* Your assessment is very important for improving the work of artificial intelligence, which forms the content of this project
Download The Citric Acid Cycle
Size-exclusion chromatography wikipedia , lookup
Basal metabolic rate wikipedia , lookup
Multi-state modeling of biomolecules wikipedia , lookup
Lactate dehydrogenase wikipedia , lookup
Butyric acid wikipedia , lookup
Photosynthesis wikipedia , lookup
Phosphorylation wikipedia , lookup
Fatty acid synthesis wikipedia , lookup
Metalloprotein wikipedia , lookup
Mitochondrial replacement therapy wikipedia , lookup
Fatty acid metabolism wikipedia , lookup
Amino acid synthesis wikipedia , lookup
Glyceroneogenesis wikipedia , lookup
Nicotinamide adenine dinucleotide wikipedia , lookup
Evolution of metal ions in biological systems wikipedia , lookup
Biosynthesis wikipedia , lookup
Microbial metabolism wikipedia , lookup
Mitochondrion wikipedia , lookup
Light-dependent reactions wikipedia , lookup
Adenosine triphosphate wikipedia , lookup
Photosynthetic reaction centre wikipedia , lookup
Electron transport chain wikipedia , lookup
Biochemistry wikipedia , lookup
NADH:ubiquinone oxidoreductase (H+-translocating) wikipedia , lookup
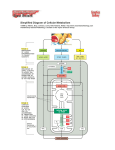
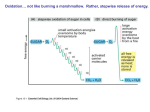
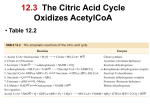
The Citric Acid Cycle The aerobic processing of glucose start with the complete oxidation of glucose derivatives to CO2. This oxidation takes place in the citric acid cycle, a series of reactions also known as the tricarboxylic acid (TCA) cycle or the Krebs cycle. The citric acid cycle is the final common pathway for the oxidation of fuel molecules. It also serves as a source of building blocks for biosyntheses. Most fuel molecules enter the cycle as acetyl CoA. The link between glycolysis and the citric acid cycle is the oxidative decarboxylation of pyruvate to form acetyl CoA. In eukaryotes, this reaction and those of the cycle take place inside mitochondria, in contrast with glycolysis, which takes place in the cytosol. Biosynthetic Roles of the Citric Acid Cycle. Intermediates drawn off for biosyntheses (shown by red arrows) are replenished by the formation of oxaloacetate from pyruvate. Mitochondrion. The double membrane of the mitochondrion is evident in this electron micrograph. The numerous invaginations of the inner mitochondrial membrane are called cristae. The oxidative decarboxylation of pyruvate and the sequence of reactions in the citric acid cycle take place within the matrix. Pyruvate Transport (Antiporter) Pyruvate Pyruvate Carrier OH - Overview of the Citric Acid Cycle. The citric acid cycle oxidizes twocarbon units, producing two molecules of CO2, one molecule of GTP, and highenergy electrons in the form of NADH and FADH2. The function of the citric acid cycle is the harvesting of high energy electrons from carbon fuels. Note that the citric acid cycle itself neither generate large amount of ATP nor includes oxygen as a reactant. Instead, it removes electrons from acetyl CoA and uses these electrons to form NADH and FADH2. The formation of Acetyl Coenzyme A from Pyruvate. Pyruvate is oxidatively decarboxylated by the pyruvate dehydrogenase complex to form acetyl CoA. This irreversible reaction is the link between glycolysis and citric acid cycle. Pyruvate dehydrogenase complex of E. coli Enzyme Abbreviation Number of chains Prosthetic group Reaction catalyzed Pyruvate dehydrogenase component E1 24 TPP Oxidative decarboxylation of pyruvate Dihydrolipoyl transacetylase E2 24 Lipoamide Transfer of the acetyl group to CoA Dihydrolipoyl dehydrogenase E3 12 FAD Regeneration of the oxidized form of lipoamide The Formation of Acetyl Coenzyme A from Pyruvate The reaction requires the participation of the three enzymes of the pyruvate dehydrogenase complex, each composed of several polypeptide chains, and five coenzymes: – – – – – B1 thiamine pyrophosphate (TPP), lipoic acid, FAD, CoA NAD+. Acetyl Coenzyme A (Acetyl CoA). B5 The conversion of pyruvate into acetyl CoA consists of three steps: 123- Decarboxylation, Oxidation, Transfer of the acetyl group to CoA. Thiazole ring Carbanion Mechanism of the Decarboxylation Reaction of E1 , The Pyruvate Dehydrogenase Component of the Pyruvate Dehydrogenese Complex. The hydroxyethyl group attached to TPP is oxidized to form an acetyl group and concomitantly transferred to lipoamide, a derivative of lipoic acid that is linked to the side chain of a lysine residue by an amide linkage. The oxidant in this reaction is the disulfide group of lipoamide, which is reduced to its disulfhydryl form. This reaction, also catalyzed by the pyruvate dehydrogenase component E1, yields acetyllipoamide. Third, the acetyl group is transferred from acetyllipoamide to CoA to form acetyl CoA. Dihydrolipoyl transacetylase (E2) catalyzes this reaction. The pyruvate dehydrogenase complex cannot complete another catalytic cycle until the dihydrolipoamide is oxidized to lipoamide. In a fourth step, the oxidized form of lipoamide is regenerated by dihydrolipoyl dehydrogenase (E3). Two electrons are transferred to an FAD prosthetic group of the enzyme and then to NAD+. Citrate Synthase Forms Citrate from Oxaloacetate and Acetyl Coenzyme A The citric acid cycle begins with the condensation of a fourcarbon unit, oxaloacetate, and a two-carbon unit, the acetyl group of acetyl CoA. Oxaloacetate reacts with acetyl CoA and H2O to yield citrate and CoA. This reaction, which is an aldol condensation followed by a hydrolysis, is catalyzed by citrate synthase. Citrate is isomerized into isocitrate to enable the sixcarbon unit to undergo oxidative decarboxylation. The isomerization of citrate is accomplished by a dehydration step followed by a hydration step. The result is an interchange of a hydrogen atom and a hydroxyl group. The enzyme catalyzing both steps is called aconitase because cis-aconitate is an intermediate. Isocitrate Is Oxidized and Decarboxylated to α-Ketoglutarate The oxidative decarboxylation of isocitrate is catalyzed by isocitrate dehydrogenase. The intermediate in this reaction is oxalosuccinate, an unstable β-ketoacid. While bound to the enzyme, it loses CO2 to form α-ketoglutarate. Succinyl Coenzyme A Is Formed by the Oxidative Decarboxylation of α-Ketoglutarate α-Ketoglutarate dehydrogenase A High Phosphoryl-Transfer Potential Compound Is Generated from Succinyl Coenzyme A The cleavage of the thioester bond of succinyl CoA is coupled to the phosphorylation of a purine nucleoside diphosphate, usually GDP. This reaction is catalyzed by succinyl CoA synthetase (succinate thiokinase). Oxaloacetate Is Regenerated by the Oxidation of Succinate The final stage of the citric acid cycle. A methylene group is converted into a carbonyl group in three steps; – Oxidation (Succinate dehydrogenase) – Hydration (Fumarase) – Second oxidation (Malate dehydrogenase) Not only is oxaloacetate thereby regenerated for another round of the cycle, but also more energy is extracted in the form of FADH2 and NADH. Stoichiometry of Citric Acid Cycle. Acetyl CoA + 3NAD + FAD + GDP + Pi + 2H2O 2 CO2 + 3NADH + FADH2 + GTP + 2H+ +CoA Reactions of the Pyruvate Dehydrogenase Complex. At the top (center), the enzyme (represented by a yellow, a blue, and two red spheres) is unmodified and ready for a catalytic cycle. (1) Pyruvate is decarboxylated to form the hydroxyethyl TPP. (2) The dihydrolipoyl arm of E2 moves into the active site of E1. (3) E1 catalyzes the transfer of the two-carbon group to the dihydrolipoyl group to form the acetyl-lipoyl complex. (4) E2 catalyzes the transfer of the acetyl moiety to CoA to form the product acetyl CoA. The disulfhydryl lipoyl arm then swings to the active site of E3. E3 catalyzes (5) the reduction of the lipoic acid and (6) the transfer of the protons and electrons to NAD+ to complete the reaction cycle. 1. Pyruvate is decarboxylated at the active site of E1, forming the substituted TPP intermediate, and CO2 leaves as the first product. This active site lies within the E1 complex, connected to the enzyme surface by a 20-Å-long hydrophobic channel. 2. E2 inserts the lipoyl-lysine arm of the lipoamide domain into the channel in E1. 3. E1 catalyzes the transfer of the acetyl group to the lipoamide. The acetylated lipoyl-lysine arm then leaves E1 and enters the E2 cube through 30 Å windows on the sides of the cube to visit the active site of E2, located deep in the cube at the subunit interface. 4. The acetyl moiety is then transferred to CoA, and the second product, acetyl CoA, leaves the cube. The reduced lipoyl-lysine arm then swings to the active site of the E3 flavoprotein. 5. At the E3 active site, the lipoamide acid is oxidized by coenzyme FAD. 6. The final product, NADH, is produced with the reoxidation of FADH2, and the reactivated lipoamide is ready to begin another reaction cycle. The structural integration of three kinds of enzymes makes the coordinated catalysis of a complex reaction possible. The proximity of one enzyme to another increases the overall reaction rate and minimizes side reactions. All the intermediates in the oxidative decarboxylation of pyruvate are tightly bound to the complex and are readily transferred because of the ability of the lipoyllysine arm of E2 to call on each active site in turn. Step Reaction Prosthetic group Type* Aconitase Fe-S b Aconitase Fe-S c 2a Citrate 2b cis-Aconitate+ H2O 3 Isocitrate + NAD+ 4 α-Ketoglutarate + NAD+ + CoA NADH 5 Succinyl CoA + Pi + GDP 6 Succinate + FAD (enzyme-bound) (enzyme-bound) 7 Fumarate + H2O l-malate Furmarase c 8 l-Malate + NAD+ oxaloacetate + NADH + H+ Malate dehydrogenase e * cis-aconitate + H2O Enzyme isocitrate α-ketoglutarate + CO2 + NADH succinyl CoA + CO2 + succinate + GTP + CoA fumarate + FADH2 Isocitrate dehydrogenase α-Ketoglutarate dehydrogenase complex d+e Lipoic acid, FAD, TPP Succinyl CoA synthetase Succinate dehydrogenase d+e f FAD, Fe-S e Reaction type: (a) condensation; (b) dehydration; (c) hydration; (d) decarboxylation; (e) oxidation; (f) substrate-level phosphorylation. 1. Two carbon atoms enter the cycle in the condensation of an acetyl unit (from acetyl CoA) with oxaloacetate. Two carbon atoms leave the cycle in the form of CO2 in the successive decarboxylations catalyzed by isocitrate dehydrogenase and αketoglutarate dehydrogenase. Interestingly, the results of isotope-labeling studies revealed that the two carbon atoms that enter each cycle are not the ones that leave. 2. Four pairs of hydrogen atoms leave the cycle in four oxidation reactions. Two molecules of NAD+ are reduced in the oxidative decarboxylations of isocitrate and α-ketoglutarate, one molecule of FAD is reduced in the oxidation of succinate, and one molecule of NAD+ is reduced in the oxidation of malate. 3. One compound with high phosphoryl transfer potential, usually GTP, is generated from the cleavage of the thioester linkage in succinyl CoA. 4. Two molecules of water are consumed: one in the synthesis of citrate by the hydrolysis of citryl CoA and the other in the hydration of fumarate. Glycolysis has both an aerobic and an anaerobic mode, whereas the citric acid cycle is strictly aerobic. Glycolysis can proceed under anaerobic conditions because NAD+ is regenerated in the conversion of pyruvate into lactate. Control of the Citric Acid Cycle The citric acid cycle is regulated primarily by the concentration of ATP and NADH. The key control points are the enzymes: isocitrate dehydrogenase and α-ketoglutarate dehydrogenase. Oxidative Phosphorylation The NADH and FADH2 formed in glycolysis, fatty acid oxidation, and the citric acid cycle are energy-rich molecules because each contains a pair of electrons having a high transfer potential. When these electrons are used to reduce molecular oxygen to water, a large amount of free energy is liberated, which can be used to generate ATP. Oxidative phosphorylation is the process in which ATP is formed as a result of the transfer of electrons from NADH or FADH2to O2 by a series of electron carriers carriers.. This process, which takes place in mitochondria, is the major source of ATP in aerobic organisms For example, oxidative phosphorylation generates 26 of the 30 molecules of ATP that are formed when glucose is completely oxidized to CO2 and H2O. The flow of electrons from NADH or FADH2 to O2 through protein complexes located in the mitochondrial inner membrane leads to the pumping of protons out of the mitochondrial matrix. The resulting uneven distribution of protons generates a pH gradient and a transmembrane electrical potential that creates a proton-motive force. ATP is synthesized when protons flow back to the mitochondrial matrix through an enzyme complex. Thus, the oxidation of fuels and the phosphorylation of ADP are coupled by a proton gradient across the inner mitochondrial membrane. Sequence of Electron Carriers in the Respiratory Chain. Oxidative phosphorylation is the culmination of a series of energy transformations that are called cellular respiration or simply respiration in their entirety. First, carbon fuels are oxidized in the citric acid cycle to yield electrons with high transfer potential. Then, this electron-motive force is converted into a proton-motive force and, finally, the proton-motive force is converted into phosphoryl transfer potential. The conversion of electron-motive force into proton-motive force is carried out by three electron-driven proton pumps: – – – – NADH-Q oxidoreductase, Q-cytochrome c oxidoreductase, and cytochrome c oxidase. These large transmembrane complexes contain multiple oxidation-reduction centers, including quinones, flavins, iron-sulfur clusters, hemes, and copper ions. Complex I Complex II FADH2 Complex III Complex IV Does not pump protons Cyt C (reduced) Cyt C (oxidized) 4H+ Fe O2 4H+ (Pumped protons) Cu 2 H2 O Four “chemical” protons are Taken up from the matrix side to reduce one molecule of O2 to two molecules of H2O. Four additional “pumped” protons are transported out of the matrix and released on the cytosolic side in the course of the reaction. The pumped protons double the efficiency of free-energy storage in the form of a proton gradient. 4H+ (chemical protons) A Proton Gradient Powers the Synthesis of ATP The final phase of oxidative phosphorylation is carried out by ATP synthase, an ATP-synthesizing assembly that is driven by the flow of protons back into the mitochondrial matrix. Components of this remarkable enzyme rotate as part of its catalytic mechanism. Oxidative phosphorylation vividly shows that proton gradients are an interconvertible currency of free energy in biological systems. In oxidative phosphorylation, the synthesis of ATP is coupled to the flow of electrons from NADH or FADH2 to O2 by a proton gradient across the inner mitochondrial membrane. Electron flow through three asymmetrically oriented transmembrane complexes results in the pumping of protons out of the mitochondrial matrix and the generation of a membrane potential. ATP is synthesized when protons flow back to the matrix through a channel in an ATPsynthesizing complex, called ATP synthase (also known as F0F1-ATPase). Oxidative phosphorylation exemplifies a fundamental theme of bioenergetics: the transmission of free energy by proton gradients. The flow of electrons through Complexes I, III, and IV leads to the transfer of protons from the matrix side to the cytosolic side of the inner mitochondrial membrane. A proton-motive force consisting of a pH gradient (matrix side basic) and a membrane potential (matrix side negative) is generated. The flow of protons back to the matrix side through ATP synthase drives ATP synthesis. The enzyme complex is a molecular motor made of two operational units: a rotating component and a stationary component. The rotation of the g subunit induces structural changes in the b subunit that result in the synthesis and release of ATP from the enzyme. Proton influx provides the force for the rotation. The flow of two electrons through NADH-Q oxidoreductase, Q-cytochrome c oxidoreductase, and cytochrome c oxidase generates a gradient sufficient to synthesize 1, 0.5, and 1 molecule of ATP, respectively. Hence, 2.5 molecules of ATP are formed per molecule of NADH oxidized in the mitochondrial matrix, whereas only 1.5 molecules of ATP are made per molecule of FADH2 oxidized because its electrons enter the chain at QH2, after the first proton-pumping site. about 30 molecules of ATP are formed when glucose is completely oxidized to CO2; this value supersedes the traditional estimate of 36 molecules of ATP. Most of the ATP, 26 of 30 molecules formed, is generated by oxidative phosphorylation. Recall that the anaerobic metabolism of glucose yields only 2 molecules of ATP. Sites of Action of Some Inhibitors of Electron Transport. Uncoupling of Oxidative Phosphorylation. 2,4 –Dinitrophenol, a lipid-soluble substance, can carry protons across the inner mitochondrial membrane. Figure 18.45. Action of an Uncoupling Protein. Uncoupling protein-1 (UCP-1) generates heat by permitting the influx of protons into the mitochondria without the synthesis of ATP. Regulated Uncoupling Leads to the Generation of Heat The uncoupling of oxidative phosphorylation is a means of generating heat to maintain body temperature in hibernating animals, in some newborn animals (including human beings), and in mammals adapted to cold. Brown adipose tissue, which is very rich in mitochondria (often referred to as brown fat mitochondria), is specialized for this process of nonshivering thermogenesis. The inner mitochondrial membrane of these mitochondria contains a large amount of uncoupling protein (UCP), here UCP-1, or thermogenin, a dimer of 33-kd subunits that resembles ATP-ADP translocase. UCP-1 forms a pathway for the flow of protons from the cytosol to the matrix. In essence, UCP-1 generates heat by short-circuiting the mitochondrial proton battery. This dissipative proton pathway is activated by free fatty acids liberated from triacylglycerols in response to hormonal signals, such as b-adrenergic agonists . Many Shuttles Allow Movement Across Mitochondrial Membranes. Electrons from Cytosolic NADH Enter Mitochondria by Shuttles. Recall that the glycolytic pathway generates NADH in the cytosol in the oxidation of glyceraldehyde 3-phosphate, and NAD+ must be regenerated for glycolysis to continue. How is cytosolic NADH reoxidized under aerobic conditions? NADH cannot simply pass into mitochondria for oxidation by the respiratory chain, because the inner mitochondrial membrane is impermeable to NADH and NAD+. The solution is that electrons from NADH, rather than NADH itself, are carried across the mitochondrial membrane. One of several means of introducing electrons from NADH into the electron transport chain is the glycerol 3-phosphate shuttle. The first step in this shuttle is the transfer of a pair of electrons from NADH to dihydroxyacetone phosphate, a glycolytic intermediate, to form glycerol 3-phosphate.This reaction is catalyzed by a glycerol 3phosphate dehydrogenase in the cytosol. Glycerol 3-phosphate is reoxidized to dihydroxyacetone phosphate on the outer surface of the inner mitochondrial membrane by a membrane-bound isozyme of glycerol 3-phosphate dehydrogenase. An electron pair from glycerol 3-phosphate is transferred to a FAD prosthetic group in this enzyme to form FADH2. This reaction also regenerates dihydroxyacetone phosphate. Figure 18.37. Glycerol 3-Phosphate Shuttle. Electrons from NADH can enter the mitochondrial electron transport chain by being used to reduce dihydroxyacetone phosphate to glycerol 3-phosphate. Glycerol 3-phosphate is reoxidized by electron transfer to an FAD prosthetic group in a membrane-bound glycerol 3-phosphate dehydrogenase. Subsequent electron transfer to Q to form QH2 allows these electrons to enter the electron-transport chain. MalateAspartate Shuttle. In the heart and liver, electrons from cytosolic NADH are brought into mitochondria by the malate-aspartate shuttle, which is mediated by two membrane carriers and four enzymes. Electrons are transferred from NADH in the cytosol to oxaloacetate, forming malate, which traverses the inner mitochondrial membrane and is then reoxidized by NAD+ in the matrix to form NADH in a reaction catalyzed by the citric acid cycle enzyme malate dehydrogenase. The resulting oxaloacetate does not readily cross the inner mitochondrial membrane, and so a transamination reaction is needed to form aspartate, which can be transported to the cytosolic side. Mitochondrial glutamate donates an amino group, forming aspartate and α-ketoglutarate. In the cytoplasm, aspartate is then deaminated to form oxaloacetate and the cycle is restarted. This shuttle, in contrast with the glycerol 3-phosphate shuttle, is readily reversible. Consequently, NADH can be brought into mitochondria by the malate- aspartate shuttle only if the NADH/NAD+ ratio is higher in the cytosol than in the mitochondrial matrix. This versatile shuttle also facilitates the exchange of key intermediates between mitochondria and the cytosol. The Entry of ADP into Mitochondria Is Coupled to the Exit of ATP by ATP-ADP Translocase The major function of oxidative phosphorylation is to generate ATP from ADP. However, ATP and ADP do not diffuse freely across the inner mitochondrial membrane. How are these highly charged molecules moved across the inner membrane into the cytosol? A specific transport protein, ATP-ADP translocase (also called adenine nucleotide translocase or ANT), enables these molecules to traverse this permeability barrier. Most important, the flows of ATP and ADP are coupled. ADP enters the mitochondrial matrix only if ATP exits, and vice versa. The reaction catalyzed by the translocase, which acts as an antiporter. Mechanism of Mitochondrial ATP-ADP Translocase. The translocase catalyzes the coupled entry of ADP and exit of ATP into and from the matrix. The reaction cycle is driven by membrane potential. The actual conformational change corresponding to eversion of the binding site could be quite small. The membrane potential and hence the protonmotive force are decreased by the exchange of ATP for ADP, which results in a net transfer of one negative charge out of the matrix. ATP/ADP exchange is energetically expensive; about a quarter of the energy yield from electron transfer by the respiratory chain is consumed to regenerate the membrane potential that is trapped by this exchange. The inhibition of this process lead to the subsequent inhibition of cellular respiration as well. Mitochondrial Transporters. Transporters (also called carriers) are transmembrane proteins that move ions and charged metabolites across the inner mitochondrial membrane. Reaction sequence ATP yield per glucose molecule Glycolysis: Conversion of glucose into pyruvate (in the cytosol) Phosphorylation of glucose -1 Phosphorylation of fructose 6-phosphate -1 Dephosphorylation of 2 molecules of 1,3-BPG +2 Dephosphorylation of 2 molecules of phosphoenolpyruvate +2 2 molecules of NADH are formed in the oxidation of 2 molecules of glyceraldehyde 3-phosphate Conversion of pyruvate into acetyl CoA (inside mitochondria) 2 molecules of NADH are formed Citric acid cycle (inside mitochondria) 2 molecules of guanosine triphosphate are formed from 2 molecules of succinyl CoA +2 6 molecules of NADH are formed in the oxidation of 2 molecules each of isocitrate, α-ketoglutarate, and malate 2 molecules of FADH2 are formed in the oxidation of 2 molecules of succinate Oxidative phosphorylation (inside mitochondria) 2 molecules of NADH formed in glycolysis; each yields 1.5 molecules of ATP (assuming transport of NADH by the glycerol 3-phosphate shuttle) +3 2 molecules of NADH formed in the oxidative decarboxylation of pyruvate; each yields 2.5 molecules of ATP +5 2 molecules of FADH2 formed in the citric acid cycle; each yields 1.5 molecules of ATP +3 6 molecules of NADH formed in the citric acid cycle; each yields 2.5 molecules of ATP + 15 net yield per molecule of glucose + 30 The Pentose Phosphate Pathway. Many endergonic reactions, notably the reductive biosynthesis of fatty acids , and cholesterol, as well as photosynthesis, require NADPH in addition to ATP. Despite their close chemical resemblance, NADPH and NADH are not metabolically interchangeable. Whereas NADH participates in utilizing the free energy of metabolite oxidation to synthesize ATP (oxidative phosphorylation), NADPH is involved in utilizing the free energy of metabolite oxidation for otherwise endergonic reductive biosynthesis. Pathways Requiring NADPH Synthesis: – – – – Fatty acid biosynthesis. Cholesterol biosynthesis. Neurotransmitter biosynthesis. Nucleotide biosynthesis. Detoxification: – Reduction of oxidized glutathione. – Cytochrome P50 monooxygenases. NADPH is generated by the oxidation of G6P via an alternative pathway to glycolysis, the pentose phosphate pathway (also called the hexose monophosphate (HMP) shunt or the phosphogluconate pathway.