* Your assessment is very important for improving the work of artificial intelligence, which forms the content of this project
Download The Calcium-Binding Activity of a Vacuole
Plant virus wikipedia , lookup
Biochemistry wikipedia , lookup
Point mutation wikipedia , lookup
Ultrasensitivity wikipedia , lookup
Biochemical cascade wikipedia , lookup
Gene expression wikipedia , lookup
Clinical neurochemistry wikipedia , lookup
Lipid signaling wikipedia , lookup
Ancestral sequence reconstruction wikipedia , lookup
Paracrine signalling wikipedia , lookup
Magnesium transporter wikipedia , lookup
Expression vector wikipedia , lookup
G protein–coupled receptor wikipedia , lookup
Bimolecular fluorescence complementation wikipedia , lookup
Interactome wikipedia , lookup
Protein structure prediction wikipedia , lookup
Signal transduction wikipedia , lookup
Phosphorylation wikipedia , lookup
Metalloprotein wikipedia , lookup
Nuclear magnetic resonance spectroscopy of proteins wikipedia , lookup
Protein–protein interaction wikipedia , lookup
Two-hybrid screening wikipedia , lookup
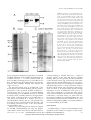
The Calcium-Binding Activity of a Vacuole-Associated, Dehydrin-Like Protein Is Regulated by Phosphorylation1 Bruce J. Heyen2, Muath K. Alsheikh, Elizabeth A. Smith, Carl F. Torvik, Darren F. Seals3, and Stephen K. Randall* Department of Biology, Indiana University-Purdue University at Indianapolis, 723 West Michigan Street, Indianapolis, Indiana 46202–5132 A vacuole membrane-associated calcium-binding protein with an apparent mass of 45 kD was purified from celery (Apium graveolens). This protein, VCaB45, is enriched in highly vacuolate tissues and is located within the lumen of vacuoles. Antigenically related proteins are present in many dicotyledonous plants. VCaB45 contains significant amino acid identity with the dehydrin family signature motif, is antigenically related to dehydrins, and has a variety of biochemical properties similar to dehydrins. VCaB45 migrates anomalously in sodium dodecyl sulfate-polyacrylamide gel electrophoresis having an apparent molecular mass of 45 kD. The true mass as determined by matrix-assisted laser-desorption ionization time of flight was 16.45 kD. VCaB45 has two characteristic dissociation constants for calcium of 0.22 ⫾ 0.142 mm and 0.64 ⫾ 0.08 mm, and has an estimated 24.7 ⫾ 11.7 calcium-binding sites per protein. The calcium-binding properties of VCaB45 are modulated by phosphorylation; the phosphorylated protein binds up to 100-fold more calcium than the dephosphorylated protein. VCaB45 is an “in vitro” substrate of casein kinase II (a ubiquitous eukaryotic kinase), the phosphorylation resulting in a partial activation of calcium-binding activity. The vacuole localization, calcium binding, and phosphorylation of VCaB45 suggest potential functions. The vacuole is a reservoir for calcium (Machlon, 1984) and consequently plays an important role in calcium homeostasis (Miller et al., 1990; Allen and Sanders, 1995; Sanders et al., 1999). Regulation of vacuole calcium levels is complex involving a variety of calcium channels and pumps (Sanders et al., 1999; Sze et al., 2000). Sustained elevated levels of cytosolic calcium can be toxic (Hepler and Wayne, 1985), so under normal conditions, cytosolic calcium levels increase only transiently. Proteinaceous calcium buffers may serve as homeostats to attenuate the signal transduction system. Well-characterized protein calcium buffers include calreticulin and calsequestrin (Ostwald and MacLennon, 1974; Campbell et al., 1983b). Homologs of calsequestrin (Krause et al., 1989; Xing et al., 1994), calreticulin (Chen et al., 1994; Napier et al., 1995; Nelson et al., 1997), and calnexin (Li et al., 1998) have been identified in plants. These calcium-binding proteins can bind on the order of 20 to 50 calcium ions with both high- (1–3 sites per protein) and low- (20–50 sites per protein) affinity 1 This work was supported in part by Purdue University (Research Fellowship) and by the U.S. Department of AgricultureNational Research Initiative Competitive Grants Program (grant no. 99 –35100 –7668 to S.K.R.). 2 Present address: Departments of Biology and Chemistry, Tabor College, 400 South Jefferson, Hillsboro, KS 67063. 3 Present address: Van Andel Institute, 333 Bostwick Avenue NE, Grand Rapids, MI 49503. * Corresponding author; e-mail [email protected]; fax 317–274 – 2846. Article, publication date, and citation information can be found at www.plantphysiol.org/cgi/doi/10.1104/pp.002550. sites. The levels of calcium binding proteins may have a significant impact on signaling processes and may regulate second messenger transmission (Camacho and Lechleiter, 1995; Mery et al., 1996; Coppolino et al., 1997). In an alternative role, calcium-dependent interactions of calnexin and calreticulin have been characterized with a variety of proteins (Nigam et al., 1994; Peterson et al., 1995) and both are implicated in the promotion of correct protein folding (Hebert et al., 1996). These latter activities clearly suggest a molecular chaperone role. Recently, a high-capacity, low-affinity calcium-binding protein was localized to the radish (Raphanus sativus) root vacuole. This protein did not show sequence resemblance to EF handcontaining calcium-binding proteins, calsequestrin, or dehydrins (Yuasa and Maeshima, 2000). Most members of the dehydrin superfamily of related genes are expressed during periods of low water content or during exposure to environmental stresses where osmotic stress is a component of the stress mechanism (Skriver and Mundy, 1990; Close et al., 1993b; Close, 1997). Included in the dehydrin superfamily are several late embryogenesis-abundant mRNA-encoded proteins (LEAs), defined by the presence of the K domain (the prototypical sequence being EKKGIMDKIKEKLPG). Many dehydrins additionally contain an S domain (a tract of six–seven Sers) upstream of the amino-terminal-most K domain. Dehydrins have potential phosphorylation sites and can be phosphorylated (Vilardel et al., 1990; Plana et al., 1991; C.F. Torvik and S.K. Randall, unpublished data). Despite the progress in understanding the gene regulation affecting expression of the dehydrin proteins, the Downloaded from on www.plantphysiol.org June 15, 2017 - Published by www.plantphysiol.org Plant Physiology, October 2002, Vol. 130, pp. 675–687, © 2002 American Society of Plant Biologists Copyright © 2002 American Society of Plant Biologists. All rights reserved. 675 Heyen et al. biochemical function of these proteins and their role in cryoprotection have remained speculative. Calcium ligand blots, a valuable technique for the identification of novel calcium-binding proteins (Campbell et al., 1983a, 1983b; Maruyama and Nonomura, 1984), specifically identifies a variety of calcium-binding proteins including the EF handcontaining calmodulin (Chen et al., 1994), the 14-3-3 calcium-binding putative transcription factor (Lu et al., 1994), and the calcium buffers calreticulin (Chen et al., 1994) and calsequestrin (Franceschi et al., 1993). Calcium ligand blots revealed only a limited number of calcium-binding proteins (four–five) associated with plant membranes (Randall, 1992). In this report, we characterize one of these proteins, the vacuolar calcium-binding protein known as VCaB45. VCaB45 is likely a member of the dehydrin family of proteins. These findings suggest that calcium binding could be a general property of dehydrins and suggest potential functions of dehydrins as calcium buffers or perhaps as calcium-dependent chaperones. We further demonstrate that the calcium-binding activity of VCaB45 is dependent upon its phosphorylation status. RESULTS Purification of VCaB45 The presence of a 45-kD calcium-binding protein in vacuole-enriched membrane fractions was noted previously (Randall, 1992). This report describes the purification, identification, and characterization of this protein. The 45-kD protein was purified from a vacuole-enriched membrane fraction, first by permeabilizing membrane vesicles with 0.2% (w/w) Triton X-100, resulting in a fraction highly enriched in vacuole lumenal proteins (see methods). These proteins were further fractionated by anion-exchange chromatography (Randall, 1992). Peak fractions were resolved on two-dimensional gels. Figure 1 shows a typical two-dimensional gel separation of the 0.2% (w/w) Triton X-100 extract of vacuole membranes; analyzed by protein and by 45Ca ligand blots. At all purification stages, VCaB45 was identified by the 45 Ca ligand-blotting method. The final purified protein had an apparent molecular mass of 45 kD and an observed pI of 5.2 ⫾ 0.2 (average of five independent determinations). Although VCaB45 represents a significant portion of the 0.2% (w/w) Triton X-100soluble vacuole proteins, it is likely not a major vacuole protein constituent (Randall, 1992; based upon the purification, see discussion in Fig. 2A). Antibody was raised to the purified protein in mice by injection of spots of protein excised from blots of several two-dimensional gels. The enrichment of VCaB45 through the various purification stages is illustrated by using this antibody (Fig. 2A). A semiquantitative estimate of purification based upon the western blot indicated a 400- to 500-fold purification 676 Figure 1. Two-dimensional analysis of 0.2% (w/w) Triton X-100extracted vacuole membranes. After the Triton X-100 treatment, the soluble phase was separated first by isoelectric focusing and then by SDS-PAGE (O’ Farrell, 1975). The pH gradient was established in the first dimension with 0.5% (w/v) ampholytes (3.5–5 pH range), 0.75% (w/v) ampholytes (4–6 pH range), and 0.75% ampholytes (5–7 pH range). After electrophoresis and transfer to nitrocellulose, the blot was first probed with 45calcium (B, as described) and later stained with Ponceau S to detect protein (A). The arrows in A indicate the protein spots that correspond to the calcium-binding activity detected in B. The white circles in B indicate calcium-binding activity. The pH values were obtained by slicing a parallel first dimension gel, incubating the slices in deionized water, and then measuring the pH with an pH meter. The average value of VCaB45 was deduced to be 5.2 ⫾ 0.2 (average of five determinations). Molecular mass standards are indicated on the left. (from initial homogenate to anion-exchange peak fraction). The enrichment of VCaB45 in total membranes compared with total cellular extract (approximately 10-fold) is indicative of a predominantly membrane/ organelle localization for VCaB45. Though this purifi- Downloaded from on June 15, 2017 - Published by www.plantphysiol.org Copyright © 2002 American Society of Plant Biologists. All rights reserved. Plant Physiol. Vol. 130, 2002 Vacuolar Calcium-Binding Protein, VCaB45 tion (Figs. 2A and 3A); the lower band was never seen in tissues extracted in hot SDS-PAGE buffer (Fig. 2, B and C). Characterization of VCaB45. Localization and Distribution of VCaB45 Figure 2. A, Enrichment of VCaB45 during purification. Equal amounts of each fraction (2 g protein) were fractionated by SDSPAGE. A western blot was probed with a 1:2,000 (v/v) dilution of antibody raised against purified VCaB45. Lane 1, Whole celery (Apium graveolens) homogenate (VCaB45 is not detectable at this exposure); lane 2, microsomal membranes; lane 3, vacuolar membrane fraction; lane 4, Triton X-100 extract of vacuole membrane fraction (soluble vacuolar proteins); lane 5, peak fraction from DEAESepharose column. B, Various plant tissues contain proteins of similar molecular mass that are immunologically related to VCaB45. Celery vacuolar membranes (0.05 g, lane 1) and tissue homogenates of pea (Pisum sativum; lane 2), soybean (Glycine max; lane 3), and maize (Zea mays; lane 4) seedlings (9 g each), were probed with anti-VCaB45. C, VCaB45 accumulates to high levels in cortical tissues of celery petioles. Cortical (C) and vascular (V) tissues were isolated separately and homogenized directly into 2⫻ SDS-PAGE buffer. Gels were loaded with equivalent amounts of protein (10 g) and blots were probed with anti-VCaB45. Molecular mass standards (in kD) are indicated on the right. cation procedure was used for eliciting antisera, the large-scale purification methods used in all subsequent experiments described in this paper are outlined later. VCaB45 was susceptible to proteolytic degradaPlant Physiol. Vol. 130, 2002 Immunoreactive proteins were found in all dicotyledonous species tested (celery, pea, soybean, beets [Beta vulgaris], potato [Solanum tuberosum], carrot [Daucus carota], tobacco [Nicotiana tabacum], and Arabidopsis), but were markedly absent in the two monocotyledonous species (maize and oats [Avena sativa]) tested (Fig. 2B). To determine whether VCaB45 protein levels might be correlated with the presence of vacuoles, we exploited the characteristic of celery petioles that allows, with relative ease, the physical separation of vascular tissues from cortical tissue. The parenchyma cells in the cortical tissues tend to be extensively vacuolated, whereas the cells in the vascular tissues are much less so. VCaB45 was observed to be at low levels in vascular tissues but was highly abundant in the cortical tissues, consistent with a vacuole location and function (Fig. 2C). VCaB45 appears to be present predominantly in the low-density fractions (Fig. 3, 0%/4% [w/w] dextran interface) enriched in vacuole membranes (Randall, 1992). Although significant amounts of VCaB45 also appear in higher density gradient fractions (4%/7% [w/w] dextran), it is not clear whether this is due to the presence of vacuole membranes in this fraction or whether VCaB45 is also localized in the endoplasmic reticulum (which is enriched in the 4%/7% [w/w] fraction). The low-density location is consistent with observations of the calcium-binding activity (observed at 45 kD) made previously (Randall, 1992). To further examine the subcellular localization of VCaB45, we took an additional approach. We had previously developed methods to evacuolate tobacco protoplasts from suspension-cultured tobacco BY2 cells and to isolate intact vacuoles (Seals and Randall, 1997). Using these methods, vacuole markers in evacuolated protoplasts were reduced 10- to 12-fold and in isolated vacuoles were enriched approximately 3- to 4-fold (Seals and Randall, 1997). Consistent with a vacuole location, a tobacco protein (Tb45), immunologically related to the celery VCaB45, is largely depleted in evacuolated protoplasts and, conversely, is enriched in isolated vacuoles from tobacco cells (Fig. 3B). However, based on all these data, we cannot conclude that VCaB45 localization is restricted to the vacuole. VCaB45 could be quantitatively removed from the low-density membranes by treatment with 0.2% (w/w) Triton X-100 (Fig. 3A), a concentration insufficient to solubilize integral membrane proteins (Randall and Sze, 1986). Treatment of membranes with 0.5 m KI, a chaotropic agent often used to dissociate peripheral proteins from membranes (Lai et al., Downloaded from on June 15, 2017 - Published by www.plantphysiol.org Copyright © 2002 American Society of Plant Biologists. All rights reserved. 677 Heyen et al. Figure 3. A, VCaB45 is enriched in low-density fractions and is released by 0.2% (w/w) Triton X-100. Membranes obtained from dextran step gradients (0%/4% [w/v] dextran interface, 4%/7% [w/v] dextran interface, etc., see Randall, 1992) were permeabilized with 0.2% (w/w) Triton X-100 (for 30 min at 4 C) and then centrifuged at 214,200g for 40 min. Equivalent portions of untreated membranes (M), the Triton X-100-solubilized supernatants (S), and membrane pellets (P) were separated by SDS-PAGE. The western blot was probed with anti-VCaB45. B, VCaB45-related protein (designated Tb45) is depleted in evacuolated tobacco protoplasts and is enriched in isolated vacuoles. Protoplasts (P) were produced from tobacco BY2 cells and vacuoles were selectively removed by ultracentrifugation resulting in evacuolated protoplasts (EV). In a separate preparation, vacuoles (V) were isolated and purified from protoplasts (P). Identical quantities of protein were resolved by SDS-PAGE. Tb45 indicates a tobacco protein immunologically related to celery VCaB45. Blots were probed with anti-VCaB45. Molecular mass standards (in kD) are indicated on the right. 1988), did not release VCaB45 (data not shown). These data are consistent with either a lumenal location or a very weak hydrophobic association of VCaB45 with the membrane. To better understand the potential functions of VCaB45, we determined whether this protein was disposed on the cytosolic side or the lumenal side of the vacuole membrane. We first needed to determine that the low amount of Triton X-100 could release lumenally localized proteins. Treatment of membranes with 0.2% (w/w) Triton X-100 released 8.5-fold more of the lumenal enzyme acid phosphatase and 6.4-fold more of the VCaB45 protein than membranes not treated with detergent (Fig. 4A). The small amount of activity solubilized in the untreated membranes is likely due to either membrane damage during the initial membrane preparation or the subsequent centrifugation steps. Overall, the data are consistent with Triton X-100-permeabilized membranes releasing lumenally localized proteins. To further define the location of VCaB45, freshly isolated membranes were treated with proteinase K (Fig. 4B). If VCaB45 was located inside the membrane vesicle, then one would expect no protein degradation unless the membranes were disrupted by detergent. In the detergent-treated membranes, VCaB45 was readily proteolysed to a smaller size by proteinase K (at higher concentrations of protease VCaB45 was completely degraded; data not shown). The purified VCaB45 was also susceptible to proteinase K digestion (data not shown). However, in the absence of detergent, the insensitivity of VCaB45 to the protease suggests the membrane is 678 protecting it and, thus, argues strongly for a lumenal location of VCaB45. Sequence and General Properties of VCaB45 After a cyanogen bromide cleavage and subsequent sequencing by in-line HPLC/mass spectrometry, we obtained a mixed sequence, (M) EKEDEKLPGGVKTVE (major species) and (M) KKIKHDXSKVGAKTF (minor species). These sequences were searched utilizing the program FASTF (searching the database National Center for Biotechnology Information/BLAST NR). The FASTF algorithm takes various combinations of the two mixed sequences and searches the database finding the best match. The top match obtained was found within a carrot sequence (carrot dehydrin, accession no. AB010898) MEKIKEKLPGGGKKVE, a perfect match (Table I). Note that this sequence contains a close match to the canonical “K” domain, the dehydrin signature motif (EKKGIMDKIKEKLPG; Close et al., 1993a, 1993b; Close, 1997). The carrot dehydrin does not contain any perfect “K” domain (Tan et al., 2000). The remaining amino acids matched a second sequence in the same protein (MKKEEKDETKVIATEF, 10 amino acids identical, four similar, one not similar, and one amino acid unidentified in the experimentally obtained sequence). Overall, these two matches represent 26 identical amino acids of a total of 32 amino acids (81% identity) and 30 similar amino acids of 32 total (94% similarity). In addition, the top 10 sequences returned from the database search were dehydrins from various organisms. Downloaded from on June 15, 2017 - Published by www.plantphysiol.org Copyright © 2002 American Society of Plant Biologists. All rights reserved. Plant Physiol. Vol. 130, 2002 Vacuolar Calcium-Binding Protein, VCaB45 Figure 4. Localization of VCaB45 in vacuole membranes. A, Supernatants of vacuole membranes treated with or without 0.2% (w/w) Triton X-100, were obtained after centrifugation at 100,000g for 30 min. Supernatants were separated by SDS-PAGE, blotted, and probed with anti-VCaB45. Note that to visualize VCaB45 in untreated membrane supernatants it was necessary to load twice the proportion of that loaded for Triton-treated supernatants. The numerical data for VCaB45 represent densitometric analysis (arbitrary units) of the western blot, factoring the different gel loads. Acid phosphatase activity of the supernatants of membranes treated either with or without Triton X-100 were 17 and 2 A405 min⫺1 l extract⫺1, respectively. B, Vacuole membranes were treated with or without 0.2% (w/w) Triton X-100 and simultaneously with or without 2 mg mL⫺1 proteinase K. After a 30-min incubation at 4°C, a portion of the entire sample was separated by SDS-PAGE, blotted, and probed with anti-VCaB45. Molecular mass standards (mass in kD) are shown at the left. Further, in the recent screening of an Arabidopsis expression library with the antibody raised against celery VCaB45 (S.K. Randall, unpublished data), we have obtained only cDNA clones that encode a dehydrin protein (i.e. ERD14). Based on these data and together with reactivity to the anti-K domain serum (see below), we have concluded that celery VCaB45 is a dehydrin-like protein. One prediction of VCaB45 properties based upon its hypothetical identity as a dehydrin-like protein is that of solubility after heat treatment. A number of the dehydrin family members remain soluble after a 90°C heat treatment (Lin et al., 1990; Close et al., 1993b). VCaB45 remained soluble after heat treatment (Fig. 5A). We took advantage of the solubility after heat treatment of VCaB45 to develop an alternative (rapid, with little proteolytic breakdown) purification procedure for VCaB45. This procedure involved isolation of vacuole-enriched membranes, extraction with 0.2% (w/w) Triton X-100, heat treatPlant Physiol. Vol. 130, 2002 ment of the extract, recovery of the soluble phase after heat treatment, and, finally, anion-exchange chromatography. A substantial enrichment of VCaB45 was achieved through this heat treatment procedure (Fig. 5, A and B). In addition, the calcium-binding activity (Fig. 5C) was conserved during this procedure and was consistent with the enrichment of the immunoreactive polypeptide. The overall increase in purification efficiency facilitated the processing of the approximately 8 kg of petiole material (per experiment) required for the calcium-binding studies discussed below. We do not have the full-length sequence for VCaB45 and because several dehydrins behave anomalously in SDS-PAGE, we determined the mass of purified VCaB45 by matrix-assisted laser-desorption ionization time of flight (MALDI-TOF). The major molecular ion was found to be 16.449 kD, whereas a minor species (13% of the major) was 19.043 kD. No larger molecular species were observed. We conclude it likely that the true mass of VCaB45 is approximately 16.5 kD. This overestimate of mass by SDS-PAGE is consistent with that of other dehydrins (Gilmour et al., 1992; Welin et al., 1995; Svensson et al., 2000). The confirmation of VCaB45’s identity as a dehydrin-like protein suggested protein levels might be regulated by environmental factors. In seedlings, VCaB45 levels are increased by cold stress, the phytohormone abscisic acid (ABA), and by drought stress (Fig. 6). However, mature celery plants did not regulate levels of VCaB45 by cold stress (Fig. 6). The regulation by environmental stress in seedlings is consistent with VCaB45’s proposed identity as a dehydrin-like protein. Phosphorylation of VCaB45 and Calcium-Binding Properties The dehydrin Rab17 can be phosphorylated in the “S” domain (Vilardel et al., 1990; Plana et al., 1991; Jensen et al., 1998) and most dehydrins have potential CKII phosphorylation sites (in the “S” domain). Because unpublished data from our lab suggested other dehydrins were also phosphorylated, we decided to test whether VCaB45 was phosphorylated and, if so, whether the phosphorylation state of the enzyme might influence calcium binding. Treatment of celery VCaB45 with shrimp alkaline phosphatase (SAP) resulted in a small but discernible shift in apparent molecular mass (of approximately 4–6 kD) on SDS-PAGE gels, whereas denatured SAP had no effect (Fig. 7A). Such shifts are often used to assess the phosphorylation status of proteins (Kitta et al., 2001; Rivedal and Opsahl, 2001). The action of alkaline phosphatase on VCaB45 could be prevented by 200 m vanadate, an inhibitor of phosphatase activity (Fig. 7B, I), indicating the gel shift requires an enzymatically active phosphatase. These results suggest that the purified VCaB45 is phosphorylated. A logical Downloaded from on June 15, 2017 - Published by www.plantphysiol.org Copyright © 2002 American Society of Plant Biologists. All rights reserved. 679 Heyen et al. Table I. A mixed amino acid sequence obtained from a cyanogen bromide digest and subsequent sequencing by in-line HPLC/mass spectrometry of VCaB45 is aligned with the carrot dehydrin sequence (accession no. AB010898) The Mets were inferred (cyanogen bromide cleaves peptides after Mets). When these sequences were searched on GenBank (plant sequences, nonredundant, utilizing the program FASTF) the greatest similarity was found with the carrot sequence, which encodes a dehydrin protein. Out of the mixed sequence was obtained a perfect match (VCaB45 Seq 2, from amino acids 142–157) and a second match (VCaB45 Seq 1, from amino acids 29 – 44), using the remaining amino acids, with 62% identity and 87% similarity. The alignment between the carrot dehydrin and the two celery VCaB45 sequences are highlighted in black (identical amino acids) and in gray (similar amino acids). extension of this conclusion is that this protein is normally phosphorylated in planta. Even more exciting is the observation that treatment of VCaB45 with alkaline phosphatase greatly reduces the ability of this protein to bind calcium when measured by the calcium ligand blot method (Fig. 7B, II). The presence of potential casein kinase II phosphorylation sites on other known dehydrins suggested to us that VCaB45 might also be phosphorylated by CKII. It appears that VCaB45 is an “in vitro” substrate for CKII and that the phosphorylation of previously dephosphorylated VCaB45 shifts the protein back to a higher apparent molecular mass, and restores, at least in part, the calcium-binding activity (Fig. 7C). To confirm that the polypeptide that shifted after treatment with alkaline phosphatase was the same dehydrin-like protein, an antibody specific to dehydrins was utilized (Fig. 7D). The dehydrin antibody detected a similar shift as that observed with the VCaB45 antibody (Fig. 7D). The specificity of the antibody interaction was confirmed by the reaction against the known Arabidopsis dehydrin, ERD14, and competition with the K peptide. The antibody detection of both ERD14 and VCaB45 was prevented by the blocking K peptide (Fig. 7D). It is also significant that the antibody raised against VCaB45 recognized ERD14, consistent with the identity of VCaB45 as a dehydrin-like protein. The reactivity of the antibody raised against the K peptide (the signature sequence of dehydrins) confirmed that the shifting phenomena was due to a change in the phosphorylation status of the dehydrin-like protein, VCaB45, which resulted in altered calcium binding (Fig. 7, B and C). Thus, the calcium-binding activity of VCaB45 is influenced by its phosphorylation status. 680 The initial characterization of VCaB45 was based on the premise that calcium binding measured by the ligand-blot assay reflects a “true” functional activity. This method has been used in the past to identify a variety of calcium-binding proteins (Ostwald and MacLennon, 1974; Campbell et al., 1983a, 1983b). We have also compared calcium-binding activity (on ligand blots) with a vacuolar annexin protein, VCaB42 (Seals and Randall, 1994, 1997). Although the vacuolar annexin binds calcium in the native state, with an apparent dissociation constant (Kd) of 60 nm (Seals and Randall, 1994), it does not bind calcium by the calcium ligand-blot assay (Fig. 8). This is consistent with the known properties of the endonexin fold, the calcium-binding domain found in the family of annexin proteins. This analysis also demonstrates that VCaB45 binds calcium with some degree of specificity (also note the paucity of calcium-binding proteins in crude preparations; Fig. 5). Although the blotbased ligand-binding assay has been very convenient for the initial identification of calcium-binding proteins and for rapid analysis during purification, it does not give information about the calcium-binding activity of the native protein. To quantitatively analyze the calcium-binding parameters of native VCaB45, we have conducted equilibrium dialysis calcium-binding assays. Equilibrium dialysis experiments indicated apparent saturable binding of calcium to native VCaB45 (Fig. 9A). Higher calcium concentrations than those shown in Figure 9 encouraged precipitation of VCaB45. Scatchard plots (Fig. 9D) indicated the presence of two distinct binding sites of different affinity. Average values for the Kds of these sites were 0.22 ⫾ 0.142 and 0.64 ⫾ 0.08 mm (average of nine experi- Downloaded from on June 15, 2017 - Published by www.plantphysiol.org Copyright © 2002 American Society of Plant Biologists. All rights reserved. Plant Physiol. Vol. 130, 2002 Vacuolar Calcium-Binding Protein, VCaB45 Figure 5. Comparison of calcium-binding activity and total protein obtained after heat treatment. A, Celery VCaB45 remains soluble after heat treatment. The Triton X-100 extract (Total) was heat treated (20 min at 80°C–90°C) and then chilled to 4°C (10 min). A supernatant and a pellet were obtained after centrifugation of the extract at 100,000g for 30 min. Equal portions of all fractions were separated by SDS-PAGE and blots probed with anti-VCaB45. Greater than 90% of total protein precipitated after the heat treatment (not shown). B, Lane 1 contained 22.5 g of protein; the other lanes contained a corresponding portion equivalent to the membrane volume containing 22.5 g of protein. B, Onemillimeter-thick gel stained with Coomassie Brilliant Blue. C, The same samples (but twice the amounts of protein were loaded compared with the gel in B) separated on a 2-mm-thick SDS-PAGE gel and 45calcium ligand-blotted. Lanes 1, 2, and 3 are equivalent fractions in both B and C. Lane 1, 0%/4% (w/v) dextran membranes; lane 2, Triton-extracted supernatant; lane 3, heat-treated supernatant. Though run on different gels, the major Coomassie-staining band in B, lane 3 corresponds to the calciumbinding band (arrow indicates VCaB45) in C, lane 3 (by protein staining of the polyvinylidene difluoride [PVDF] blot after the calcium blot; data not shown). Molecular mass standards (B, stainable; C, prestained; Bio-Rad Laboratories, Hercules, CA) are indicated on the left. Arrowhead indicates VCaB45. ments using three different preparations of purified VCaB45). Estimates of the number of binding sites on VCaB45 were 24.7 ⫾ 11.7 mol calcium bound per mole VCaB45 (assuming the mass of VCaB45 is 16.5 kD). A Hill plot (Fig. 9B) indicated little cooperativity between these multiple binding sites because the slope was near 1. The phosphorylation state of VCaB45 had a dramatic effect on the calcium-binding activity. Dephosphorylation of the purified VCaB45 resulted in a large decrease (Fig. 9, A and C) in calcium binding (corresponding to a 30–100-fold decrease in calcium bound per mole VCaB45). Similar to the ligand blotting results, rephosphorylation of VCaB45 with casein kinase II resulted in a modest recovery of calcium-binding activity (Fig. 9C). The phosphorylation state seems to similarly affect both the high- and low-affinity (measured at 0.1 or 0.8 mm calcium) calcium-binding sites. Various ions were tested for the ability to compete for VCaB45 calcium-binding sites (Fig. 10). Magnesium and the monovalent cations had little effect on Plant Physiol. Vol. 130, 2002 calcium binding to VCaB45. However, a variety of divalent cations (at 0.2 mm) did decrease calcium binding. Zinc gave the greatest amount of inhibition (of the divalent cations; Fig. 10 and inset). Zinc interaction with the calcium-binding site was not surprising because zinc seems to have affinity for a number of calcium binding sites in a variety of proteins. Of those divalent cations tested, only manganese and magnesium are likely to be physiological ligands (though manganese is toxic at millimolar levels; Foy et al., 1978). The concentration at which the other cations were tested is unlikely to be achieved in living plants. Lanthanum (a trivalent cation), an often-used nonphysiological inhibitor for calcium-binding sites and in particular calcium channels (Friedman et al., 1998; Dennison and Spalding, 2000), was strongly inhibitory to calcium binding by VCaB45. DISCUSSION VCaB45 is likely a vacuole-localized member of the dehydrin protein family. In support of that conclu- Downloaded from on June 15, 2017 - Published by www.plantphysiol.org Copyright © 2002 American Society of Plant Biologists. All rights reserved. 681 Heyen et al. Figure 6. Expression of VCaB45 protein in celery during low temperature (LT), ABA, and drought (DR) treatments. Older celery plants were treated at 6°C for 1 week and younger seedlings at 5°C for 2 d, 100 M ABA (in 0.01% [v/v] Tween 20, 0.26% [v/v] methanol, sprayed on leaves 2 successive d) for 2 d, and drought stress (not watered for 11 d). Age indicated is the time after planting (germination is approximately 2 weeks after planting). The minus signs indicate the respective controls for each of the treatments. Total proteins were extracted as described in “Materials and Methods.” A, Blots were probed with antiVCaB45. Left, Older plants; performed in a different experiment and the blots were developed separately from those shown in the younger plants. Both of these experiments were performed at least twice with similar results. B, Coomassiestained gel indicating total protein from the plants treated in A. The major band present is Rubisco large subunit, mass of approximately 53 kD. sion is an array of evidence substantiating the similarities between VCaB45 and dehydrins: (a) VCaB45 contains a region that is very similar to the dehydrin “signature” motif, the “K” domain, and another region very similar to a K-rich sequence in a carrot dehydrin (Table I); (b) VCaB45 is specifically recognized by an antibody raised to the K domain (Fig. 6D), and, conversely, an antibody raised against VCaB45 recognized a known dehydrin; (c) When an Arabidopsis expression library was screened with the monospecific antibody for VCaB45, only dehydrin-encoding cDNAs were obtained (ERD14; S.K. Randall, unpublished data); (d) VCaB45 is soluble after heating to denaturing conditions (90°C for 20 min), a characteristic of the dehydrin family; (e) VCaB45 shows an anomalous migration on SDSPAGE, a characteristic of some dehydrins; and (f) The accumulation of VCaB45 in response to the various environmental stresses is also consistent with the pattern of dehydrin expression. Both data from density gradients (in celery) and the isolation of intact vacuoles (from tobacco) suggest that VCaB45 is at least partially vacuole localized. The proteolysis protection experiments and the 0.2% (w/w) Triton X-100 extractability are consistent with a lumenal location of this protein. Although dehydrins were initially discovered over a decade ago, the physiological function of dehydrins has remained enigmatic. It has been speculated that dehydrins might bind ions (particularly phosphate or sulfate ions; Dure, 1993), or stabilize (chaperone) cytoplasmic proteins against denaturation, or stabilize supermolecular structures or membranes (Close et al., 1993a, 1993b; Kaye and Guy, 1995; Close, 1997). We now have evidence that the celery dehydrin-like 682 protein binds calcium. An important question is whether ion binding is a general property of dehydrins. This hypothesis appears to be consistent with the purification of dehydrins on immobilized metal columns (Svensson et al., 2000). Although most members of the dehydrin superfamily of related genes are transcriptionally activated during periods of osmotic stress (Skriver and Mundy, 1990; Close, 1993b, 1997), several dehydrins appear to be constitutively expressed (Welin et al., 1994; Nylander et al., 2001). It is interesting that mature celery plants appear to constitutively express VCaB45, whereas in young seedlings, levels of VCaB45 are responsive to environmental stress (Fig. 7). Calcium Binding as a Property of VCaB45 The VCaB45 protein has a high capacity for calcium (binding an average of 25 mol calcium mol protein⫺1) and has at least two distinct types of binding sites with Kds of approximately 0.22 and 0.64 mm. Calcium buffer proteins like calreticulin, calsequestrin, and calnexin use extensive arrays of acidic amino acidrich regions (at the carboxyl terminus) to bind a large number of calcium ions per protein molecule. Calreticulin has a single high-affinity (1.6 M) calciumbinding site in the P domain (Pro rich) and highcapacity/low-affinity (0.3–2 mm) binding sites at the C terminus (Michalak et al., 1992). The latter site is characterized by 37/55 residues that are acidic. Consistent with the acidic nature of calcium-binding sites in calreticulin, VCaB45 is a rather acidic protein, having a pI of 5.2. Although manganese and zinc are likely candidates to bind VCaB45 based on in vitro Downloaded from on June 15, 2017 - Published by www.plantphysiol.org Copyright © 2002 American Society of Plant Biologists. All rights reserved. Plant Physiol. Vol. 130, 2002 Vacuolar Calcium-Binding Protein, VCaB45 Figure 7. Treatment of purified celery VCaB45 with SAP results in a shift in apparent molecular mass and a decrease in calcium-binding activity. A, VCaB45, purified by heat treatment followed by anion-exchange chromatography, was treated for 60 min with SAP. Controls (0 time) were obtained by adding SAP and immediately adding SDS-PAGE sample buffer and boiling. After SDS-PAGE, blots were probed with anti-VCaB45. B, VCaB45 was treated for 60 min as in A, either without SAP, with SAP, or with SAP plus 200 M sodium (ortho) vanadate. Reactions were terminated by the addition of hot SDS-PAGE sample buffer and heating at 90°C for 5 min. After SDS-PAGE, gels were stained with Coomassie Brilliant Blue (I) or used for calcium ligand blots (II). C, VCaB45 was dephosphorylated with SAP as in A and B, then was repurified by anion-exchange chromatography, and incubated for 3 h at 30°C in the presence or absence of casein kinase II (CKII). Samples were analyzed by Coomassie staining of gels (I) or by calcium ligand blots (II). D, Gel shifts monitored by anti-VCaB45 and antidehydrin (DHN). The first two lanes of each panel contained VCaB45, whereas the third contained the purified Arabidopsis dehydrin ERD14. VCaB45 was treated with SAP as in B. Competition of the anti-DHN was with 2.5 mg mL⫺1 K peptide (TGEKKGIMDKIKEKLPGQH). ERD14 (Arabidopsis ecotype Columbia) was expressed in Escherichia coli and purified by heat treatment followed by anion-exchange chromatography. assays (Fig. 10), both of these are micronutrients and are unlikely to reach millimolar concentrations in plant cells. Although magnesium can be found in cells at millimolar levels, it does not appear to compete as well for calcium binding (Fig. 10). Thus, the predominant physiologically relevant ligand for VCaB45 appears to be calcium. For these reasons, we suggest that the physiological role of VCaB45 may involve calcium binding. Because VCaB45 is a dehydrin-like protein, it is of great interest to determine whether other proteins of the dehydrin family also bind calcium and whether calcium binding is a distinct property related to the function of dehydrins. We are presently testing that hypothesis. Plant Physiol. Vol. 130, 2002 Regulation of Calcium Binding by Phosphorylation Because calcium-binding properties of VCaB45 are dependent on its phosphorylated state, we were interested in whether the phosphorylation of VCaB45 was regulated by stress conditions. We addressed this question indirectly by examining the apparent mass of VCaB45 under cold stress conditions or when it is constitutively expressed (and additionally exposed to cold stress). We could visualize the phosphorylation status by a shift in apparent mass (Fig. 7). By this method, under all conditions we have tested, VCaB45 is always present in vivo in the phosphorylated state (Fig. 6). We see no evidence of a Downloaded from on June 15, 2017 - Published by www.plantphysiol.org Copyright © 2002 American Society of Plant Biologists. All rights reserved. 683 Heyen et al. MATERIALS AND METHODS Plant Material and Vacuole Isolation Figure 8. VCaB45 binds calcium in the ligand blot but the vacuole annexin, VCaB42, does not. VCaB42 was obtained as previously described (Seals and Randall, 1994) and loaded in the first lane of each gel. VCaB45 was eluted from a nitrocellulose spot cut from a two-dimensional gel. The nitrocellulose was boiled in SDS-PAGE buffer and loaded directly into the well with the sample buffer (the second lane of each gel). Left, Coomassie-stained SDS-PAGE. Right, Calcium ligand blot. Position of molecular mass standards (in kD) are indicated. cold-induced alteration of dephosphorylation or phosphorylation. However, it is important to note that subtle changes in phosphorylation would be difficult to detect using this assay. At this time, it is not clear whether the phosphorylation of VCaB45 is a reversible phosphorylation or whether it is a constitutive phosphorylation. The issue of where VCaB45 is phosphorylated, and by what kinase, are intriguing questions yet to be answered. Multiple perfect casein kinase II phosphorylation sites {[ST]-x(2)[DE]} can be found in some dehydrins (four of eight possible sites within or adjacent to the S domain in ERD14, for example). The presence of these sites in other dehydrins was our rationale for testing the affect of CKII phosphorylation on calcium-binding activity in VCaB45. Although CKII (a ubiquitous kinase) could phosphorylate VCaB45 “in vitro” and activate the protein to bind calcium, the reactivation was incomplete (Fig. 9C). The reason for this incomplete activation is unclear. Regardless, the identity of the activating phosphorylation target site may help to illuminate the identity of the responsible kinase. Based upon our findings that VCaB45 can bind calcium (in preliminary experiments, calcium binding has also been observed in other known dehydrins (S.K. Randall, unpublished data), we propose two alternative hypotheses for the function of VCaB45 specifically, and possibly dehydrins in general. First, a protective function of these proteins may be conferred by their high capacity for calcium binding. Thus, they may help alleviate the elevated intracellular calcium concentrations caused by leakage across membranes that occurs during environmental stress. Alternatively, they may play a protein chaperone role that is calcium dependent, similar to the function of calnexin and calreticulin (Nigam et al., 1994; Peterson et al., 1995; Hebert et al., 1996). 684 For most experiments, celery (Apium graveolens) was obtained from a local grocery; for seedling experiments (Fig. 7), celery cv Tall Utah was used. The procedure for vacuolar membrane isolation was as described in Randall (1992) with slight modifications. Homogenization buffer composed of 250 mm d-mannitol, 3 mm EGTA, 50 mm HEPES, and 1 mm dithiothreitol (DTT), pH 7.4. To reduce protease activities, a cocktail comprising 1 mm phenylmethylsulfonyl fluoride (0.05% [w/v] dimethylsulfoxide, final concentration), 1 mm benzamidine, 10 g mL⫺1 aprotinin, 1 g mL⫺1 leupeptin, and 10 m pepstatin, was present during homogenization. Celery petioles were homogenized in a blender (Waring, Winsted, CT) for 15 s at top speed with a ratio of 2.5 mL of homogenization buffer per gram fresh weight petioles. The supernatant obtained from two low-speed centrifugations (10,000g for 10 min) was centrifuged for 35 min at 58,000g. The membranes obtained in the pellet were resuspended in 2.5 mm HEPES (pH 7.2), 250 mm d-mannitol, and 1 mm DTT (resuspension buffer), and separated on dextran gradients. The 0%/4% (w/w) dextran interface is highly enriched in vacuole membranes, the 4%/7% and 7%/12% (w/w) dextran interfaces are enriched in the endoplasmic reticulum, and the membranes pelleted thorough the 12% (w/v) dextran cushion are enriched in chloroplasts and mitochondria (Randall, 1992). Dextran gradient fractions were diluted and recentrifuged. The pellets were resuspended in resuspension buffer and stored at ⫺80°C. For purposes of producing homogenates with minimal proteolysis, tissues were homogenized directly into hot 2⫻ SDS-PAGE sample buffer, at 1 mL g fresh weight⫺1 plant material. The homogenates were centrifuged for 10 min at 10,000g. Supernatants obtained were boiled for 5 min, and then stored at ⫺80°C. In this case, estimates of protein were made using the Amido Black assay (Kaplan and Pedersen, 1985). Triton X-100 Permeabilization of the Vacuole Membranes The highly enriched vacuolar membrane fraction (see Randall, 1992) was permeabilized with a low concentration of Triton X-100 detergent to release the contents of the vacuole membrane vesicles. The vacuolar membrane fraction was treated with 0.2% (w/w) Triton X-100 and 1 mm DTT for 30 min at 4°C, with gentle rotation. Permeabilization was conducted in the presence of the proteinase inhibitor cocktail described above. On some occasions, the Triton X-100-treated membranes were sonicated for 4 min and then centrifuged for 30 min at 214,200g at maximal radius (type 90 Ti rotor). Later, it became clear that sonication was unnecessary. The resulting supernatant, containing soluble lumenal vacuolar proteins, was saved for further purification steps and stored at ⫺80°C. For the localization experiments, fresh vacuolar membranes isolated from the 0%/5% (w/w) dextran gradient were treated (or not) with 0.2% (w/w) Triton X-100 and then centrifuged at 135,000g (at maximal radius, in a TL Ultracentrifuge, Beckman Instruments, Fullerton, CA) for 1 h at 4°C. The supernatants were recovered and diluted 100-fold for the acid phosphatase assay or analyzed by SDS-PAGE. In some cases, membranes were treated with proteinase K in the presence or absence of 0.2% (w/w) Triton X-100. The digestion was stopped by the addition of phenylmethylsulfonyl fluoride (5 mm final concentration) and then the samples were adjusted to 1⫻ SDS-PAGE sample buffer and boiled. Fractionation of Triton X-100 Supernatant (Lumenal Vacuolar Contents) by Anion-Exchange Chromatography The Triton X-100 supernatant was diluted 10-fold with 20 mm Tris-HCl buffer, pH 8.2 (at 4 C), and loaded at 0.2 mL min⫺1 onto a 50-mL packed bed volume of DEAE-Sepharose (Amersham-Pharmacia Biotech, Uppsala) anion-exchange column. The proteins were eluted with a linear 0 to 500 mm NaCl gradient generated by a Waters 650E Advanced Protein Purification System (Millipore, Bedford, MA). All fractions were assayed for calciumbinding activity with the 45Ca⫹2 ligand-blot assay described previously (Randall, 1992). Downloaded from on June 15, 2017 - Published by www.plantphysiol.org Copyright © 2002 American Society of Plant Biologists. All rights reserved. Plant Physiol. Vol. 130, 2002 Vacuolar Calcium-Binding Protein, VCaB45 Figure 9. Calcium binding to purified VCaB45 estimated by equilibrium dialysis. A, Calcium binding was estimated by equilibrium dialysis. Calcium binding to purified VCaB45 (white squares) is the average of three independent experiments from a single preparation of purified VCaB45. The SAP-treated calcium-binding data (white circles) were obtained from two experiments using the same preparation. Error bars represent SDs; where not shown, the SDs were smaller than the symbol indicating the data point. B, Hill plot (log % VCaB45 bound/100% ⫺ % VCaB45 bound, plotted against log [Ca])k derived from the data in A. Line drawn through the 50% bound region had a slope of 0.961, indicating little cooperativity in calcium binding. C, VCaB45 was untreated (gray bar), treated with SAP to dephosphorylate the protein (black bars), or was dephosphorylated followed by rephosphorylation with casein kinase II (hatched bar). Calcium binding was performed at concentrations estimated to measure high-affinity sites (100 M) or low-affinity sites (800 M). D, Scatchard plot (nY/X versus nY, where nY is mol calcium bound per mol VCaB45 and X is free calcium) derived from the data shown in A. Kds derived from the shown Scatchard plot were 0.41 and 0.75 mM calcium, while the maximum number of binding sites was 27 (assuming mass of VCaB45 is 16.5 kD). Data from three different preparations of purified VCaB45 (nine experiments) indicated average Kds of 0.22 ⫾ 0.142, 0.64 ⫾ 0.08, and the maximal number of binding sites was 24.7 ⫾ 11.7. Molecular Mass Estimates Mass estimates were performed on a Voyager-DE Pro MALDI-TOF (PerSeptive/Applied Biosystems, Foster City, CA) operated in a positive ion mode with sinapinnic acid as the matrix. Immunization Schedule and Western Blotting The calcium-binding protein (VCaB45) purified by anion-exchange chromatography and subsequently on large-scale two-dimensional gels (O’Farrell, 1975) was then transferred to nitrocellulose. Spots amounting to Plant Physiol. Vol. 130, 2002 5 g of protein were excised and dissolved in 100 L of dimethyl sulfoxide. The solubilized nitrocellulose/protein mixture was mixed well with 100 L of Freund’s adjuvant. Injections of approximately 1 g of protein were in Balb/C mice, intraperitoneally. Subsequent boosts were carried out every 3 to 4 weeks. Blood was drawn 10 to 14 d after each boost and was incubated at 4°C overnight. Serum samples were collected after centrifugation of the incubated blood for 5 min at 3,000g. Antidehydrin (DHN) antiserum and dehydrin K peptideTGEKKGIMDKIKEKLPGQH were kindly supplied by Dr. Timothy J. Close (Close et al., 1993a). For western blotting, samples were separated by SDS-PAGE (10% [w/w] acrylamide), transferred to either nitrocellulose or PVDF membranes and blocked with 5% (w/v) nonfat dry Downloaded from on June 15, 2017 - Published by www.plantphysiol.org Copyright © 2002 American Society of Plant Biologists. All rights reserved. 685 Heyen et al. for 5 min at room temperature. The reaction was initiated by the addition of 30 L of 10 mm p-nitrophenyl phosphate. The reaction was stopped (after a 30-min incubation, previously determined to be within the linear range) by the addition of 300 L of 1.0 m Na2CO3. The absorbance was read at 405 nm. Data was expressed as change in A min⫺1 l extract ⫺1. Alkaline Phosphatase and Kinase Treatments Typically, purified VCaB45 (approximately 0.6 g) was treated for up to 60 min with 2.4 units of SAP (Roche Diagnostics, Indianapolis) in a total volume of 18 L. The incubation buffer was composed of 0.05 m Tris-Cl and 5 mm magnesium chloride, pH 8.0, at 20°C and approximately 50 mm NaCl contributed by the purified VCaB45. Proteinase inhibitors (final concentrations: 10 mm benzamidine, 5 g mL⫺1 aprotinin, and 50 g mL⫺1 leupeptin) were also present. Denaturation of SAP was accomplished by heating for 15 min at 65°C. When VCaB45 was to be subsequently rephosphorylated by CKII, the dephosphorylation mixture (scaled up to 75 L) was first mixed with anion-exchange beads (approximately 10 L of packed volume of DEAE-Sepharose) for 2 h at 4°C. Under these conditions, SAP bound to the beads, whereas the majority of VCaB45 did not. The unbound fraction was then heat treated (as above) to denature any residual SAP and cooled on ice. To 22 L of the heat-treated mixture, 3 L of 10⫻ kinase buffer (supplied by the manufacturer), proteinase inhibitors (as above), and 250 units of casein kinase II (CKII, New England Biolabs, Beverly, MA) were added (final volume 30 L). The kinase reaction was initiated by the addition of ATP to a final concentration of 5 mm and incubation was continued for 3 h at 30°C. Figure 10. Inhibition of calcium binding by cations. Calcium binding was estimated by equilibrium dialysis. Calcium binding was performed as described, in the presence of 0.2 mM calcium. Competition was examined by adding an additional 0.2 mM of the indicated metal ion. The valence of the metal added is indicated. The bar value for an additional 0.2 mM calcium added was calculated based upon a 2-fold dilution of isotope and the binding data from Figure 9. Inset, Competition of calcium-binding by zinc. SDs (where greater than 5%) are indicated. Data are average of three experiments. milk in either Tris-buffered saline or phosphate-buffered saline. To demonstrate the competition by the K peptide (Fig. 6D), equal volumes of antiDHN serum and K peptide (5 mg mL⫺1) were mixed and pre-incubated at room temperature for 30 min. Anti-mouse or anti-rabbit IgG conjugated to horseradish peroxidase were used as secondary antibodies for anti-VCaB45 and anti-DHN, respectively. Antibody detection procedures were essentially as described by the manufacturers of Western Blot Chemiluminescence (PerkinElmer Life Sciences Inc., Boston). Calcium-Binding Assays Calcium-binding activity was determined using either a ligand-blotting method or by equilibrium dialysis. Ligand blotting was performed after SDS-PAGE separation and western-blot transfer to PVDF membranes as described (Maruyama and Nonomura, 1984) with some modifications (Randall, 1992). Equilibrium dialysis assays for calcium binding were performed with Quizsep Micro Dialyzer cells (all teflon surfaces, 100-L capacity, Mid-West Scientific, St. Louis) and Spectra/Por molecular weight cutoff (6,000–8,000) membranes. Typically, 50 to 75 L (approximately 15 g of protein) of purified VCaB45 was dialyzed for 5 h against 20 mL of 20 mm Tris-Cl (pH 7.0) at 4°C. To reduce the possibility of spurious ions originating from glassware, polypropylene-ware, teflon dialysis cells, and highly purified water were utilized for these experiments. Generally added to the dialysis buffer were 0.05 to 1.0 mm calcium chloride and 2 to 4 Ci 45calcium chloride. After dialysis, at least two 20-L aliquots were taken from the both the dialysate and the dialysis buffer and counted by liquid scintillation. Acid Phosphatase Assays Acid phosphatase activity was determined as described previously (Boller and Kende, 1979). In brief, 100 L of membrane extract, 320 L of water, and 50 L of 1 m succinic acid (pH 5.0 with NaOH) were incubated 686 ACKNOWLEDGMENTS The molecular mass estimate by MALDI-TOF was kindly performed courtesy of Dr. Jeffrey Patrick at (Eli Lilly and Company, Indianapolis). We also thank Dr. Alan Mahrenhotz of the Biochemistry Biotechnology Facility (Indiana University School of Medicine, Indianapolis) for the amino acid sequencing and MALDI-TOF analysis. The antidehydrin and K peptide were graciously supplied by Dr. Timothy Close (University of California, Riverside). Received January 12, 2002; returned for revision February 28, 2002; accepted June 11, 2002. LITERATURE CITED Allen GJ, Sanders D (1995) Calcineurin, a type 2B protein phosphatase, modulates the calcium-permeable slow vacuolar ion channel of stomatal guard cells. Plant Cell 7: 1473–1483 Boller T, Kende H (1979) Hydrolytic enzymes in the central vacuole of plant cells. Plant Physiol 63: 1123–1132 Camacho P, Lechleiter JD (1995) Calreticulin inhibits repetitive intracellular calcium waves. Cell 82: 765–771 Campbell KP, MacLennan DH, Jorgensen AO (1983a) Staining of calciumbinding proteins, calsequestrin, calmodulin, troponin C, and S-100, with the cationic carbocyanine dye, “stains-all.” J Biol Chem 258: 11267–11273 Campbell KP, MacLennan DH, Jorgensen AO (1983b) Purification and characterization of calsequestrin from canine cardiac sarcoplasmic reticulum and identification of the 53,000 dalton glycoprotein. J Biol Chem 258: 1197–1204 Chen F, Hayes PM, Mulrooney DM, Pan A (1994) Identification and characterization of cDNA clones encoding plant calreticulin in barley. Plant Cell 6: 835–843 Close TJ (1997) Dehydrins: a commonality in the response of plants to dehydration and low temperature. Physiol Plant 100: 291–296 Close TJ, Fenton RD, Moonan F (1993a) A view of plant dehydrins using antibodies specific to the carboxyl terminal peptide. Plant Mol Biol 23: 279–286 Close TJ, Fenton RD, Yang A, Asghar R, DeMason DA, Crone DE, Meyer NC, Moonan F (1993b) Plant responses to cellular dehydration during environmental stress. Curr Top Plant Physiol 10: 104–118 Coppolino MG, Woodside MJ, Demaurex N, Grinstein S, St-Arnaud R, Dedhar S (1997) Calreticulin is essential for integrin-mediated calcium signalling and cell adhesion. Nature 386: 843–847 Downloaded from on June 15, 2017 - Published by www.plantphysiol.org Copyright © 2002 American Society of Plant Biologists. All rights reserved. Plant Physiol. Vol. 130, 2002 Vacuolar Calcium-Binding Protein, VCaB45 Dennison KL, Spalding EP (2000) Glutamate-gated calcium fluxes in Arabidopsis. Plant Physiol 124: 1511–1514 Dure L III (1993) Structural motifs in Lea proteins. Plant responses to cellular dehydration during environmental stress. Curr Top Plant Physiol 10: 91–103 Foy CD, Chaney RL, White MC (1978) The physiology of metal toxicity in plants. Annu Rev Plant Physiol 29: 511–566 Franceschi VR, Li X, Zhang D, Okita TW (1993) Calsequestrin-like calciumbinding protein is expressed in calcium-accumulating cells of Pistis stratiotes. Proc Natl Acad Sci USA 90: 6986–6990 Friedman H, Meir S, Rosenberger I, Halevy AH, Kaufman PB, PhilosophHadas S (1998) Inhibition of the gravitropic response of snapdragon spikes by the calcium-channel blocker lanthanum chloride. Plant Physiol 118: 483–492 Gilmour SJ, Artus NN, Thomashow MF (1992) cDNA sequence analysis and expression of two cold-regulated genes of Arabidopsis thaliana. Plant Mol Biol 18: 13–21 Hebert DN, Foellmer B, Helenius A (1996) Calnexin and calreticulin promote folding, delay oligomerization, and suppress degradation of influenza hemagglutinin in microsomes. EMBO J 15: 2961–2968 Hepler PK, Wayne RO (1985) Calcium and plant development. Annu Rev Plant Physiol 36: 397–439 Jensen AB, Goday A, Figueras M, Jessop AC, Page SM (1998) Phosphorylation mediates the nuclear targeting of maize Rab17 protein. Plant J 13: 691–697 Kaplan RS, Pedersen PL (1985) Determination of microgram quantities of protein in the presence of milligram levels of lipid with amido black 10B. Anal Biochem 150: 97–104 Kaye C, Guy CL (1995) Perspectives of plant cold tolerance: physiology and molecular responses. Sci Prog 78: 271–299 Kitta K, Clement SA, Remeika J, Blumberg JB, Suzuki YJ (2001) Endothelin-1 induces phosphorylation of GATA-4 transcription factor in the HL-1 atrial-muscle cell line. Biochem J 359: 375–380 Krause KH, Chou M, Thomas MA, Sjolund RD, Campbell KP (1989) Plant cells contain calsequestrin. J Biol Chem 264: 4269–4272 Lai S, Randall SK, Sze H (1988) Peripheral and integral subunits of the tonoplast H⫹-ATPase from oat roots. J Biol Chem 263: 16731–16737 Li X, Su RTC, Hsu H-T, Sze H (1998) The molecular chaperone calnexin associates with the vacuolar H⫹-ATPase from oat seedlings. Plant Cell 10: 119–130 Lin CT, Gou WW, Everson E, Thomashow MF (1990) Cold acclimation in Arabidopsis and wheat: response associated with expression of related genes encoding “boiling stable” polypeptides. Plant Physiol 94: 1078–1083 Lu G, Sehnke PC, Ferl RJ (1994) Phosphorylation and calcium binding properties of Arabidopsis GF14 brain protein homolog. Plant Cell 6: 501–510 Machlon AES (1984) Calcium fluxes at plasmalemma and tonoplast. Plant Cell Environ 7: 423–429 Maruyama K, Nonomura Y (1984) High molecular weight calcium-binding protein in the microsome of scallop striated muscle. J Biochem 96: 859–870 Mery L, Mesaeli N, Michalak M, Opas M, Lew DP, Krause KH (1996) Overexpression of calreticulin increases intracellular calcium storage and decreases store-operated calcium influx. J Biol Chem 271: 9332–9339 Michalak M, Miller RE, Burns K, Opas M (1992) Calreticulin. Biochem J 285: 681–692 Miller AJ, Vogg G, Sanders D (1990) Cytosolic calcium homeostasis in fungi: roles of plasma membrane transport and intracellular sequestration of calcium. Proc Natl Acad Sci USA 87: 9348–9352 Napier RM, Trueman S, Henderson J, Boyce JM, Hawes C, Fricker MD, Venis MA (1995) Purification, sequencing, and functions of calreticulin from maize. J Exp Bot 46: 1603–1623 Plant Physiol. Vol. 130, 2002 Nelson DE, Glaunsinger B, Bohnert HJ (1997) Abundant accumulation of the calcium-binding molecular chaperone calreticulin in specific floral tissues of Arabidopsis thaliana. Plant Physiol 114: 29–37 Nigam SK, Goldberg AL, Ho S, Rohde MF, Bush KT, Sherman MYU (1994) A set of endoplasmic reticulum proteins possessing properties of molecular chaperones includes calcium-binding proteins and members of the thioredoxin superfamily. J Biol Chem 269: 1744–1749 Nylander M, Svensson J, Palva ET, Welin BV (2001) Stress-induced accumulation and tissue specific localization of dehydrins in Arabidopsis. Plant Mol Biol 45: 263–279 O’Farrell PH (1975) High resolution two-dimensional electrophoresis of proteins. J Biol Chem 250: 4007–4021 Ostwald TJ, MacLennon DH (1974) Isolation of a high affinity calciumbinding protein from sarcoplasmic reticulum. J Biol Chem 249: 974–979 Peterson JR, Ora A, Van PN, Helenius A (1995) Transient, lectin-like association of calreticulin with folding intermediates of cellular and viral glycoproteins. Mol Biol Cell 6: 1173–1184 Plana M, Itarte E, Eritja R, Goday A, Pages M, Martinez MC (1991) Phosphorylation of the maize RAB-17 protein by casein kinase 2. J Biol Chem 266: 22510–22514 Randall SK (1992) Characterization of vacuolar calcium-binding proteins. Plant Physiol 100: 859–867 Randall SK, Sze H (1986) Properties of the partially purified tonoplast H⫹-pumping ATPase from oat roots. J Biol Chem 261: 1364–1371 Rivedal E, Opsahl H (2001) Role of PKC and MAP kinase in EGF- and TPA-induced connexin43 phosphorylation and inhibition of gap junction intercellular communication in rat liver epithelial cells. Carcinogenesis 22: 1543–1550 Sanders D, Brownlee C, Harper JF (1999) Communicating with calcium. Plant Cell 11: 691–706 Seals DF, Randall SK (1994) A 43 kDa annexin-like protein is associated with plant vacuoles. Plant Physiol 106: 1403–1412 Seals DF, Randall SK (1997) A vacuole-associated annexin protein, VCaB42, correlates with the expansion of tobacco cells. Plant Physiol 115: 753–761 Skriver K, Mundy J (1990) Gene expression in response to abscisic acid and osmotic stress. Plant Cell 2: 503–512 Svensson J, Palva ET, Welin B (2000) Purification of recombinant Arabidopsis dehydrins by metal ion affinity chromatography. Protein Expr Purif 20: 169–178 Sze H, Liang F, Hwang I, Cunan AC, Harper JF (2000) Diversity and regulation of plant calcium pumps: insights from expression in yeast. Annu Rev Plant Physiol Plant Mol Biol 51: 433–462 Tan S-K, Sage-Ono K, Kamada H (2000) Cloning and characterization of ECPP44, a cDNA encoding a 44-kilodalton phosphoprotein relating to somatic embryogenesis in carrot. Plant Biotechnol 17: 61–68 Vilardel J, Goday A, Freire MA, Torrent M, Martinez MC, Torre JM, Pages M (1990) Gene sequence, developmental expression, and protein phosphorylation of RAB-17 in maize. Plant Mol Biol 14: 423–432 Welin BV, Olson A, Nylander M, Palva ET (1994) Characterization and differential expression of dhn/lea/rab-like genes during cold acclimation and drought stress in Arabidopsis thaliana. Plant Mol Biol 26: 131–144 Welin BV, Olson A, Palva ET (1995) Structure and organization of two closely related low-temperature-induced dhn/lea/rab-like genes in Arabidopsis thaliana L. Heynh. Plant Mol Biol 29: 391–395 Xing T, Williams LE, Nelson SJ, East JM, Hall JL (1994) Immunological detection and localization of a calsequestrin-like protein in red beet and cucumber cells. Protoplasma 179: 158–165 Yuasa K, Maeshima M (2000) Purification, properties, and molecular cloning of a novel calcium-binding protein in radish vacuoles. Plant Physiol 124: 1069–1078 Downloaded from on June 15, 2017 - Published by www.plantphysiol.org Copyright © 2002 American Society of Plant Biologists. All rights reserved. 687