* Your assessment is very important for improving the workof artificial intelligence, which forms the content of this project
Download MOLECULAR BASIS FOR MEMBRANE PHOSPHOLIPID
Survey
Document related concepts
Membrane potential wikipedia , lookup
Mechanosensitive channels wikipedia , lookup
Protein phosphorylation wikipedia , lookup
Cytokinesis wikipedia , lookup
Organ-on-a-chip wikipedia , lookup
Theories of general anaesthetic action wikipedia , lookup
Protein moonlighting wikipedia , lookup
SNARE (protein) wikipedia , lookup
Magnesium transporter wikipedia , lookup
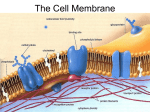
Lipid bilayer wikipedia , lookup
Signal transduction wikipedia , lookup
Model lipid bilayer wikipedia , lookup
Cell membrane wikipedia , lookup
Western blot wikipedia , lookup
Transcript
P1: rpk/mkv May 5, 1997 P2: rpk 14:30 Annual Reviews AR032-07 32-07 Annu. Rev. Biochem. 1997. 66:199–232 c 1997 by Annual Reviews Inc. All rights reserved Copyright MOLECULAR BASIS FOR MEMBRANE PHOSPHOLIPID DIVERSITY: Why Are There So Many Lipids? W. Dowhan Department of Biochemistry and Molecular Biology, University of Texas–Houston, Medical School, Houston, Texas 77225; e-mail: [email protected] KEY WORDS: phospholipid function, phosphatidylethanolamine, phosphatidylglycerol, cardiolipin, membrane proteins, lipid polymorphism ABSTRACT Phospholipids play multiple roles in cells by establishing the permeability barrier for cells and cell organelles, by providing the matrix for the assembly and function of a wide variety of catalytic processes, by acting as donors in the synthesis of macromolecules, and by actively influencing the functional properties of membrane-associated processes. The function, at the molecular level, of phosphatidylethanolamine, phosphatidylglycerol, and cardiolipin in specific cellular processes is reviewed, with a focus on the results of combined molecular genetic and biochemical studies in Escherichia coli. These results are compared with primarily biochemical data supporting similar functions for these phospholipids in eukaryotic organisms. The wide range of processes in which specific involvement of phospholipids has been documented explains the need for diversity in phospholipid structure and why there are so many membrane lipids. CONTENTS INTRODUCTION . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . PHOSPHOLIPID METABOLISM . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . Phospholipid Synthesis in Escherichia coli . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . Phospholipid Synthesis in Eukaryotic Organisms . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . FUNCTIONS OF ANIONIC PHOSPHOLIPIDS . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . Roles of Phosphatidylglycerol and Cardiolipin . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . FUNCTIONS OF ZWITTERIONIC PHOSPHOLIPIDS . . . . . . . . . . . . . . . . . . . . . . . . . . . . . Roles of Phosphatidylethanolamine . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 200 201 201 205 207 208 216 216 199 0066-4154/97/0701-0199$08.00 P1: rpk/mkv May 5, 1997 P2: rpk 14:30 200 Annual Reviews AR032-07 32-07 DOWHAN LIPID POLYMORPHISM . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 222 SUMMARY AND FUTURE DIRECTIONS . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 227 INTRODUCTION It is widely recognized that phospholipids play multiple roles in cell processes. Their primary function is to define the permeability barrier of cells and organelles by forming a phospholipid bilayer. This bilayer serves as the matrix and support for a vast array of proteins involved in important functions of the cell such as energy transduction, signal transduction, solute transport, DNA replication, protein targeting and trafficking, cell-cell recognition, secretion, etc. Phospholipids do not play a static role in these processes but are active participants that influence the properties of the proteins associated with the membrane and serve as precursors to important cellular components. Because phospholipids have no inherent catalytic activity, a major challenge has been to develop approaches to investigate the specific role phospholipids play in cellular functions. The majority of evidence establishing functions for phospholipids has been a secondary product of the study of cellular processes in in vitro reconstituted systems. This approach does yield important clues about function but can be fraught with artifact and may lead to conclusions that have no relationship to the in vivo state. Therefore, in vivo approaches are needed to validate the observations made in vitro. A classic approach to defining in vivo function has been to use genetics. However, genetically dissecting phospholipid function in vivo presents several complications: (a) The genetic approach is indirect because mutations cannot be made directly in a phospholipid but rather must be made in the biosynthetic pathway leading to a particular phospholipid. (b) Mutations in “phospholipid genes” may compromise not only cell integrity but also several cell functions, so elimination of or drastic alteration in the level of a particular phospholipid might be expected to affect many cellular processes simultaneously or, worse yet, to compromise cell integrity before affecting cell function. (c) The more complex the organism and its related phospholipid make-up, the more difficult it is to deal with the limitations imposed by the first two constraints. Fortunately, recent studies utilizing a set of mutants in phospholipid metabolism in Escherichia coli (1) have demonstrated that meaningful data can be obtained using a combined molecular genetic and biochemical approach both to validate functions in vivo initially postulated on the basis of in vitro data and to uncover previously undocumented functions. The successful application of these molecular genetic approaches to E. coli should be generally applicable to more complicated biological systems such as yeast and somatic cells. Although dissecting the molecular basis for phospholipid function will be more difficult in P1: rpk/mkv May 5, 1997 P2: rpk 14:30 Annual Reviews AR032-07 32-07 PHOSPHOLIPID DIVERSITY 201 eukaryotic systems than in bacteria, the basic principles underlying the function of phospholipids in E. coli should be relevant to phospholipid function in more complex organisms and will be useful guides in designing experimental approaches in these latter organisms. Because an understanding of the multiple roles of phospholipids in general cell physiology and specific cellular processes is essential for understanding normal cell function, information of this type also lies at the core of understanding cellular dysfunction. In this review, I concentrate on those findings in E. coli for which genetic approaches in concert with biochemical studies have resulted in detailed understanding of phospholipid functions at the molecular level. Important contributions from other bacterial systems are noted, as well as those from both yeast and somatic cell systems about which much of what is known is based on in vitro biochemical studies. The role of phospholipids as messengers in signal transduction is not covered because many excellent reviews (2–5) are available on this subject, and genetic approaches have not been used extensively to validate in vivo function. PHOSPHOLIPID METABOLISM Initial definition of phospholipid biosynthetic pathways in E. coli was based on biochemical studies reported by Kennedy and coworkers (5a, 5b). Subsequent genetic analysis of these metabolic pathways has confirmed the biochemical findings, but more important, the detailed development of the genetics of phospholipid metabolism in E. coli has made possible a combined molecular genetic and biochemical approach to define the role of individual phospholipid species at the molecular level (1, 6). Findings that contribute to a detailed understanding of phospholipid genetics in E. coli (7–9) are being closely followed by similar information about Saccharomyces cerevisiae (10, 11). Rapid and dramatic progress is being made in somatic cell systems toward defining the genetics of phospholipid metabolism (12). Therefore, the approaches currently being applied in prokaryotic systems will shortly be applicable to more complex organisms. Phospholipid Synthesis in Escherichia coli In all organisms, phospholipid biosynthesis (see Figure 1) begins with two acylation steps of sn-glycerol-3-P to form phosphatidic acid [see (8) for the most recent descriptions of genes and gene products in E. coli]. Genetic manipulation at the first acyltransferase (encoded by the plsB gene) can be used to affect the protein-to-phospholipid ratio of the cell membrane of E. coli, but only a limited number of studies on cell properties have been reported (13, 14) in which this ratio has been altered. In bacteria, phosphatidic acid (PA) is converted (Step a) P1: rpk/mkv P2: rpk October 9, 1997 202 17:12 Annual Reviews AR032-07n 32-07 DOWHAN Figure 1 Phospholipid biosynthetic pathway in E. coli and the associated genes. The substituents attached to the “X” position of phosphatidic acid define the various phospholipids listed. The substituent for CDP-DAG is CMP and for CL, phosphatidylglycerol. The gene encoding the enzyme catalyzing each step is listed. to the central liponucleotide intermediate CDP-diacylglycerol (CDP-DAG), which is the precursor (Steps b and c) to the major zwitterionic phospholipid phosphatidylethanolamine (PE), found in gram-negative bacteria such as E. coli and gram-positive bacteria represented by the Bacilli (15); the major anionic phospholipids phosphatidylglycerol (PG) and cardiolipin (CL) are found in almost all bacteria (Steps d–f). Gram-negative bacteria with a high content of unsaturated fatty acids and intracellular membrane systems contain phosphatidylcholine (PC) (16), which is made by methylation of PE via S-adenosyl methionine as in the photosynthetic organism Rhodobacter sphaeroides (17). Many gram-positive bacteria lack PE and PC but contain derivatives of PG in which one of the free hydroxyls of glycerol is acylated with an amino acid transferred from amino acyl tRNA (18), resulting in either a zwitterionic or net positively charged phospholipid; several species of bacilli have been shown to contain these derivatives of PG in addition to PE (15). Little is known about the function and properties of these phospholipids. Gram-positive bacteria lacking PE usually contain high levels of uncharged diacylglycerols with mono- and disaccharides at the sn-3 position (19–21); the function of these lipids is discussed later. Gram-negative organisms contain an additional class P1: rpk/mkv May 5, 1997 P2: rpk 14:30 Annual Reviews AR032-07 32-07 PHOSPHOLIPID DIVERSITY 203 of phosphate-containing lipids that are the lipid A-based (β, 10 -6-linked disaccharide of glucosamine decorated with six fatty acids and two phosphates) portion of lipopolysaccharide (LPS) (22) and make up the outer lipid monolayer of the outer membrane (23); phospholipids make up the inner monolayer of the outer membrane and the bilayer of the inner membrane (24). The head-group composition of the phospholipids of E. coli is relatively invariant under a broad spectrum of growth conditions (PE at 70–80%, PG at 20–25%, and CL at 5% or less); only CL levels rise, at the expense of PG, as cells enter the stationary phase (25). Position 1 of the phospholipid backbone is occupied predominately by palmitic acid, and position 2 by the dominant monounsaturated fatty acids palmitoleic and cis-vaccenic acids (8). In cells grown at 37◦ C, the ratio of saturated to unsaturated fatty acids is about 1:1, but at 17◦ C the ratio is about 1:2 (26). If one considers the added diversity of different chain lengths for fatty acids, and fatty acids with varying degrees of saturation, then the number of individual phospholipid species even in a simple prokaryotic organism such as E. coli is in the hundreds (7, 27), even though the major phospholipid classes account for 95% of the total glycerophosphatebased phospholipids. Since most of these individual phospholipid species can form a bilayer permeability barrier under physiological conditions, why are there so many phospholipid species, and what is the molecular basis for the wide diversity in membrane phospholipid structure? E. coli does not readily take up phospholipids from the growth medium (28), so isolation of phospholipid auxotrophs has not been an effective mutant screening technique, although a mutant with an elevated Michaelis constant for the first acyltransferase is a glycerol-3-phosphate auxotroph (13). The isolation of the first mutations in the pssA, pgsA, and cds genes relied on massive screening by autoradiography of colonies defective in in situ phospholipid synthesis (6) rather than on screening and selection for defects in in vivo biosynthesis. Subsequent cloning of these genes led to large overproduction of gene products and construction of mutants carrying null alleles of pssA (29) (Step b) and pgsA (30) (Step d). Expression of the pssA or pgsA gene under conditions that allowed the measurement of limiting levels of gene product necessary to maintain normal phospholipid composition indicated that these enzymes are produced in large excess over the minimum required to sustain growth. Regulation of phospholipid metabolism appears to occur not at the level of gene expression but at the point of enzyme regulation. It is remarkable that for all of the enzymes responsible for phospholipid biosynthesis, overproduction of 10- to 100-fold has little effect on total phospholipid content or phospholipid composition (7). The only effective means of significantly altering E. coli phospholipid composition has been to decrease the level of functional gene product. Even with large alterations in phospholipid composition brought about by genetic manipulation, P1: rpk/mkv May 5, 1997 P2: rpk 14:30 204 Annual Reviews AR032-07 32-07 DOWHAN E. coli cells are surprisingly resilient and remain viable under defined conditions, which has made possible the correlation of cell phenotypes other than loss of membrane barrier function or cell viability with a particular phospholipid content or composition. Therefore, important progress has been possible in defining the roles of PE, PG, and CL in E. coli. About 5% of the head group of PE and 25% of the head group of PG turn over per generation. The inner core of LPS is decorated with ethanolamine phosphate and ethanolamine pyrophosphate, which are derived directly from the head group of PE (31). A major end product of the turnover in PE and PG is the decoration of the periplasmic membrane–derived oligosaccharides (MDO) with ethanolamine-P (not shown in Figure 1) and sn-1-glycerol-P (Step g), respectively, by direct transfer from phospholipid (32). Although stabilization of the cell against changes in the osmolarity of the growth medium appears to be a function of MDO, MDO is not essential, presumably owing to multiple mechanisms to deal with such changes. The lipid byproduct of MDO synthesis is DAG, which is recycled back to PA (Step h) by a specific DAG kinase encoded by the dgk gene. The dgk gene is normally not essential except under conditions of low osmolarity or artificial stimulation of the DAG cycle resulting in high accumulation of DAG, which is detrimental (33). Thus far DAG has not been associated with any regulatory process in E. coli, but it is interesting that E. coli has a cycle (7) analogous to the phosphatidylinositol (PI) signaling pathway of eukaryotic cells (2, 3) involving DAG, and an anionic phospholipid (PG instead of PI) that responds to external signals such as changes in osmolarity. Additional turnover of PG and PE comes from the posttranslational modification of several lipoproteins, of which the major outer membrane lipoprotein (lpp gene product) is the best studied (34). More than 70,000 copies of this protein are in the cell envelope (23). Recently this posttranslational event was established to be a transfer of DAG from PG to form a thioether linkage to a cysteine residue near the N-terminus of the preprotein form of the lipoprotein (35) prior to removal of the signal sequence N-terminal to the modified cysteine. The newly exposed N-terminal cysteine is N-acylated by fatty acid from the 1-position of the phospholipid pool (36); due to its predominance, PE is the major donor (37). This protein is assembled in trimers associated with the inner monolayer of the outer membrane minimally through its lipid moiety, and roughly one third of the monomers are covalently bound via their C-terminus to the peptidoglycan (34). Although cells lacking this protein are viable, they have a distorted outer membrane and leak many of the periplasmic components. Among the many other similar lipoproteins, some may be essential, or loss of these proteins as a whole may be lethal (34). Only a limited number of mutants or genes relating to phospholipid metabolism in other bacteria have been isolated. R. sphaeroides is a gram-negative P1: rpk/mkv May 5, 1997 P2: rpk 14:30 Annual Reviews AR032-07 32-07 PHOSPHOLIPID DIVERSITY 205 bacterium that contains PC and sulfolipids also found in plants. At low oxygen tension this organism undergoes a differentiation of the cytoplasmic membrane with the formation of an intracytoplasmic membrane containing the newly synthesized photosynthetic machinery utilized for energy production under these conditions (38). Pre-existing phospholipids are transferred from the cytoplasmic membrane to the intracytoplasmic membrane at specific times during the life cycle, whereas the proteins of this latter membrane are continually inserted. Sulfolipids and PC might be expected to play some role in photoheterotrophic growth of this organism, but mutants defective in their synthesis have no obvious phenotypes (17, 39). The methylase responsible for PC biosynthesis has been cloned and functionally expressed in E. coli (17). The presence of PC at 20% of the total phospholipid had no apparent effect on E. coli cells. Therefore, heterologous expression of eukaryotic membrane proteins in E. coli either with or without PC could be used to study the potential role of PC in both their assembly and function. Because PI can also reach up to 20% of E. coli phospholipids with no apparent deleterious effect by introduction of the yeast gene encoding PI synthase (40), similar experiments are possible to probe the requirements for PI by specific eukaryotic proteins. The pssA gene homologue has been identified and cloned from Bacillus subtilis (41). Although the B. subtilis gene can functionally replace the E. coli gene, the former gene product appears to function quite differently in E. coli. Rather than remaining at 70–80%, PE levels are over 90% when the B. subtilis enzyme replaces the E. coli enzyme, further supporting the conclusion that much of the regulation of E. coli phospholipid metabolism occurs at the level of enzyme regulation. This experiment again demonstrates the added information gained from such heterologous expression of genes encoding phospholipid biosynthetic enzymes. On the other hand, the R. sphaeroides pgsA gene functionally replaces the E. coli gene with no apparent change in E. coli properties (42). With further development of the genetics of phospholipid metabolism in diverse organisms, the ability to manipulate and design membrane architecture will expand. Heterologous expression of gene products from more complex systems in bacteria with controlled phospholipid composition has great potential to expand our understanding of the structure and function of membrane proteins from more complex organisms. Phospholipid Synthesis in Eukaryotic Organisms Phospholipid metabolism in eukaryotic cells (Figure 2) is quite similar to the bacterial pathways but more complex, owing to the added diversity in phospholipid head-group and fatty-acid composition in eukaryotic cells and, in some cases, to the presence of multiple isoforms for the activities associated with phospholipid synthesis [see (10–12, 43) for recent summaries of phospholipid P1: rpk/mkv May 5, 1997 P2: rpk 14:30 206 Annual Reviews AR032-07 32-07 DOWHAN Phosphatidic Acid 1 EtNH2 Cho 5 DAG 4 2 CDP-Cho 3 CDP-EtNH2 10 PI 9 CDP-DAG 11 12 PG, CL PC 6 Serine 7 3Me PE PS + Cho PS + EtNH2 8 Figure 2 Phospholipid biosynthetic pathway in eukaryotic cells. The full name of each lipid can be found in the text. Cho, EtNH2 , and Me refer to choline, ethanolamine, and methyl, respectively. Steps 4, 5, 6, and 11 involve multiple reactions. metabolism in eukaryotic cells including the yeast S. cerevisiae]. In eukaryotic cells, a critical branch point in metabolism lies at PA, which is partitioned between DAG (Step 1) and CDP-DAG (Step 9); the former leads to PC (Steps 2 and 4) and PE (Steps 3 and 5) via the CDP-choline (CDP-Cho) and CDPethanolamine (CDP-EtNH2 ) pathways, respectively, and the latter leads to PI (Step 10) and the mitochondrial phospholipids PG and CL (Step 11). The CTP:phosphocholine cytidylyltransferase (the second reaction in Step 4) has been the target of intense study because this enzyme is the rate limiting determinant in PC biosynthesis (12). The gene encoding this activity has been isolated from yeast (44), and several cDNAs from mammalian sources are available (45, 46); there appears to be only one isoform derived from each gene. PI is the precursor to the polyphosphorylated inositols central to lipid-dependent signal transduction. Phosphatidylserine (PS) decarboxylation (Step 8) and the methylation of PE (Step 6) occur in all eukaryotic cells. Synthesis of phospholipids in yeast proceeds by the same pathways as in somatic cells, but in addition yeast has the prokaryotic system for making PS from CDP-DAG (Step 12), which is missing in all higher eukaryotic cells. Somatic cells synthesize PS by head-group exchange between PE or PC and serine (Step 7); mutants of and cDNAs encoding these exchange enzymes have been isolated (47, 48). The recent completion of the yeast genome sequence and the growing library of somatic cell cDNA sequences will result in a rapid expansion in the cataloging of homologous genes and eventual identification of many of these genes in somatic cells responsible for phospholipid biosynthesis. Using P1: rpk/mkv May 5, 1997 P2: rpk 14:30 Annual Reviews AR032-07 32-07 PHOSPHOLIPID DIVERSITY 207 sequence homologies between species has already lead to the identification and cloning of genes or cDNAs encoding CDP-DAG synthases (Step 9) in yeast (49), Drosophila (50), and humans (51). Similarly, genes or cDNAs encoding PS decarboxylases (Step 8) have been isolated from yeast (52–55), Chinese hamster ovary cells (56), and humans (PJ Trotter & DR Voelker, unpublished results). The initial mutants in yeast phospholipid metabolism were choline auxotrophs that proved to be mutants in the CHO1/PSS gene (encodes PS synthase, Step 12) that could be bypassed in media containing choline through utilization of the CDP choline–dependent (Step 2) pathway (11). PSD mutants (Step 8) were not identified in these screens because two nonhomologous genes encode PS decarboxylases (54, 55). Although these two gene products have different subcellular locations—PSD1 gene product in the inner mitochondrial membrane and PSD2 gene product in a vacuolar-like organelle—they still substitute for each other. Why two nonhomologous decarboxylases with different locations are required by cells is still not clear but may be related to a need for phospholipid synthesis in several organelles of the cell rather than just at the major sites of synthesis, i.e. the endoplasmic reticulum and the mitochondria. In Drosophila, there are multiple isoforms of CDP-DAG synthase (Step 9), apparently made by alternate RNA splicing paths from a single CDS gene transcript (50). A mutant in one isoform specific to the photoreceptor membrane of the eye dramatically affects PI-dependent signal transduction and photoactivated responses resulting in blindness. CDP-DAG metabolism appears to be normal in other membranes and cells of this mutant. In the CDS and PSD examples, a genetic approach was used to selectively inactivate an organelle-specific pathway of phospholipid synthesis, allowing the dissection of multiple pathways and the roles of specific phospholipids in individual organelles. Targeting mitochondrial phospholipid metabolism has the greatest potential for defining phospholipid function in specific processes in eukaryotic cells. Particularly in yeast, cells are viable with mitochondria that are dysfunctional in many mitochondrial-localized processes, and CL has been thought to be essential for many of these functions. Peroxidation of CL and reduction in the steady state content of mitochondrial CL have been associated with several disease states (57). Many of these potential roles for CL can be tested once the appropriate mutants in CL synthesis are available. FUNCTIONS OF ANIONIC PHOSPHOLIPIDS In most membrane systems, the balance of charged phospholipids favors the zwitterionic phospholipids (or uncharged DAG sugars), as less than 30% of the membrane phospholipids are anionic. Evidence from a broad range of organisms and systems supports the involvement of anionic phospholipid head P1: rpk/mkv May 5, 1997 P2: rpk 14:30 208 Annual Reviews AR032-07 32-07 DOWHAN groups in the membrane association of many cytoplasmic proteins. Detergent phospholipid mixed micelles and the application of substrate dilution kinetics (58) were used to define the specific role and stoichiometry of the association of protein kinase Cs with, and their activation by, a complex of PS, DAG, and Ca2+ (2, 3). Membrane association results in a large conformational change in the kinases that exposes the active site and activates the enzymes. Whether islands of anionic phospholipids exist in membranes or are induced by association of positively charged domains of peripheral membrane proteins is not clear, but such segregation of negative charges would afford a more attractive binding site than segregation of zwitterionic phospholipids. Roles of Phosphatidylglycerol and Cardiolipin PG and CL are found in virtually all bacterial membranes but are largely confined to the mitochondrial membrane of eukaryotic cells. Specific requirements for the membrane association and activation of cytoplasmic proteins of E. coli have been demonstrated for these lipids, but because null mutants in the cls gene (which contain less than 0.1% CL) show few phenotypes (25), these functions can be assumed by PG alone. The development of a useful “biological reagent” in which the anionic phospholipid content of E. coli can be regulated has supplied the in vivo evidence for the involvement of PG in several processes. First, the construction of a null allele of the pgsA gene (Figure 1, Step d) established that anionic phospholipids are essential to E. coli (30). Because the mutant cells were not viable, it was not possible to determine the molecular basis for this requirement. Next, a single copy of the pgsA gene (59) was placed under regulation of lacOP (lactose operator-promoter) so that the level of pgsA gene expression and thus the steady state level of anionic phospholipids, PG and CL, could be varied as a function of the specific inducer of the lacOP, isopropyl thiogalactoside (IPTG). In such cells, the level of these two phospholipids can be varied from about 3% (below the limiting level necessary for growth of a wild type strain) to 20%. Surprisingly, only cell growth and not cell viability is dependent on the anionic phospholipid content. This result indicates that lack of PG does not compromise membrane integrity but does limit other functions. One such function is a source of components for modification of other membrane constituents, primarily MDO (Figure 1, Step g) and the lipoproteins. However, mutations in MDO synthesis (32) or the major outer membrane lipoprotein (34) are not lethal. A mutation lacking the major outer membrane protein (lpp gene product) is a suppressor of “leaky” pgsA mutants (60), i.e. mutants that still express low levels of synthase activity, which include the lacOP-pgsA mutant grown in the absence of IPTG (59); pgsA null mutants are not suppressed by lack of lipoprotein synthesis (30). Therefore, E. coli has an absolute requirement for PG, but there are several processes that P1: rpk/mkv May 5, 1997 P2: rpk 14:30 Annual Reviews AR032-07 32-07 PHOSPHOLIPID DIVERSITY 209 require PG at different levels. Lack of lipoprotein appears to relieve a drain on the limited PG pool in leaky pgsA mutants, allowing functions necessary for cell growth to proceed. Using this biological reagent to control cellular PG content, it has been possible to identify some of these functions of PG by both verifying in vitro observations on the putative needs for anionic phospholipids and characterizing the molecular basis for phenotypes observed in cells with low PG. INITIATION OF DNA REPLICATION In E. coli, extensive biochemical and morphological evidence exists for association of the genome with the membrane at the initiation, elongation, and termination sites (61, 62). In vitro evidence supports a requirement of anionic phospholipids in the initiation of DNA replication dependent on the DnaA protein (63–67). DnaA protein in association with either ATP or ADP (with equally high affinity and slow off rates) binds specifically to the DNA sequence at the initiation site (oriC) for DNA replication. Only the ATP form allows the remainder of the initiation complex to form at oriC and replication to begin. Slow conversion of ATP to ADP associated with DnaA protein bound to oriC inhibits continuous rounds of initiation of DNA replication. However, addition of PG or CL was found to stimulate continuous rounds of initiation; highly saturated phospholipids were not effective (68), which is consistent with the inhibition of initiation when fatty acid auxotrophs of E. coli are grown on saturated fatty acids (69). The mechanistic basis for this stimulation in vitro appears to be association of DnaA protein with anionic phospholipid–containing vesicles resulting in an increase in the rate of ADP/ATP exchange, which allows reactivation of DnaA protein for subsequent rounds of replication. Although the in vitro data supported involvement of PG and/or CL in initiation of DNA replication, the effect was not anionic phospholipid–species specific and could even be induced using nonionic detergents. Therefore, in vivo evidence was needed to establish the physiological significance of these observations. If PG is necessary for DnaA protein function, then established suppressor mutations that bypass the need for DnaA protein should also suppress the IPTGgrowth dependence of cells expressing the pgsA gene under lacOP control (70). Mutations in RNase HI (rnhA gene) bypass DnaA protein–dependent initiation at oriC by allowing constitutive stable DNA replication, i.e. random initiation divorced from cell cycle control (71–73). RNase HI normally degrades all RNA primers necessary for initiation of DNA replication, except the one at the oriC site protected by DnaA protein, thereby restricting initiation to oriC. However, if primers are available at several alternate oriT sites, constitutive stable DNA replication will initiate replication at these sites independent of DnaA protein but now dependent on RecA (recA gene) protein (74). The requirement for RecA P1: rpk/mkv May 5, 1997 P2: rpk 14:30 210 Annual Reviews AR032-07 32-07 DOWHAN protein can be suppressed by an unknown mechanism in a lexA(Def) mutant (75). Introduction of an rnhA mutation into the above lacOP-pgsA mutant suppressed its IPTG requirement for growth, and this suppression was lost in a recA mutant background (70). Introduction of a lexA(Def) mutation into the rnhA recA double mutant restored IPTG-independent growth. These mutations had no effect on phospholipid composition or the reduced expression of the pgsA gene. Interestingly, constitutive stable DNA replication cannot support growth of a pgsA null strain even in a lpp mutant background, indicating that some basal level of PG is still required to maintain other functions requiring PG. An ideal mutant to further establish the in vivo relationship between DnaA protein function and anionic phospholipids would be an altered DnaA protein that suppressed the IPTG dependence of the lacOP-pgsA mutant directly. Further evidence for a critical role of anionic phospholipids in DnaA protein (ATP-bound form) function comes from proteolysis experiments carried out on DnaA protein (76). Tryptic digestion of the 52-kDa DnaA protein yields a 30-kDa (residues 115–381) fragment in the presence of PG-containing phospholipid vesicles, and a 29-kDa fragment (residues 115–372) in the absence of vesicles. Both fragments retain high affinity for nucleotides, but only the larger fragment interacts with anionic phospholipids (not zwitterionic PC) to release tightly bound nucleotide; a 35-kDa chymotryptic fragment (residues 118–458) behaves similarly to the 30-kDa fragment. Inspection of the amino acid sequence in the region of the 1-kDa difference in the above two digestion products reveals an 18–amino acid stretch that, when displayed as an helical wheel, shows a highly hydrophobic side capable of interacting with the core of the bilayer. The specificity of functional interaction of DnaA protein with phospholipids is in marked contrast to the highly specific interaction of protein kinase Cs with PS and DAG (2). In vitro, the minimum requirement for activation of DnaA protein appears to be a fluid membrane surface with a net negative charge. Non–E. coli lipids such as PI, acidic (but not neutral) gangliosides, and acidic sphingolipids will substitute for the major anionic phospholipids of E. coli (76) in affecting the affinity of DnaA protein for ATP in vitro. This lack of specificity would suggest that DnaA protein either recognizes or recruits a domain of several anionic phospholipids on the membrane surface. The identification of a hydrophobic region associated with phospholipid interaction (76) would indicate that DnaA protein may partially insert into the membrane bilayer, following the model for other peripheral membrane proteins (2, 77, 78). This insertion may provide the energy for a conformational change, resulting in a change in affinity for nucleotides. Further support for clustering of anionic phospholipids in binding and activation of DnaA protein comes from studies with mixtures of model cationic and anionic lipid analogs with similar and P1: rpk/mkv May 5, 1997 P2: rpk 14:30 Annual Reviews AR032-07 32-07 PHOSPHOLIPID DIVERSITY 211 different hydrophobic domains (79). Independent of head-group chemistry, the analogs with similar hydrophobic domains disperse randomly within the surface of vesicles, whereas mixtures of analogs with different hydrophobic domains tend to phase-separate in clusters with similar hydrophobic domains if the energy gained from cluster formation exceeds electrostatic repulsion between like head groups. Anionic lipid analogs and not cationic lipid analogs affect DnaA protein interaction with nucleotides in the same manner as PG and CL. Mixtures of anionic and cationic analogs in which the anionic analogs cluster are an order of magnitude more effective than mixtures that do not cluster. Model studies with multivalent positively charged peptides support a cooperative interaction of the peptides with membranes dependent on the surface concentration of anionic phospholipids (80). Results with these model systems support a requirement for a critical number of anionic phospholipids to form a DnaA protein binding site, a finding consistent with a required threshold level of PG for initiation of DNA synthesis and cell growth. NUCLEOTIDE BINDING PROTEINS Is the involvement of anionic phospholipids with DnaA protein an isolated example or a more general phenomenon among DNA binding proteins? Antibodies made against either DNA or CL recognize both DNA and CL (81, 82). Single-stranded and left-handed Z DNA binding proteins (83), including RecA protein of E. coli (84), also show high affinity for PG and CL. SV40 T antigen, the initiator of SV40 DNA replication, is inhibited in vitro specifically by CL out of several anionic and zwitterionic phospholipids tested (85). Both prokaryotic (86) and eukaryotic (87) DNA topoisomerases I have affinity for anionic phospholipids. The drug chlorpromazine, which binds anionic phospholipids, relieves the inhibition by PG and CL of the in vitro DNA relaxation activity of the enzyme, and addition of chlorpromazine to growing cells relaxes plasmid DNA dependent on the presence of the topoisomerase. These results suggest that inactive enzyme is stored bound to membranes and is activated in response to stimuli that affect membrane phospholipid structure. Associations of anionic phospholipids with mitochondrial and nuclear DNA polymerases have been reported that, in many cases, also resulted in inhibition of function (88, 89). Finally, the signaling lipid, phosphatidylinositol 4,5-bisphosphate, specifically accelerates GTP/GDP exchange and stimulates GTPase activity when bound to the G-proteins dynamin (90) and ADP-ribosylation factor I (91) in a manner analogous to the effect of anionic phospholipids on DnaA protein interaction with ATP. This abbreviated list of defined effects of anionic phospholipids on enzyme functions shows that serious consideration should be given to more extensive studies on the interaction and effects of anionic phospholipids on many of the enzymes that bind DNA or nucleotides. P1: rpk/mkv May 5, 1997 P2: rpk 14:30 212 Annual Reviews AR032-07 32-07 DOWHAN TRANSLOCATION OF PROTEINS ACROSS MEMBRANES There are many features common to prokaryotic and eukaryotic cells in the mechanisms for distribution of proteins synthesized in the cytoplasm to their final site of function (92). Proteins must contain encoded information to direct them to their final location and to interact with the membrane-associated machinery responsible for translocation of these proteins across the membrane of the target organelles. Because membranes are an integral part of these distribution processes, the specific role of phospholipids cannot be ignored. The most detailed understanding of the role of anionic phospholipids at the molecular level in the translocation of proteins across membranes comes from studies in E. coli [see (93, 94) for a summary of this process]. Preproteins are targeted to the membrane-associated preprotein translocase complex by their N-terminal leader sequences characterized by a positively charged domain followed by a hydrophobic domain. Preproteins are retarded in their folding by their N-terminal leader sequences and recognized by various molecular chaperones, such as SecB, that are required to deliver unfolded precursors to the membrane (95, 96). This translocase complex is composed of the integral membrane proteins SecYEG, which are sufficient for in vitro function, and possibly additional proteins, such as SecD and SecF (97), required for optimal efficiency in vivo. A critical cytoplasmic component of the translocase is SecA, which binds to the chaperone-precursor complex and to SecY, dependent on the presence of anionic phospholipids in the membrane (98). Translocation of precursor protein is an energy-driven process that uses cycles of ATP binding and hydrolysis to drive a large portion of SecA across the membrane to the periplasmic side (99) and is postulated to be coupled to export of segments of preproteins in association with SecA (93). Thus, not only is SecA bound through anionic phospholipids to the membrane surface, but it is transiently a transmembrane protein. The process is further rendered irreversible by removal of the leader sequence in the periplasm by leader peptidase. Although considerable in vitro biochemical data suggested that anionic phospholipids may play an important role in protein translocation, definitive in vivo data first came from the observation that in cells (pgsA3 lpp) growing near limiting levels of anionic phospholipids (100), the precursors to outer membrane proteins such as OmpA and PhoE accumulated in the cytoplasm, indicating a significant retardation in their translocation. Reconstitution of protein translocation in vitro using PG-depleted inverted membrane vesicles showed these vesicles to be defective in supporting protein translocation; mutants lacking only CL were normal. The cytoplasmic SecA component of the translocase complex has low ATPase activity, which is activated upon membrane association. In a lacOP-pgsA strain, the rate of translocation of precursors across the membrane in vivo is proportional to the level of IPTG induction of anionic P1: rpk/mkv May 5, 1997 P2: rpk 14:30 Annual Reviews AR032-07 32-07 PHOSPHOLIPID DIVERSITY 213 phospholipid biosynthesis (101, 102). Isolated membranes from cells grown at different IPTG levels demonstrated a direct relationship between their anionic phospholipid content and (a) the affinity of cytoplasmic SecA for isolated membranes, (b) the level of stimulation of latent ATPase activity by these membranes, and (c) the effectiveness of these membranes in supporting in vitro reconstituted protein translocation. Membranes depleted in anionic phospholipids and defective in in vitro protein translocation can be reactivated (102, 103) in a dose-response manner by the incorporation of anionic, but not zwitterionic, phospholipids. As with the DnaA protein, the only requirement is head-group charge with no specificity for structure of the head group. SecA will bind to anionic phospholipid–containing vesicles and become activated in the absence of other proteins. However, the nature of SecA and its affinity for membranes are enhanced by the assembly of a complete translocation complex including the precursor protein (101, 103). Hydrolysis of ATP tightly coupled to translocation requires the complete translocase complex (93), suggesting that SecA association with the membrane is a multistep process. Initial weak association with the membrane appears to be ionic, via positively charged domains on this mostly acidic protein, followed by a conformational change, evidenced by an increased sensitivity to trypsin (78, 104). Insertion of part of the protein into the membrane has been verified by changes in tryptophan fluorescence (78) and by exposure of the protein on the periplasmic side of the membrane during active protein translocation (99, 105). Direct insertion into the hydrophobic domain of phospholipid monolayers has been demonstrated, and ATP antagonizes association and penetration of SecA (77). This membrane association is strengthened and given direction and purpose through the added specificity of association of SecA with the complete translocase complex. Although translocation of preproteins appears to occur through a proteinaceous pore in most membranes (106, 107), nascent precursor proteins have been shown to come in contact with the core of the phospholipid bilayer at an early step during entry into the endoplasmic reticulum (107). Leader sequence association with the membrane appears to involve anionic phospholipids in SecA-independent protein translocation, suggesting that such an interaction may also be important for efficient SecA-dependent protein translocation. Phage M13 procoat protein is translocated across membranes independent of the SecA system. However, this translocation is still dependent on a consensus leader sequence and anionic phospholipids (108). In model systems, association of procoat with phospholipid vesicles is dependent on acidic phospholipids and the positive character of the leader sequence. Using the lacOP-pgsA strain, a dependence on anionic phospholipids for procoat translocation and formation of coat protein was demonstrated both in vivo and in vitro. Incorporation of anionic phospholipids into PG-depleted membranes P1: rpk/mkv May 5, 1997 P2: rpk 14:30 214 Annual Reviews AR032-07 32-07 DOWHAN restores efficient procoat translocation in vitro. Variation in both the positive charge content of leader sequences of precursor proteins and the anionic phospholipid content of target membranes demonstrated a direct correlation between these two parameters and translocation efficiency (109). In model phospholipid vesicle systems, the leader sequences of precursor proteins have been demonstrated to bind specifically to anionic phospholipids and insert into the bilayer with the formation of defined helix-break-helix secondary structure (110–112); this structure also appears to be assumed by the leader sequence as part of the complete precursor protein (113). A theoretical stereochemical analysis of the general structural motifs characteristic of leader peptides and various phospholipids supports the formation of a hydrophobic α-helical leaderpeptide–anionic-phospholipid complex during the insertion and translocation of leader sequences across the membrane (114). PG showed preference over CL in stabilizing this complex. Therefore, it is quite clear that anionic phospholipids play multiple and specific roles in the complex process of protein translocation in prokaryotic cells. ANIONIC PHOSPHOLIPIDS IN EUKARYOTIC CELLS There are many examples in the literature, mainly from in vitro studies, of involvement of anionic phospholipids in eukaryotic cells similar to that seen in prokaryotic systems. What is generally lacking is genetic evidence to verify the physiological significance of these in vitro results. Leader sequences for mitochondrial precursor proteins encoded by nuclear genes have a basic/hydrophobic amino acid motif similar to that found in bacteria (92). These sequences have been shown to interact specifically with anionic phospholipid vesicles as amphipathic helices and insert into the bilayer independent of other protein factors (115–117). Import of proteins into mitochondria is inhibited by andriamycin, an antibiotic with high affinity for anionic phospholipids (118, 119). Such results suggest that anionic phospholipids of the mitochondria (PG and CL in somatic cells and primarily CL in yeast) are involved in assembling the protein translocase of the mitochondria and/or interacting directly with the leader sequences. Due to its intracellular distribution, CL has been postulated to be an essential and specific component of many mitochondrial functions such as electron transport, ion permeability, membrane integrity, protein import, and solute transport, and has been intensively studied as such (120). Most of the evidence for the requirement of several mitochondrial proteins for CL comes from extensive purification resulting in loss of activity, which could be restored by the addition of CL. The ADP/ATP carrier, the most abundant protein in mitochondrial membranes, has six CLs tightly bound to lysines and possibly several more loosely associated CLs (121). Removal of these tightly bound lipids renders the carrier inactive, but activity can be reconstituted by addition of CL. A mutant of the P1: rpk/mkv May 5, 1997 P2: rpk 14:30 Annual Reviews AR032-07 32-07 PHOSPHOLIPID DIVERSITY 215 carrier has been isolated that functions normally in vivo but after purification is active only when supplemented with CL (122). The purified mutant protein was found to contain very little tightly bound CL, suggesting a reduced affinity for CL only revealed when phospholipids are removed during purification. In the presence of Ca2+ , the ADP/ATP carrier has been shown to also function as a large unselective conductance channel in both isolated mitochondria and in liposomes reconstituted with the purified protein (123). Since CL has higher affinity for Ca2+ than for Mg2+ , it was postulated that specific binding of Ca2+ to tightly associated CL disrupts its interaction with lysine residues on the protein, resulting in a conformational change that opens the channel. Not considered by the authors is the unique property of CL to form nonbilayer structures in the presence of divalent cations, which may also alter the local lipid structure and induce changes in protein conformation. The requirement for CL by the cytochrome c oxidase, the terminal enzyme in the oxidative phosphorylation process, has been extensively studied. Its substrate, cytochrome c, also interacts in vitro with CL and PG (124, 125). CL-depleted cytochrome oxidase (0.2 mol CL per mol) was fully active in the non-ionic detergent Tween 80, but in the absence of detergent, it required phospholipid with a distinct preference for CL over dimyristoyl-PC (126). Although the tight binding of CL to the oxidase has always been assumed to be due to an essential role in its function, the substitution of Tween 80 indicates that no unique property of CL is necessary for activity. In reconstituted proteoliposomes, CL may have a higher affinity for the surface of the oxidase than other phospholipids. In addition, the interaction of its substrate, cytochrome c, with anionic phospholipid vesicles may play a role in the formation of the enzyme substrate complex. The recently available crystal structure of bovine heart cytochrome oxidase does not show any specifically bound CL, but does show PE and PG specifically bound (127). Still unresolved in the core of the structure is remaining phosphate-containing material that may be CL. Preliminary genetic evidence for a role of the anionic phospholipids PG and CL in mitochondrial function is available (128, 129). Mutants of Chinese hamster ovary cells with a conditionally lethal temperature-sensitive defect in PG/CL biosynthesis have been reported. Mutants shifted to the restrictive temperature showed multiple defects in mitochondrial function. Complex I (rotenone-sensitive NADH oxidase) was the most compromised of the aerobic energy–producing complexes of the mitochondria. The mitochondria also showed gross changes in morphology. Although these lipid mutants have properties similar to those of many mutants of yeast with specific defects in electron transport, they differ in that the lethality of the mutation cannot be suppressed by growth on glucose. Thus, PG and CL are required for more than respiration. The gross morphological changes in the mutant might be related to P1: rpk/mkv May 5, 1997 P2: rpk 14:30 216 Annual Reviews AR032-07 32-07 DOWHAN reduced mitochondrial ATP (the source would then be glycolysis) as a result of a dysfunctional ATP/ADP carrier that appears to require CL for function (122). Because mammalian mitochondria have significant levels of both PG and CL, the defects in this mutant cannot be attributed specifically to lack of either phospholipid, and as in E. coli, many functions may be supported by either lipid. What is needed are tighter mutants that completely shut off anionic phospholipid biosynthesis, and mutants analogous to the lacOP-pgsA allele of E. coli in which lipid composition can be controlled. Given the complexity of mammalian cells, a better system for studying the role of these anionic phospholipids might be yeast. FUNCTIONS OF ZWITTERIONIC PHOSPHOLIPIDS The zwitterionic phospholipids, mainly PC and/or PE, together comprise the majority of the membrane phospholipids of eukaryotic cells, gram-negative bacteria, and many gram-positive bacteria. PC and PE have been treated as interchangeable in many experimental designs. Due to the more desirable physical properties of PC in forming vesicles and defined structures in solution, PC has been preferentially used in many in vitro studies. However, there are significant and important differences in chemistry and properties between these two lipids. PE has a smaller head group, can hydrogen bond through its ionizable amine, and has the unique property shared with divalent cation–CL complexes of undergoing a bilayer-to-nonbilayer physical transition (discussed below) influenced by its fatty acid content and the temperature (130). Both PC and PE possess a dipole moment across their respective head groups that can be oriented by the membrane electrochemical potential (131). Since only PE can hydrogen bond, the response to the electrochemical potential would be influenced differently by the immediate environment. It is clear that PC and PE are not exchangeable with respect to their properties, but it is also now clear that they are not functionally exchangeable in supporting biological processes. Therefore, experimental design involving lipids must be governed by the lipid specificity of the system and not by the convenience of using a particular lipid in an in vitro experiment. This is especially true in studies of bacterial systems, such as E. coli, that do not contain PC. Roles of Phosphatidylethanolamine The only system in which both genetic and biochemical evidence exists for specific roles of PE in cell function is E. coli. Conditional lethal mutations in the pssA (132, 133) or psd (134) gene of E. coli (Figure 1, Steps b and c) have established a critical requirement for PE that is not unreasonable because this lipid accounts for the majority of the membrane phospholipids. However, it was P1: rpk/mkv May 5, 1997 P2: rpk 14:30 Annual Reviews AR032-07 32-07 PHOSPHOLIPID DIVERSITY 217 observed that supplementation of the growth medium with 20–50 mM Mg2+ suppressed the growth defect of these mutants, which subsequently grew at considerably reduced levels of PE. The surprising result was that a strain carrying a null allele of pssA is viable dependent on divalent cation supplementation of the growth medium (29). This mutant lacks PE (<0.1% versus the normal 70–80%) and PS but has a normal fatty acid composition and phospholipid-toprotein ratio. No new phospholipid species were present, although there was some elevation in the minor lipids PA and CDP-DAG. PG and CL account for 90% of the total phospholipid in about equal molar amounts when grown in Mg2+ or Sr2+ and in about a 2:1 molar ratio when grown in Ca2+ (29, 135). The lipid A component of LPS-to-phospholipid ratio is also normal (J Williamson & CRH Raetz, personal communication), indicating that the outer membrane bilayer is intact. However, the LPS fraction showed altered mobility on sodium dodecyl sulfate (SDS) polyacrylamide gels, indicating a change in charge probably due to lack of decoration with ethanolamine normally derived from PE (136). Although the mutant grows in rich medium (supplemented with divalent cations) with generation times only twice that of wild type cells, it displays a complex phenotype including filamentous growth. Growth in minimal defined media is very poor and requires supplementation with amino acids including tryptophan. The cells enter the stationary phase at a much reduced cell density, die and lyse when divalent cations are removed, have an increased sensitivity to several antibiotics, have defects in electron transport (137), are compromised in several secondary transport systems for sugars and amino acids (138), have defects in the Cpx membrane–associated signal transduction system (139), and are defective in motility and chemotaxis (140). Therefore, basic life functions can be maintained without PE, but many normally important but nonessential life processes are compromised. This biological reagent poses on the one hand a challenge to sort out biochemically the complex pleiotropic phenotype of this mutant, but on the other hand it affords an excellent opportunity to relate its phenotypes to in vitro–determined biochemical requirements for PE. SOLUTE TRANSPORT Detailed evidence shows that PE has a specific involvement in supporting active transport by the lactose permease of E. coli in reconstituted proteoliposomes (141, 142). Proteoliposomes lacking PE and made from PG and CL could only support facilitated transport by the permease. Interestingly, PC could not substitute for PE in supporting active transport, and monomethyl- and dimethyl-PE were progressively less effective than PE. The turnover of substrate rather than substrate binding was the step requiring unsaturated PE (143). Lipid composition of these vesicles did not affect the direction or magnitude of an artificially imposed electrochemical gradient across the P1: rpk/mkv May 5, 1997 P2: rpk 14:30 218 Annual Reviews AR032-07 32-07 DOWHAN membranes, but clearly PE was necessary for the permease to couple transport with this gradient. The E. coli mutant carrying the null allele of pssA and lacking PE was used to investigate the requirement for PE for active transport in vivo (138). Active uptake of both lactose and its analogs was reduced 5- to 10-fold in both rate and extent in PE-deficient cells as compared to the mutant strain carrying a plasmid-borne copy of the pssA gene and normal PE levels. Nonenergy-dependent, facilitated transport was normal in the absence of PE. Uphill efflux from energized inverted membrane vesicles preloaded with substrate was supported by vesicles isolated from PE-containing but not PE-deficient cells. The defect in PE-containing cells and membranes was not due to a reduced proton electrochemical potential across the membrane (137, 138, 144) or to a reduced amount of permease assembled in the membrane. Several other transport systems have been reported to require or be stimulated by PE. These include the high affinity proline transport protein (145) and the melibiose transport protein of E. coli (146), the sodium-dependent leucine transport system of Pseudomonas aeruginosa (147), the branched-chain amino acid carrier of E. coli (148), and several amino acid transport systems of bacilli species (149). In the PE-deficient mutant of E. coli, active uptake of proline, tryptophan, and lysine could not be detected (M Bogdanov & W Dowhan, unpublished result). Therefore, the original in vitro observations suggesting a specific role for PE in supporting active transport by the lactose permease, which has now been verified to be important in vivo, appear to be of broad importance to the function of several solute transport systems. The molecular basis for this uncoupling of metabolic energy from solute transport remains to be determined. MEMBRANE PROTEIN ASSEMBLY If large changes in the anionic phospholipid content affect secretion and assembly of membrane proteins, then one might expect additional effects in membranes lacking PE. The SecA-dependent protein translocase system appears to function normally in E. coli lacking PE (144). However, the requirement for divalent cations is carried over to the in vitro reconstituted system made up of PE-deficient membranes. This requirement appears to be a structural one for CL in membranes lacking PE, which is discussed below in the section on Lipid Polymorphism. However, because the membrane phospholipid matrix replaces the cytoplasm as the solvent for integral membrane proteins, it should not be surprising that phospholipids play a large role in the folding and assembly of membrane proteins. It is clear that a growing number of auxiliary proteins are required during the assembly of membrane proteins to guide the folding path for the proteins and to aid in the transition from the cytoplasmic to the membrane environment (150). These chaperones generally maintain a partially unfolded state for the proteins P1: rpk/mkv May 5, 1997 P2: rpk 14:30 Annual Reviews AR032-07 32-07 PHOSPHOLIPID DIVERSITY 219 to prevent the initiation of dead-end folding pathways prior to membrane insertion. Therefore, chaperones act in a transient manner during the assembly process but are not required once the protein is assembled. The strict definition of a chaperone excludes the furnishing of additional structural information during the protein assembly process. What has not been considered is whether phospholipids act as molecular chaperones in guiding the assembly process but are no longer necessary once the properly folded state is attained. Certainly the general amphipathic property of all ionic lipids influences and determines membrane protein structure, but this solvent property of phospholipids can be substituted in many cases by detergents once the protein is properly folded. Do individual phospholipid species have specific roles in the membrane protein assembly process? The lack of full function of lactose permease assembled in the absence of PE appears to be due in part to its misassembly. The first indication of this misassembly came from studies (151) using a monoclonal antibody directed against a conformationally sensitive continuous epitope in the periplasmic loop of the permease between transmembrane domains VII and VIII (152). Binding of monoclonal antibody to spheroplasts or right-side-out inner membrane vesicles of E. coli uncoupled permease function from the proton electrochemical potential but still allowed facilitated transport, as did assembly of the permease in membranes lacking PE (138). This monoclonal antibody did not bind to the permease in spheroplasts or right-side membrane vesicles prepared from PE-deficient (pssA null allele) E. coli cells (151). The monoclonal antibody still detected permease from wild type cells after SDS polyacrylamide gel electrophoresis and transfer to a solid support in a standard Western blot analysis, but it did not recognize the permease derived from PE-deficient cells; there appeared to be no phospholipid associated with the permease prior to transfer to the solid support. These results suggest that PE is necessary during the assembly of the permease in order to form the proper conformation within this periplasmic loop, but once formed, PE is no longer necessary to maintain this conformation. Development of a novel adaptation of the Western blot procedure allowed the reconstitution of partially denatured permease in vitro in the presence of phospholipids. Phospholipids were first applied to the solid support (153) prior to transfer of the proteins from the polyacrylamide gel (151). During this transfer, SDS was removed electrophoretically as the partially denatured proteins refolded in the presence of hydrated phospholipids. Remarkably, permease derived from PE-deficient cells regained its conformationally sensitive epitope if renatured specifically in the presence of either total E. coli phospholipids or PE alone. Neither PG, CL, lyso-PE, nor PC could substitute for PE. Therefore, PE appears to be specifically required and sufficient for the proper folding of this conformationally sensitive epitope. Misfolding was not due to the high content P1: rpk/mkv May 5, 1997 P2: rpk 14:30 220 Annual Reviews AR032-07 32-07 DOWHAN of PG and CL but to the lack of a positive determinant (PE) of proper folding. Renaturation of membrane proteins from detergents in the presence of phospholipids has been widely used, but few examples exist of such a specificity for a particular phospholipid. This modified Western blotting procedure utilizing phospholipids should be generally applicable to other membrane proteins to probe lipid-dependent conformations or activities directly on a solid support. PE appears to play an even more fundamental role in the assembly of the lactose permease in that its presence is required for proper topological organizing of the permease in the inner membrane. Encoded within the primary structure of membrane proteins are topogenic signals that must interact with topological determinants in the membrane and the protein assembly machinery to properly orient the transmembrane helices characteristic of many membrane proteins (105, 154–157). Lactose permease structure is a paradigm for secondary transport proteins throughout nature in that it has 12 hydrophobic α-helical transmembrane spanning domains (158, 159). Orientation of the alternating periplasmic and cytoplasmic hydrophilic loops between these domains follows the general “positive inside rule” in that the net charge for cytoplasmic loops is positive and the net charge for periplasmic loops is either neutral or negative (156). Several mechanisms have been proposed for attainment of this orientation including charge interaction of the net positive loops with the anionic phospholipids on the cytoplasmic side of the membrane (160). In PE-deficient membranes this interaction would be amplified, so if this were the only factor in determining membrane protein topology, proteins would still be properly oriented. However, at least for the lactose permease, this appears not to be the case. A derivative of the permease containing an engineered Factor Xa protease site in the cytoplasmic domain between transmembrane domains VI and VII is fully functional and assembled properly in PE-containing membranes, as shown by proteolysis studies of oriented membrane vesicles (161). However, when assembled in PE-deficient membranes, this cytoplasmic domain is now exposed to the periplasmic side of the membrane (162). Clearly, not all membrane proteins are misassembled or misoriented in PE-deficient cells. The essential leader peptidase of E. coli has been shown to be properly oriented in PE-deficient membranes (144). However, the lack of proper functioning of several secondary transport systems in PE-deficient membranes (M Bogdanov & W Dowhan, unpublished results) suggests that additional proteins with structural homology to lactose permease may also be misassembled. Because only a subset of structurally similar proteins may be affected by lack of PE during assembly, specific interactions between topogenic signals on these proteins with a topological determinant in the membrane such as PE may be the missing signal for proper assembly. However, the current data do not rule out an indirect effect of the lack of PE on the integral membrane P1: rpk/mkv May 5, 1997 P2: rpk 14:30 Annual Reviews AR032-07 32-07 PHOSPHOLIPID DIVERSITY 221 protein assembly machinery. The assembly of integral membrane proteins is not as well understood as the SecA-dependent translocation of proteins across membranes. Lactose permease assembly appears not to be dependent on the SecA-dependent translocase (164). However, the export of the periplasmic domain of inner membrane–localized leader peptidase (165) and the export of alkaline phosphatase fused to inner membrane proteins (166) require the SecAdependent translocase complex, which does function normally in PE-deficient cells (144). So any indirect effect of PE would have to be on the remaining uncharacterized machinery for integral membrane proteins and would only affect a subset of membrane proteins. Uncovering these novel effects of PE on the assembly of the lactose permease in particular and possibly other membrane proteins would have been very difficult without the development of strains of E. coli lacking PE. Now that such effects have been recognized, more detailed studies are warranted to determine the molecular basis for misassembly of proteins in membranes lacking PE. Several examples of a specific chaperone-like function for lipids have been reported. The nuclear encoded mitochondrial protein rhodanese is assisted in its folding by protein chaperones as well as detergents (167). Although the denatured protein can be refolded in an active form in the presence of detergent alone, inclusion of CL in the micelles resulted in a slower rate of renaturation but an increased yield of active protein in a CL dose–responsive manner (168); inclusion of PC had no effect on the renaturation process. Denatured but not native rhodanese showed highly specific interaction with CL vesicles apparently trapping a folding intermediate that required the addition of detergent to complete the renaturation process (169, 170). Therefore, it is possible that CL acts as a molecular chaperone during the movement of rhodanese across the inner mitochondrial membrane and maintains a specific unfolded intermediate that is delivered to chaperones in the matrix of mitochondria to complete the folding process. Similarly, LPS may act as a molecular chaperone in the assembly of outer membrane proteins of E. coli (171). In vitro synthesis of the outer membrane protein PhoE in the presence of very low amounts of the detergent Triton X-100 results in a transient assembly-competent intermediate that requires the presence of LPS and divalent cation to proceed to a stable folded monomer form of the protein. However, the final assembly of the protein into its native trimer is inhibited by LPS. Incubation of these monomers in the presence of increased amounts of Triton X-100 and purified outer membranes results in formation of the final trimer associated with the membranes with trimerization preceding membrane insertion. These results are consistent with LPS acting transiently as a molecular chaperone in the formation of the stable monomer because dissociation of LPS appears to be required prior to trimerization and insertion into the outer membrane, which of course is the P1: rpk/mkv May 5, 1997 P2: rpk 14:30 222 Annual Reviews AR032-07 32-07 DOWHAN location of previously made LPS. These results are supported by the fact that altered LPS from deep-rough mutants will not support stable monomer formation, but outer membranes from deep-rough mutants are competent recipients of the trimer (171). Previous data also indicate that newly synthesized LPS, rather than preformed LPS, is necessary for proper assembly of outer membrane proteins (172, 173). LIPID POLYMORPHISM Classical representations of phospholipid organization in membranes (Figure 3) focus on lamellar or bilayer structures such as the ordered gel state (Lβ ) or fluid liquid crystalline state (Lα ). However, some natural phospholipids in vitro can assume nonbilayer structures such as the reversed hexagonal (HII ) phase, as has been documented with X-ray crystallography and nuclear magnetic resonance (NMR) (174). Although an extensive treatment of the physical chemistry of lipid polymorphism is beyond the scope of this review (see 27, 174–176), the dominant structural feature allowing nonbilayer structures for lipids is a small Figure 3 Structural representation of the arrangement of phospholipids. Arrangement of the head-group and fatty-acid domains of phospholipids in the fluid liquid crystalline state (Lα ), the ordered gel state (Lβ), and the reversed hexagonal state (HII ). P1: rpk/mkv May 5, 1997 P2: rpk 14:30 Annual Reviews AR032-07 32-07 PHOSPHOLIPID DIVERSITY 223 polar head group associated with a large hydrophobic domain, i.e. a cone shape for the lipid. Extensive and permanent amounts of such nonbilayer structures would compromise the barrier function of membranes. However, bilayer lipids have a different intrinsic radius of curvature from nonbilayer lipids, so changes in the proportion of these two types of lipids will change the collective physical properties of the membrane. The HII phase is favored by high temperature and cis-unsaturated fatty acids and can be induced by cholesterol owing to its cone shape (177). The HII phase potential for a phospholipid mixture can be assessed by determining the TLH (temperature at mid-point) for the transition from lamellar to nonbilayer phase using 31 P NMR (178). The higher the TLH , the lower the potential to form the HII phase. The TLH , like the Tm (temperature at the mid-point of transition) for the Lβ -to-Lα transition, is a measure of a distinct collective property of the phospholipids making up a particular bilayer. Every biological membrane system has at least one nonbilayer-forming lipid component (27, 174), so lipid polymorphism is the rule, not the exception, in biological membranes. PC is generally considered a bilayer-forming lipid that only forms nonbilayer structures under extreme conditions or in the presence of nonbilayer-forming lipids. CL and PA in the presence of select divalent cations (mainly Ca2+ , Mg2+ , or Sr2+ , but not Ba2+ ), PE, and monogalactosyl/monoglucosyl DAGs (MGDG) can form nonbilayer structures under physiological conditions (27). The latter neutral lipids are found in high concentrations in plants, many gram-positive bacteria that lack PE, and bacteria such as Acholeplasma laidlawii, which also lacks PE and a cell wall. The potential to form nonbilayer structures may provide nonleaky discontinuity in the bilayer structure of membranes for the following important biological functions: membrane fusion and membrane vesicle formation during cell division and vesicle-mediated protein trafficking; integration of nonlipid components into the membrane bilayer; movement of macromolecules through the membrane; lateral movement of macromolecules within the bilayer; stabilization of specific membrane protein complexes; and conformational interconversions necessary to protein function. Extensive studies of lipid polymorphism have been carried out on A. laidlawii because this organism alters its mixture of bilayer and nonbilayer lipids in response to changes in growth conditions (177). The ratio of MGDG (nonbilayer forming) to DGDG (the disaccharide form of this nonionic lipid, which only assumes the bilayer configuration) determines the intrinsic radius of curvature of the membrane surface or the HII phase potential to form nonlayer structures within the membrane (179). The nonbilayer state for MGDG is favored by high temperature and unsaturation in its fatty acids. At a given growth temperature, the MGDG to DGDG ratio is inversely proportional to the unsaturated fatty acid content of MGDG. When cell growth temperature is lowered, A. laidlawii either P1: rpk/mkv May 5, 1997 P2: rpk 14:30 224 Annual Reviews AR032-07 32-07 DOWHAN increases incorporation of unsaturated fatty acids if available in the medium or increases the ratio of MGDG to DGDG to adjust the HII phase potential of its lipids to remain just below the transition from bilayer to nonbilayer. Incorporation of hydrocarbons, alcohols, detergents, and cholesterol into the membranes of this organism also alters the ratio of MGDG to DGDG consistent with maintaining a constant HII phase potential near but below the beginning of the Lα to HII phase transition (180). These adjustments appear to maintain the physical properties of the membrane well within that of the Lα state but with a constant potential for forming nonbilayer structures. Bacillus megaterium (181) and Clostridium butyricum (182, 183), which do not contain MGDG, regulate their membrane lipid polymorphism through the composition of their phospholipid fatty acids. E. coli phospholipid head-group composition is invariant under a broad range of growth conditions (7) when compared to A. laidlawii, and E. coli maintains its content of primarily nonbilayer forming lipids (PE and CL) within a narrow range (7). However, complete elimination of PE in E. coli null pssA mutants uncovered regulatory processes that appear to respond to a similar requirement for a constant but low HII phase potential as outlined above for A. laidlawii. PE-deficient E. coli require divalent cations in the following order of effectiveness to support growth: Ca2+ > Mg2+ > Sr2+ (29); neither Ba2+ nor monovalent cations will substitute (135). Although mutants of E. coli lacking either PE or CL are viable, those lacking both phospholipids are not viable even in the presence of divalent cations. Surprisingly, PE-deficient mutants regulate their CL content in response to the nonbilayer-forming potential of the divalent cation present in the growth medium (135). The lipids extracted from cells grown in 50 mM Ca2+ or Mg2+ and suspended in the same cation had a TLH (approximately 10◦ C above the growth temperature) similar to lipids extracted from wild type cells, which showed no dependence on cations. CL content of cells grown in the presence of Ca2+ was about 60% of that grown in Mg2+ , consistent with the higher potential for the former cation in inducing the HII phase. When phospholipids from cells grown in Ca2+ were suspended in Mg2+ -containing buffer, the TLH was raised by about 20◦ C, whereas suspension in Ca2+ -containing buffer of phospholipids from cells grown in Mg2+ resulted in a drop in TLH of about 20◦ C, which was well below the growth temperature. Suspensions of these phospholipids either in Ba2+ , in the absence of cations, or in high concentrations of monovalent cations did not undergo an Lα to HII phase transition, which paralleled the lack of cell growth under these ionic conditions. The surface charge screening, acyl chain order, and lipid-packing parameters of phospholipids derived from PE-deficient cells were the same in the presence of all divalent cations including Ba2+ , leaving the nonbilayer transition as the only physical parameter not supported by Ba2+ (184). The divalent P1: rpk/mkv May 5, 1997 P2: rpk 14:30 Annual Reviews AR032-07 32-07 PHOSPHOLIPID DIVERSITY 225 metal ion effects and requirements appear to be for the outer leaflet of the inner membrane and/or the inner leaflet of the outer membrane, because E. coli actively excludes Ca2+ and Sr2+ from the cytoplasm (185), whereas Mg2+ is normally maintained at high levels inside cells. Does wild type E. coli adjust the HII phase potential of its phospholipids? Although there is a large literature documenting the importance of a fluid bilayer and the relation of the Tm for the membrane lipids to the physiology of E. coli (24), few experiments have addressed the TLH as a function of growth temperature and phospholipid composition. Optimum cell function and cell viability require the Lα phase, which is achieved by maintaining the Tm of the phospholipid pool approximately 7–17◦ C below the growth temperature (186, 187) through regulation at the biosynthetic level of the degree of unsaturation of the fatty acid pool (188). However, an equally important property of the bilayer also depends on the degree of unsaturation within primarily the PE fraction that determines the TLH for the membrane lipids (26). Although the outer membrane is enriched in PE, the TLH for the inner membrane phospholipids from cells grown at several different temperatures is lower than for the total phospholipid pool (inner and outer membrane combined) owing to the enrichment of the inner membrane PE in unsaturated fatty acids, which lowers the TLH . The beginning of the Lα to HII phase transition for the inner membrane phospholipid pool is adjusted to be 10–15◦ C above the growth temperature of the cells as compared to the total phospholipid pool, beginning at 20–25◦ C above the growth temperature. This result indicates that the TLH for the outer membrane phospholipids alone is considerably higher than that of the inner membrane phospholipids, suggesting a specific requirement for nonbilayer-forming potential in the multiple functions of the inner membrane and possibly no requirement for outer membrane function. Wild type E. coli appears to balance unsaturated fatty acid content rather than phospholipid head-group content in order to maintain the Lα phase in a window between the two extremes of the Lβ phase favored by saturated fatty acids and the HII phase favored by unsaturated fatty acids (26). It is obvious why prevention of the nonbilayer state is essential, but it is not immediately apparent why HII phase potential is necessary. However, the divalent cation effects on mutants lacking PE strongly support a biological requirement for HII phase potential even when the lipids are in the Lα phase. As noted earlier, PE-deficient cells carry out normal protein translocation, but inner membrane vesicles isolated from these cells require the nonbilayer-inducing cations to be functional in protein translocation (144). In particular, the divalent cation is required on the inside of inverted membrane vesicles (periplasmic side) and can be partially compensated for in vitro by introduction of nonbilayerforming PE-derivatives into the vesicle membranes, but not by derivatives of PE (or PC) that cannot assume a nonbilayer configuration. The PE-deficient P1: rpk/mkv May 5, 1997 P2: rpk 14:30 226 Annual Reviews AR032-07 32-07 DOWHAN mutant grown in the presence of Mg2+ and then suspended in Ca2+ under conditions that would favor the HII phase for its lipids showed no disruption of the normal bilayer structure through formation of the HII phase as visualized by electron microscopy (189). However, introduction of Ca2+ into the cytoplasm using an ionophore resulted in large physical changes in the inner membrane and the formation of structures characteristic of the HII phase. Therefore, the inner leaflet of the inner membrane was able to maintain a bilayer structure in the face of the large nonbilayer potential imposed on the outer leaflet by the presence of Ca2+ . A similar scenario probably takes place at the outer membrane with the inner phospholipid leaflet possessing high nonbilayer potential (due to high PE content in wild type cells or CL in the mutant) while maintaining a bilayer configuration imposed by the outer leaflet monolayer of lipid A. Several recent reports have also suggested a role for HII phase potential in eukaryotic cells. CL is the most effective activator of the mitochondrial cytochrome P450SCC, but saturated branched-chain derivatives of PC that mimic the phospholipid polymorphic properties of CL are equally effective activators (190), indicating that the physical properties of the lipid are more important than the head-group charge. Anthracyclines such as daunomycin are effective antitumor drugs that appear to work without entering the cell. In model phospholipid studies, daunomycin significantly increased TLH but had no effect on Tm (191), thus reducing the HII phase potential. Vesicle fusion and aggregation, also dependent on nonbilayer forming lipids, were markedly decreased by daunomycin. Treatment of cells with daunomycin decreased significantly the content of plasma membrane–associated G-proteins and protein kinases. These effects were reflected in an uncoupling of ligand binding to specific receptors, which normally results in receptor association with target G-proteins. Purified protein kinase C activity is influenced by both its lipid effector molecules (2) and the physical nature of the supporting membrane made up of PC, PE, and cholesterol. No correlation between activity and lipid packing or fluidity was found. However, a clear correlation and narrow bell-shaped dependence of activity on the bilayer intrinsic radius of curvature was found (192). Therefore, protein kinase C activity would be responsive to small changes in the degree of lipid saturation, in the level of PE or cholesterol, and in lipid soluble materials such as drugs, alcohols, and anesthetics. A similar conclusion has been drawn for the factors that determine the membrane association and activity of CTP:phosphocholine cytidylyltransferase in eukaryotic cells (193). This enzymatic activity is modulated by a broad range of factors that affect the polymorphic properties of membrane lipids. Formation of the HII phase may be an important property of lung surfactant in forming a lipid monolayer at the air-water interface, which is lacking in infants with respiratory deficiency syndrome. In model monolayer systems, bilayer-forming lipids [dipalmitoyl(DP)-PC] move slowly to the air- P1: rpk/mkv May 5, 1997 P2: rpk 14:30 Annual Reviews AR032-07 32-07 PHOSPHOLIPID DIVERSITY 227 water interface, but incorporation of HII -forming lipids [dioleoyl(DO)-PE] and conditions favoring this latter phase result in rapid movement of the lipid mixture to the air-water interface (194). Mixtures of DPPC, DOPE, and cholesterol (to maintain miscibility of these two phospholipids) at temperatures favoring the lamellar phase move slowly to the interface, but at temperatures above the TLH , movement is rapid. Introduction of such artificial lipid mixtures into preterm rabbits works as well as human surfactant in restoring respiration efficiency and in substituting for natural surfactant missing at this stage of development. Clearly, the physical state of the membrane phospholipids profoundly affects membrane-associated processes. The liquid crystalline state for membrane phospholipids and a bilayer configuration have been widely recognized as essential to normal membrane phospholipid function. Although nonbilayer structures must be minimized, polymorphic structural potential is a requirement of biological membranes. The strain introduced into the curvature of the bilayer, the potential derived from the confinement of HII -forming lipids in a bilayer, and possibly the actual formation of nonbilayer structures on a transient basis are a result of the diverse spectrum of naturally occurring lipid mixtures. SUMMARY AND FUTURE DIRECTIONS A large body of information documents putative functions of phospholipids that either collectively or individually participate in and influence cellular processes. It is now clear that genetic manipulation of phospholipid metabolism can provide the in vivo information to both verify and extend in vitro observations on the functions of phospholipids. The genetic approach has the limitations of being indirect and pleiotropic in the processes affected. However, with properly controlled experiments and in combination with biochemical approaches, significant progress has been made in simple prokaryotic systems to define at the molecular level the requirements of phospholipids in several processes. Three major roles for phospholipids have emerged from these studies. Anionic phospholipids, either through charge alone or through the greater specificity of charge and structure, provide the membrane with organizational sites for multisubunit complexes composed of both integral membrane proteins and cytoplasmic proteins. Next, studies have shown that not all zwitterionic phospholipids are alike, and PE in particular has emerged as one that possesses special properties required for membrane protein assembly and for enzyme function. PE and PC should certainly not be thought of as interchangeable in in vitro studies. Finally, the importance of phospholipid polymorphism has been reinforced by the finding that the collective physical properties of membranes significantly influence processes as diverse as conformational changes in enzymes and cell division. P1: rpk/mkv May 5, 1997 P2: rpk 14:30 228 Annual Reviews AR032-07 32-07 DOWHAN With the rapid expansion of genes available from yeast and mammalian systems and the ability to synthesize eukaryotic-specific phospholipids in E. coli, new biological reagents will be available to define the role of phospholipids in more complex systems using the approaches now possible in E. coli. Biochemical studies, coupled with regulated expression of genes responsible for the synthesis of phospholipids in specific organelles and genes that make specific isozymes, are exciting approaches to better defining the roles of phospholipids and dissecting the complex interrelationship of metabolism and metabolic signaling in eukaryotic cells. ACKNOWLEDGMENTS The preparation of this review and the author’s own cited work was supported in part by NIH grant GM 20478. The author wishes to acknowledge the contributions of Eugene P. Kennedy in defining phospholipid metabolism in eukaryotic and prokaryotic systems. Visit the Annual Reviews home page at http://www.annurev.org. Literature Cited 1. Dowhan W. 1992. Methods Enzymol. 209:7–20 2. Bell RM, Burns DJ. 1991. J. Biol. Chem. 266:4661–64 3. Newton AC. 1993. Annu. Rev. Biophys. Biomol. Struct. 22:1–25 4. Maraldi NM, Cocco L, Capitani S, Mazzotti G, Barnabei O, Manzoli FA. 1994. Adv. Enzyme Regul. 34:129–43 5. Obeid LM, Hannun YA. 1995. J. Cell. Biochem. 58:191–98 5a. Kanfer K, Kennedy EP. 1963. J. Biol. Chem. 238:2919–22 5b. Kanfer K, Kennedy EP. 1964. J. Biol. Chem. 239:1720–26 6. Raetz CRH. 1986. Annu. Rev. Genet. 20:253–95 7. Raetz CRH, Dowhan W. 1990. J. Biol. Chem. 265:1235–38 8. Cronan JE Jr, Rock CO. 1996. See Ref. 195, pp. 612–36 9. Shibuya I. 1992. Prog. Lipid Res. 31:245–99 10. Carman GM, Zeimetz GM. 1996. J. Biol. Chem. 271:13293–96 11. Greenberg ML, Lopes JM. 1996. Microbiol. Rev. 60:1–20 12. Kent C. 1995. Annu. Rev. Biochem. 64:315–43 13. Bell RM. 1974. J. Bacteriol. 117:1065– 76 14. McIntyre TM, Chamberlain BK, Webster RE, Bell RM. 1977. J. Biol. Chem. 255:4487–93 15. de Mendoz D, Grau R, Cronan JE Jr. 1993. In Bacillus subtilis and Other Gram-Positive Bacteria: Biochemistry, Physiology and Molecular Genetics, ed. AL Sonenshein, JA Hoch, R Losick, pp. 411–21. Washington, DC: Am. Soc. Microbiol. 16. Goldfine H. 1984. J. Lipid Res. 25:1501– 7 17. Arondel V, Benning C, Somerville CR. 1993. J. Biol. Chem. 268:16002–8 18. Nesbitt JA, Lennarz WJ. 1968. J. Biol. Chem. 243:3088–95 19. Pieringer RA. 1968. J. Biol. Chem. 243:4894–903 20. Beining PR, Huff E, Prescott B, Theodore TS. 1975. J. Bacteriol. 121:137–43 21. Cabacungan E, Pieringer RA. 1980. Infect. Immun. 27:556–62 22. Raetz CRH. 1996. See Ref. 195, pp. 1035–63 23. Nikaido H. 1996. See Ref. 195, pp. 29– 47 P1: rpk/mkv May 5, 1997 P2: rpk 14:30 Annual Reviews AR032-07 32-07 PHOSPHOLIPID DIVERSITY 24. Kadner RJ. 1996. See Ref. 195, pp. 58– 87 25. Hiraoka S, Matsuzaki H, Shibuya I. 1993. FEBS Lett. 336:221–24 26. Morein S, Andersson A-S, Rilfors L, Lindblom G. 1996. J. Biol. Chem. 271:6801–9 27. Rilfors L, Lindblom G, Wieslander Å, Christiansson A. 1984. In Membrane Fluidity, ed. M Kates, LA Manson, pp. 205–45. London: Plenum 28. Hsu L, Jackowski S, Rock CO. 1989. J. Bacteriol. 171:1203–5 29. DeChavigny A, Heacock PN, Dowhan W. 1991. J. Biol. Chem. 266:5323–32 30. Heacock PN, Dowhan W. 1987. J. Biol. Chem. 262:13044–49 31. Hasin M, Kennedy EP. 1982. J. Biol. Chem. 257:12475–77 32. Kennedy EP. 1996. See Ref. 195, pp. 1064–71 33. Weissborn AC, Rumley MK, Kennedy EP. 1992. J. Bacteriol. 174:4856–59 34. Wu HC. 1996. See Ref. 195, pp. 1005– 14 35. Sankaran K, Wu HC. 1994. J. Biol. Chem. 269:19701–6 36. Gupta SD, Dowhan W, Wu HC. 1991. J. Biol. Chem. 266:9983–86 37. Jackowski S, Rock CO. 1986. J. Biol. Chem. 261:11328–33 38. Kiley PJ, Kaplan S. 1988. Microbiol. Rev. 52:50–69 39. Benning C, Somerville CR. 1992. J. Bacteriol. 174:2352–60 40. Xia W, Dowhan W. 1995. J. Bacteriol. 177:2926–28 41. Okada M, Matsuzaki H, Shibuya I, Matsumoto K. 1994. J. Bacteriol. 176:7456– 61 42. Dryden SC, Dowhan W. 1996. J. Bacteriol. 178:1030–38 43. Carman GM, Bae LM. 1992. Methods Enzymol. 209:298–305 44. Tsukagoshi Y, Nikawa J, Yamashita S. 1987. Eur. J. Biochem. 169:477–85 45. Kalmar GB, Kay RJ, Lachance A, Aebersold R, Cornell RB. 1990. Proc. Natl. Acad. Sci. USA 87:6029–33 46. Sweitzer TD, Kent C. 1994. Arch. Biochem. Biophys. 311:107–16 47. Kuge O, Nishijima M, Akamatsu Y. 1986. J. Biol. Chem. 261:5790–94 48. Kuge O, Nishijima M, Akamatsu Y. 1986. J. Biol. Chem. 261:5795–98 49. Shen H, Heacock PN, Clancey CJ, Dowhan W. 1996. J. Biol. Chem. 271:789–95 50. Wu L, Niemeyer B, Colley N, Socolich M, Zuker CS. 1995. Nature 373:216–22 229 51. Weeks R, Dowhan W, Shen H, Balantac N, Meegs B, et al. 1997. DNA Cell Biol. 16:281–89 52. Clancey CJ, Chang S-C, Dowhan W. 1993. J. Biol. Chem. 268:24580–90 53. Trotter PJ, Pedretti J, Voelker DR. 1993. J. Biol. Chem. 268:21416–24 54. Trotter PJ, Voelker DR. 1995. J. Biol. Chem. 270:6062–70 55. Trotter PJ, Pedretti J, Yates R, Voelker DR. 1995. J. Biol. Chem. 270:6071–80 56. Kuge O, Nishijima M, Akamatsu Y. 1991. J. Biol. Chem. 266:24184–89 57. Shigenaga MK, Hagen TM, Ames BN. 1994. Proc. Natl. Acad. Sci. USA 91:10771–78 58. Carman GM, Deems RA, Dennis EA. 1995. J. Biol. Chem. 270:18711–14 59. Heacock PN, Dowhan W. 1989. J. Biol. Chem. 264:14972–77 60. Asai Y, Katayose Y, Hikita C, Ohta A, Shibuya I. 1989. J. Bacteriol. 171:6867– 69 61. Firshein W. 1989. Annu. Rev. Microbiol. 43:89–120 62. Ryter A. 1968. Bacteriol. Rev. 32:39–52 63. Yung BY, Kornberg A. 1988. Proc. Natl. Acad. Sci. USA 85:7202–5 64. Sekimizu K, Bramhill D, Kornberg A. 1988. J. Biol. Chem. 263:7124–30 65. Sekimizu K, Kornberg A. 1988. J. Biol. Chem. 263:7131–35 66. Hwang DS, Crooke E, Kornberg A. 1990. J. Biol. Chem. 265:19244–48 67. Crooke E, Castuma CE, Kornberg A. 1992. J. Biol. Chem. 267:16779–82 68. Castuma CE, Crooke E, Kornberg A. 1993. J. Biol. Chem. 268:24665–68 69. Fralick JA, Lark KG. 1973. J. Mol. Biol. 80:459–75 70. Xia W, Dowhan W. 1995. Proc. Natl. Acad. Sci. USA 92:783–87 71. Horiuchi T, Maki H, Sekiguchi M. 1984. Mol. Gen. Genet. 195:17–22 72. Kogoma T. 1986. J. Bacteriol. 166:361– 63 73. von Meyenburg K, Boye E, Skarstad K, Koppes L, Kogoma T. 1987. J. Bacteriol. 169:2650–58 74. Kogoma T, Skarstad K, Boye E, von Meyenburg K, Steen HB. 1985. J. Bacteriol. 163:439–44 75. Torrey TA, Kogoma T. 1987. Mol. Gen. Genet. 208:420–27 76. Garner J, Crooke E. 1996. EMBO J. 15:2313–21 77. Breukink E, Demel RA, de Korte Kool G, de Kruijff B. 1992. Biochemistry 31:1119–24 78. Ulbrandt ND, London E, Oliver DB. 1992. J. Biol. Chem. 267:15184–92 P1: rpk/mkv May 5, 1997 P2: rpk 14:30 230 Annual Reviews AR032-07 32-07 DOWHAN 79. Mizushima T, Ishikawa Y, Obana E, Hase M, Kubota T, et al. 1996. J. Biol. Chem. 271:3633–38 80. Buser CA, Kim J, McLaughlin S, Peitzsch RM. 1995. Mol. Membr. Biol. 12:69–75 81. Hasselaar P, Derksen RHW, Blokzijl L, de Groot PG. 1990. Thromb. Haemost. 63:169–73 82. Shoenfeld Y, Rauch J, Massicotte H, Datta SK, Schwartz J, et al. 1983. N. Engl. J. Med. 308:414–20 83. Krishna P, Kennedy BP, Waisman DM, van de Sande JH, McGhee JD. 1990. Proc. Natl. Acad. Sci. USA 87:1292–95 84. Krishna P, van de Sande JH. 1990. J. Bacteriol. 172:6452–58 85. Hirai H, Takahashi N, Natori S, Sekimizu K. 1991. Biochim. Biophys. Acta 1090:305–10 86. Mizushima T, Natori S, Sekimizu K. 1992. Biochem. J. 285:503–6 87. Tamura H, Ikegami K, Ono K, Sekimizu S, Andoh T. 1990. FEBS Lett. 261:151– 54 88. Yoshida S, Tamiya KK, Kojima K. 1989. Biochim. Biophys. Acta 1007:61–66 89. Shoji-Kawaguchi M, Izuta S, TamiyaKoizumi K, Suzuki M, Yoshida S. 1995. J. Biochem. 117:1095–99 90. Zheng J, Cahill SM, Lemmon MA, Fushman D, Schlessinger J, Cowburn D. 1996. J. Mol. Biol. 255:14–21 91. Terui T, Kahn RA, Randazzo PA. 1994. J. Biol. Chem. 269:28130–35 92. Schatz G, Dobberstein B. 1996. Science 271:1519–26 93. Economou A, Wichner W. 1995. Cell 78:835–43 94. den Blaauwen T, Driessen AJM. 1996. Arch. Microbiol. 165:1–8 95. Diamond DL, Strobel S, Chun SY, Randall LL. 1995. Protein Sci. 4:1118–23 96. Hardy SJ, Randall LL. 1993. Philos. Trans. R. Soc. London Ser. B 339:343–54 97. Economou A, Pogliano JS, Beckwith J, Oliver DB, Wickner W. 1995. Cell 83:1171–81 98. Oliver DB. 1993. Mol. Microbiol. 7:159–65 99. Kim YJ, Rajapandi T, Oliver D. 1994. Cell 78:845–53 100. de Vrije T, de Swart RL, Dowhan W, Tommassen J, de Kruijff B. 1988. Nature 334:173–75 101. Lill R, Dowhan W, Wickner W. 1990. Cell 60:271–80 102. Kusters R, Dowhan W, de Kruijff B. 1991. J. Biol. Chem. 266:8659–62 103. Hendrick JP, Wickner W. 1991. J. Biol. Chem. 266:24596–600 104. Shinkai A, Mei LH, Tokuda H, Mizushima S. 1991. J. Biol. Chem. 266:5827–33 105. Kim H, Paul S, Jennity J, Inouye M. 1994. Mol. Microbiol. 11:819–31 106. Joly FC, Wickner W. 1993. EMBO J. 12:255–63 107. Martogilo B, Hofmann MW, Brunner J, Dobberstein B. 1995. Cell 81:207–14 108. Kusters R, Breukink E, Gallusser A, Kuhn A, de Kruijff B. 1994. J. Biol. Chem. 269:1560–63 109. Phoenix DA, Kusters R, Hikita C, Mizushima S, de Kruijff B. 1993. J. Biol. Chem. 268:17069–73 110. Keller RC, Killian JA, de Kruijff B. 1992. Biochemistry 31:1672–77 111. Demel RA, Goormaghtigh E, de Kruijff B. 1990. Biochim. Biophys. Acta 1027:155–62 112. Chupin V, Killian JA, Breg J, de Jongh HH, Boelens R, et al. 1995. Biochemistry 34:11617–24 113. Keller RC, ten Berge D, Nouwen N, Snel MM, Tommassen J, et al. 1996. Biochemistry 35:3063–71 114. Kajava AV, Bogdanov MV, Nesmeyanova MA. 1991. J. Biomol. Struct. Dyn. 9:143–57 115. Chupin V, Leenhouts JM, de Kroon AI, de Kruijff B. 1995. FEBS Lett. 373:239– 44 116. Chupin V, Leenhouts JM, de Kroon AI, de Kruijff B. 1996. Biochemistry 35:3141–46 117. Wang Y, Weiner H. 1994. Biochemistry 33:12860–67 118. Eilers M, Endo T, Schatz G. 1989. J. Biol. Chem. 264:2945–50 119. Endo T, Eilers M, Schatz G. 1989. J. Biol. Chem. 264:2951–56 120. Hoch FL. 1992. Biochim. Biophys. Acta 1113:71–133 121. Beyer K, Klingenberg M. 1985. Biochemistry 24:3821–26 122. Hoffmann B, Stöckl A, Schlame M, Beyer K, Klingenberg M. 1994. J. Biol. Chem. 269:1940–44 123. Brustovetsky N, Klingenberg M. 1996. Biochemistry 35:8483–88 124. Rytömaa M, Kinnunen PKJ. 1994. J. Biol. Chem. 269:1770–74 125. Rytömaa M, Kinnunen PKJ. 1995. J. Biol. Chem. 270:3197–202 126. Abramovitch DA, Marsh D, Powell GL. 1990. Biochim. Biophys. Acta 1020:34– 42 127. Tsukihara T, Aoyama H, Yamashita E, Tomizaki T, Yamaguchi H, et al. 1996. Science 272:1136–44 P1: rpk/mkv May 5, 1997 P2: rpk 14:30 Annual Reviews AR032-07 32-07 PHOSPHOLIPID DIVERSITY 128. Ohtsuka T, Nishijima M, Suzuki K, Akamatsu Y. 1993. J. Biol. Chem. 268:22914–19 129. Ohtsuka T, Nishijima M, Akamatsu Y. 1993. J. Biol. Chem. 268:22908– 13 130. Thurmond RL, Dodd SW, Brown MF. 1991. Biophys. J. 59:108–13 131. Seelig J. 1993. In New Developments in Lipid–Protein Interactions and Receptor Functions, ed. KWA Wirtz, L Parker, JÅ Evangelopoulos, JP Changeues, pp. 441–48. London: Plenum 132. Raetz CRH. 1976. J. Biol. Chem. 251:3242–49 133. Ohta A, Shibuya I. 1977. J. Bacteriol. 132:434–43 134. Hawrot E, Kennedy EP. 1978. J. Biol. Chem. 253:8213–20 135. Rietveld AG, Killian JA, Dowhan W, de Kruijff B. 1993. J. Biol. Chem. 268:12427–33 136. Schnaitman CA, Klena JD. 1993. Microbiol. Rev. 57:655–82 137. Mileykovskaya EI, Dowhan W. 1993. J. Biol. Chem. 268:24824–31 138. Bogdanov M, Dowhan W. 1995. J. Biol. Chem. 270:732–39 139. Mileykovskaya E, Dowhan W. 1997. J. Bacteriol. 179:1029–34 140. Shi W, Bogdanov M, Dowhan W, Zusman DR. 1993. J. Bacteriol. 175:7711– 14 141. Chen C-C, Wilson TH. 1984. J. Biol. Chem. 259:10150–58 142. Seto-Young D, Chen C-C, Wilson TH. 1985. J. Membr. Biol. 84:256–67 143. Page MGP, Rosenbusch JP, Yamato I. 1988. J. Biol. Chem. 263:15897–905 144. Rietveld AG, Koorengevel MC, de Kruijff B. 1995.EMBO J. 14:5506–13 145. Chen C-C, Wilson TH. 1986. J. Biol. Chem. 261:2599–604 146. Wilson DM, Ottina K, Newman MJ, Tsuchiya T, Ito S, Wilson TH. 1985. Membr. Biochem. 5:269–90 147. Uratani Y, Aiyama A. 1986. J. Biol. Chem. 261:5450–54 148. Driessen AJM, Hellingwerf KJ, Konings WN. 1987. J. Biol. Chem. 262:12438– 43 149. Fischer W, Nakano M, Laine RA, Bohrer W. 1978. Biochim. Biophys. Acta 528:288–97 150. Ellis RJ. 1996. In The Chaperonins, ed. RJ Ellis, pp. 1–25. London: Academic 151. Bogdanov M, Jianzhong S, Kaback HR, Dowhan W. 1996. J. Biol. Chem. 271:11615–18 152. Sun J, Wu J, Carrasco N, Kaback HR. 1996. Biochemistry 35:990–98 231 153. Taki T, Handa S, Ishikawa D. 1994. Anal. Biochem. 221:312–16 154. Andersson H, von Heijne G. 1993. J. Biol. Chem. 268:21389–93 155. Andersson H, Bakker E, von Heijne G. 1992. J. Biol. Chem. 267:1491–95 156. von Heijne G. 1992. J. Mol. Biol. 225:487–94 157. von Heijne G, Gavel Y. 1988. Eur. J. Biochem. 174:671–78 158. Kaback HR, Jung K, Jung H, Wu JH, Prive GG, Zen K. 1993. J. Bioenerg. Biomembr. 25:627–36 159. Marger MD, Saier MH Jr. 1993. Trends Biochem. Sci. 18:13–20 160. von Heijne G. 1994. In Phosphate in Microorganisms: Cellular and Molecular Biology, ed. AM Torriani, S Silver, E Yagil, pp. 247–50. Washington, DC: Am. Soc. Microbiol. 161. Consler TG, Persson BL, Jung H, Zen KH, Jung K, et al. 1993. Proc. Natl. Acad. Sci. USA 90:6934–38 162. Bogdanov M, Dowhan W. 1994. FASEB J. 8:1110 (Abstr.) 163. Deleted in proof 164. Yamato I. 1992. J. Biochem. 111:444– 50 165. Wolfe PB, Rice M, Wickner W. 1985. J. Biol. Chem. 260:1836–41 166. Pugsley AP. 1993. Microbiol. Rev. 57:50–108 167. Mendoza JA, Rogers E, Lorimer GH, Horowitz PM. 1991. J. Biol. Chem. 266:13044–49 168. Zardeneta G, Horowitz PM. 1992. J. Biol. Chem. 267:5811–16 169. Zardeneta G, Horowitz PM. 1992. Eur. J. Biochem. 210:831–37 170. Zardeneta G, Horowitz PM. 1993. Biochemistry 32:13941–48 171. de Cock H, Tommassen J. 1996. EMBO J. 15:5567–73 172. Bocquet-Pagès C, Lazdunski C, Lazdunski A. 1981. Eur. J. Biochem. 118:105– 11 173. Bolla J-M, Lazdunski C, Pagès JM. 1988. EMBO J. 7:3595–99 174. Lindblom G, Rilfors L. 1989. Biochim. Biophys. Acta 988:221–56 175. Cullis PR, de Kruijff B. 1979. Biochim. Biophys. Acta 559:399–420 176. Thurmond RL, Lindblom G, Brown MF. 1993. Biochemistry 32:5394–410 177. Wieslander Å, Christiansson A, Rilfors L, Khan A, Johansson LB-Å, Lindblom G. 1981. FEBS Lett. 124:273–78 178. Tilcock CPS, Cullis PR, Gruner SM. 1986. Biochim. Biophys. Acta 40:47– 56 179. Lindblom G, Brentel I, Sjölund M, P1: rpk/mkv May 5, 1997 P2: rpk 14:30 232 180. 181. 182. 183. 184. 185. 186. 187. 188. Annual Reviews AR032-07 32-07 DOWHAN Wikander G, Wieslander Å. 1986. Biochemistry 25:7502–10 Wieslander Å, Rilfors L, Lindblom G. 1986. Biochemistry 25:7511–17 Rilfors L, Hauksson JB, Lindblom G. 1994. Biochemistry 33:6110–20 Goldfine H, Rosenthal JJ, Johnston NC. 1987. Biochim. Biophys. Acta 904:283– 89 Goldfine H, Johnston NC, Mattai J, Shipley GG. 1987. Biochemistry 26:2814–22 Rietveld AG, Chupin VV, Koorengevel MC, Wienk HL, Dowhan W, de Kruijff B. 1994. J. Biol. Chem. 269:28670–75 Gangola P, Rosen BP. 1987. J. Biol. Chem. 262:12570–74 Nakayama H, Mitsui T, Nishihara M, Kito M. 1980. Biochim. Biophys. Acta 601:1–10 Jackson MB, Cronan JE Jr. 1978. Biochim. Biophys. Acta 512:472–79 Magnusson K, Jackowski S, Rock CO, 189. 190. 191. 192. 193. 194. 195. Cronan JE Jr. 1993. Microbiol. Rev. 57:522–42 Rietveld AG, de Kruijff B. 1997. Biochim. Biophys. Acta. 1324:263– 72 Schwarz D, Kisselev P, Wessel R, Jueptner O, Schmid RD. 1996. J. Biol. Chem. 271:12840–46 Escribá PV, Sastre M, Garcı́a-Sevilla JA. 1995. Proc. Natl. Acad. Sci. USA 92:7595–99 Slater SJ, Kelly MB, Taddeo FJ, Ho C, Rubin E, Stubbs CD. 1994. J. Biol. Chem. 269:4866–71 Jamil H, Hatch GM, Vance DE. 1993. Biochem. J. 291:419–27 Perkins WR, Dause RB, Parente RA, Minchey SR, Neuman KC, et al. 1996. Science 273:330–32 Neidhardt FC, ed. 1996. E. coli and S. typhimurium: Cellular and Molecular Biology. Washington, DC: Am. Soc. Microbiol.