activation is dependent on the identity of the odor donor rather than on the odor’s chemical composition. This might indicate that the amygdala responds to fearful stimuli in a modality-independent manner. Finally, the present results provide evidence that olfactory stimuli of high ecological relevance are processed by specialized neuronal networks similar to what has previously been demonstrated for auditory and visual stimuli. Supplementary Material Supplementary material oxfordjournals.org/. can be found at: http://www.cercor. Funding Canadian Institutes of Health Research grant (MOP 57846) to M.J.-G. and R.J.Z.; and a Swedish Research Council postdoctoral research grant (VR 2005-960) to J.N.L. Notes We wish to thank the personnel at the McConnell Brain Imaging Center for all their valuable help. Conflict of Interest: None declared. Address correspondence to Johan N. Lundström PhD, Monell Chemical Senses Center, 3500 Market Street, Philadelphia, PA 191043308, USA. E-mail: [email protected] References Amaral DG, Price JL, Pitkanen A, Carmichael ST. 1992. Anatomical organization of the primate amygdaloid complex. In: Aggleton JP, editor. The amygdala: neurobiological aspects of emotion, memory and mental dysfunction. New York: Wiley-Liss. p. 1--66. Arzy S, Seeck M, Ortigue S, Spinelli L, Blanke O. 2006. Induction of an illusory shadow person. Nature. 443:287. Bjorkman M, Juslin P, Winman A. 1993. Realism of confidence in sensory discrimination: the underconfidence phenomenon. Percept Psychophys. 54:75--81. Blanke O, Landis T, Spinelli L, Seeck M. 2004. Out-of-body experience and autoscopy of neurological origin. Brain. 127:243--258. Blanke O, Ortigue S, Landis T, Seeck M. 2002. Stimulating illusory ownbody perceptions. Nature. 419:269--270. Boehm U, Zou Z, Buck LB. 2005. Feedback loops link odor and pheromone signaling with reproduction. Cell. 123:683--695. Botvinick M, Nystrom LE, Fissell K, Carter CS, Cohen JD. 1999. Conflict monitoring versus selection-for-action in anterior cingulate cortex. Nature. 402:179--181. Boyle JA, Heinke M, Gerber J, Frasnelli J, Hummel T. 2007. Cerebral activation to intranasal chemosensory trigeminal stimulation. Chem Senses. 32:343--353. Boyle JA, Olsson MJ, Djordjevic J, Lundstrom JN, Jones-Gotman M. 2006. Contribution of the lateral orbitofrontal cortex to processing of binary odor mixtures. Chem Senses. 31:A29. Broca P. 1888. Mémoires d’Anthropologie. Paris: Reinwald. Calder AJ, Lawrence AD, Young AW. 2001. Neuropsychology of fear and loathing. Nat Rev Neurosci. 2:352--363. Cato MA, Crosson B, Gokcay D, Soltysik D, Wierenga C, Gopinath K, Himes N, Belanger H, Bauer RM, Fischler IS, et al. 2004. Processing words with emotional connotation: an FMRI study of time course and laterality in rostral frontal and retrosplenial cortices. J Cogn Neurosci. 16:167--177. Cerf-Ducastel B, Murphy C. 2001. fMRI activation in response to odorants orally delivered in aqueous solutions. Chem Senses. 26:625--637. Cerf-Ducastel B, Murphy C. 2006. Neural substrates of cross-modal olfactory recognition memory: an fMRI study. NeuroImage. 31:386--396. Chen D, Haviland-Jones J. 1999. Rapid mood change and human odors. Physiol Behav. 68:241--250. De Araujo IE, Rolls ET, Kringelbach ML, McGlone F, Phillips N. 2003. Taste-olfactory convergence, and the representation of the pleasantness of flavour, in the human brain. Eur J Neurosci. 18:2059--2068. De Araujo IE, Rolls ET, Velazco MI, Margot C, Cayeux I. 2005. Cognitive modulation of olfactory processing. Neuron. 46:671--679. Djordjevic J, Zatorre RJ, Petrides M, Boyle JA, Jones-Gotman M. 2005. Functional neuroimaging of odor imagery. NeuroImage. 24:791--801. Downing PE, Jiang Y, Shuman M, Kanwisher N. 2001. A cortical area selective for visual processing of the human body. Science. 293:2470--2473. Fredrikson M, Wik G, Fischer H, Andersson J. 1995. Affective and attentive neural networks in humans: a PET study of Pavlovian conditioning. NeuroReport. 7:97--101. Gottfried JA, Dolan RJ. 2004. Human orbitofrontal cortex mediates extinction learning while accessing conditioned representations of value. Nat Neurosci. 7:1144--1152. Gottfried JA, Smith AP, Rugg MD, Dolan RJ. 2004. Remembrance of odors past: human olfactory cortex in cross-modal recognition memory. Neuron. 42:687--695. Hummel T, Konnerth CG, Rosenheim K, Kobal G. 2001. Screening of olfactory function with a four-minute odor identification test: reliability, normative data, and investigations in patients with olfactory loss. Ann Otol Rhinol Laryngol. 110:976--981. Hummel T, Sekinger B, Wolf SR, Pauli E, Kobal G. 1997. ‘Sniffin’ sticks’: olfactory performance assessed by the combined testing of odor identification, odor discrimination and olfactory threshold. Chem Senses. 22:39--52. Jacob S, McClintock MK, Zelano B, Ober C. 2002. Paternally inherited HLA alleles are associated with women’s choice of male odor. Nat Genet. 30:175--179. Laska M, Fendt M, Wieser A, Endres T, Hernandez-Salazar LT, Apfelbach R. 2005. Detecting danger—or just another odorant? Olfactory sensitivity for the fox odor component 2,4,5-trimethylthiazoline in four species of mammals. Physiol Behav. 84:211--215. Laska M, Wieser A, Salazar LT. 2006. Sex-specific differences in olfactory sensitivity for putative human pheromones in nonhuman primates. J Comp Psychol. 120:106--112. Maddock RJ. 1999. The retrosplenial cortex and emotion: new insights from functional neuroimaging of the human brain. Trends Neurosci. 22:310--316. Martins Y, Preti G, Crabtree CR, Runyan T, Vainius AA, Wysocki CJ. 2005. Preference for human body odors is influenced by gender and sexual orientation. Psychol Sci. 16:694--701. Cerebral Cortex June 2008, V 18 N 6 1473 Mateo JM, Johnston RE. 2000. Kin recognition and the ‘armpit effect’: evidence of self-referent phenotype matching. Proc R Soc Lond B Biol Sci. 267:695--700. Morris JS, Ohman A, Dolan RJ. 1998. Conscious and unconscious emotional learning in the human amygdala. Nature. 393:467--470. Morris JS, Scott SK, Dolan RJ. 1999. Saying it with feeling: neural responses to emotional vocalizations. Neuropsychologia. 37:1155--1163. Nichols T, Brett M, Andersson J, Wager T, Poline J-B. 2005. Valid conjunction inference with the minimum statistic. NeuroImage. 25:653--660. Ohman A, Flykt A, Esteves F. 2001. Emotion drives attention: detecting the snake in the grass. J Exp Psychol Gen. 130:466--478. Pause BM, Krauel K, Schrader C, Sojka B, Westphal E, MullerRuchholtz W, Ferstl R. 2006. The human brain is a detector of chemosensorily transmitted HLA-class I-similarity in same- and opposite-sex relations. Proc Biol Sci. 273:471--478. Pause BM, Krauel K, Sojka B, Ferstl R. 1999. Body odor evoked potentials: a new method to study the chemosensory perception of self and non-self in humans. Genetica. 104:285--294. Phillips ML, Williams LM, Heining M, Herba CM, Russell T, Andrew C, Bullmore ET, Brammer MJ, Williams SC, Morgan M, et al. 2004. Differential neural responses to overt and covert presentations of facial expressions of fear and disgust. NeuroImage. 21:1484--1496. Plailly J, Bensafi M, Pachot-Clouard M, Delon-Martin C, Kareken DA, Rouby C, Segebarth C, Royet JP. 2005. Involvement of right piriform cortex in olfactory familiarity judgments. NeuroImage. 24:1032--1041. Plailly J, d’Amato T, Saoud M, Royet JP. 2006. Left temporo-limbic and orbital dysfunction in schizophrenia during odor familiarity and hedonicity judgments. NeuroImage. 29:302--313. Poellinger A, Thomas R, Lio P, Lee A, Makris N, Rosen BR, Kwong KK. 2001. Activation and habituation in olfaction—an fMRI study. NeuroImage. 13:547--560. Qureshy A, Kawashima R, Imran MB, Sugiura M, Goto R, Okada K, Inoue K, Itoh M, Schormann T, Zilles K, et al. 2000. Functional mapping of human brain in olfactory processing: a PET study. J Neurophysiol. 84:1656--1666. Royet JP, Hudry J, Zald DH, Godinot D, Gregoire MC, Lavenne F, Costes N, Holley A. 2001. Functional neuroanatomy of different olfactory judgments. NeuroImage. 13:506--519. Royet JP, Koenig O, Gregoire MC, Cinotti L, Lavenne F, Le Bars D, Costes N, Vigouroux M, Farget V, Sicard G, et al. 1999. Functional anatomy of perceptual and semantic processing for odors. J Cogn Neurosci. 11:94--109. Sabri M, Radnovich AJ, Li TQ, Kareken DA. 2005. Neural correlates of olfactory change detection. NeuroImage. 25:969--974. Savic I, Berglund H, Gulyas B, Roland P. 2001. Smelling of odorous sex hormone-like compounds causes sex-differentiated hypothalamic activations in humans. Neuron. 31:661--668. Savic I, Gulyas B, Larsson M, Roland P. 2000. Olfactory functions are mediated by parallel and hierarchical processing. Neuron. 26:735--745. 1474 Neuronal Processing of Body Odors d Lundström et al. Seifritz E, Esposito F, Neuhoff JG, Luthi A, Mustovic H, Dammann G, von Bardeleben U, Radue EW, Cirillo S, Tedeschi G, et al. 2003. Differential sex-independent amygdala response to infant crying and laughing in parents versus nonparents. Biol Psychiatry. 54: 1367--1375. Shah NJ, Marshall JC, Zafiris O, Schwab A, Zilles K, Markowitsch HJ, Fink GR. 2001. The neural correlates of person familiarity. A functional magnetic resonance imaging study with clinical implications. Brain. 124:804--815. Small DM, Jones-Gotman M, Zatorre RJ, Petrides M, Evans AC. 1997. Flavor processing: more than the sum of its parts. NeuroReport. 8:3913--3917. Sobel N, Prabhakaran V, Zhao Z, Desmond JE, Glover GH, Sullivan EV, Gabrieli JD. 2000. Time course of odorant-induced activation in the human primary olfactory cortex. J Neurophysiol. 83:537--551. Vogt BA, Vogt L, Laureys S. 2006. Cytology and functionally correlated circuits of human posterior cingulate areas. NeuroImage. 29: 452--466. Wallace P. 1977. Individual discrimination of humans by odor. Physiol Behav. 19:577--579. Weisfeld GE, Czilli T, Phillips KA, Gall JA, Lichtman CM. 2003. Possible olfaction-based mechanisms in human kin recognition and inbreeding avoidance. J Exp Child Psychol. 85:279--295. Whalen PJ, Rauch SL, Etcoff NL, McInerney SC, Lee MB, Jenike MA. 1998. Masked presentations of emotional facial expressions modulate amygdala activity without explicit knowledge. J Neurosci. 18:411--418. Wicker B, Keysers C, Plailly J, Royet JP, Gallese V, Rizzolatti G. 2003. Both of us disgusted in My insula: the common neural basis of seeing and feeling disgust. Neuron. 40:655--664. Worsley KJ, Evans AC, Marrett S, Neelin P. 1992. A three-dimensional statistical analysis for CBF activation studies in human brain. J Cereb Blood Flow Metab. 12:900--918. Yamasaki H, LaBar KS, McCarthy G. 2002. Dissociable prefrontal brain systems for attention and emotion. Proc Natl Acad Sci USA. 99:11447--11451. Zatorre RJ, Jones-Gotman M, Evans AC, Meyer E. 1992. Functional localization and lateralization of human olfactory cortex. Nature. 360:339--340. Zatorre RJ, Jones-Gotman M, Rouby C. 2000. Neural mechanisms involved in odor pleasantness and intensity judgments. NeuroReport. 11:2711--2716. Zatorre RJ, Mondor TA, Evans AC. 1999. Auditory attention to space and frequency activates similar cerebral systems. NeuroImage. 10: 544--554. Zelano C, Bensafi M, Porter J, Mainland J, Johnson B, Bremner E, Telles C, Khan R, Sobel N. 2005. Attentional modulation in human primary olfactory cortex. Nat Neurosci. 8:114--120. Zwaardemaker H. 1895. Die Physiologie des Geruchs. Leipzig: Germany W. Engelmann.