* Your assessment is very important for improving the workof artificial intelligence, which forms the content of this project
Download The presence of monoglucosylated N196
Survey
Document related concepts
Hedgehog signaling pathway wikipedia , lookup
Phosphorylation wikipedia , lookup
Signal transduction wikipedia , lookup
Magnesium transporter wikipedia , lookup
List of types of proteins wikipedia , lookup
Protein (nutrient) wikipedia , lookup
Protein phosphorylation wikipedia , lookup
Protein moonlighting wikipedia , lookup
G protein–coupled receptor wikipedia , lookup
Homology modeling wikipedia , lookup
Intrinsically disordered proteins wikipedia , lookup
Protein domain wikipedia , lookup
Protein folding wikipedia , lookup
Nuclear magnetic resonance spectroscopy of proteins wikipedia , lookup
Protein–protein interaction wikipedia , lookup
Transcript
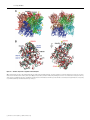
Biochem. J. (2009) 421, 87–96 (Printed in Great Britain) 87 doi:10.1042/BJ20082170 The presence of monoglucosylated N196-glycan is important for the structural stability of storage protein, arylphorin Kyoung-Seok RYU*, Jie-Oh LEE†, Taek Hun KWON*, Han-Ho CHOI‡, Hong-Seog PARK‡, Soo Kyung HWANG§, Zee-Won LEE§, Kyung-Bok LEE§, Young Hyun HAN*, Yun-Seok CHOI*, Young Ho JEON*, Chaejoon CHEONG*1 and Soohyun KIM§1 *Magnetic Resonance Team, Korea Basic Science Institute, Gwahangno 113, Daejeon 305-333, South Korea, †Department of Chemistry, Korea Advanced Institute of Science and Technology, Gwahangno 335, Daejeon 305-701, South Korea, ‡Genome Research Center, Korea Research Institute of Bioscience and Biotechnology, Gwahangno 111, Daejeon 305-806, South Korea, and §Division of Life Science, Korea Basic Science Institute, Gwahangno 113, Daejeon 305-333, South Korea Although N-glycosylation has been known to increase the stability of glycoproteins, it is difficult to assess the structural importance of glycans in the stabilization of glycoproteins. APA (Antheraea pernyi arylphorin) is an insect hexamerin that has two N-glycosylations at Asn196 and Asn344 respectively. The glycosylation of Asn344 is critical for the folding process; however, glycosylation of Asn196 is not. Interestingly, the N196glycan (glycosylation of Asn196 ) remains in an immature form (Glc1 Man9 GlcNAc2 ). The mutation of Asn196 to glutamine does not change the ecdysone-binding activity relative to that of the wild-type. In the present study, we determined the crystal structure of APA, and all sugar moieties of the N196-glycan were clearly observed in the electron-density map. Although the sugar moieties of the glycan generally have high structural flexibility, most sugar moieties of the N196-glycan were well organized in the deep cleft of the subunit interface and mediated many inter- and intrasubunit hydrogen bonds. Analytical ultracentrifugation and GdmCl (guanidinium chloride) unfolding experiments revealed that the presence of the N196-glycan was important for stabilizing the hexameric state and overall stability of APA respectively. Our results could provide a structural basis for studying not only other glycoproteins that carry an immature N-glycan, but also the structural role of N-glycans that are located in the deep cleft of a protein. INTRODUCTION the influence of this glycosylation on the overall Gibbs free H2 O ) of the protein has not been evaluated. It has energy (G unfold been reported recently that the increased protein stabilization by glycosylation originated from destabilization of the unfolded state rather than stabilization of the folded state and this effect is coupled with kinetic stabilization; the bulky polysaccharide chains inhibit formation of a residual structure in the unfolded glycoprotein [11]. Nevertheless, the analysis of glycosylation sites in the protein structure databases still emphasizes the structural role of glycosylation in the folded state, because 10 and 20 % of the potential glycosylation sites were located in a deep cleft and on the edges of grooves respectively [12]. The APA [Antheraea pernyi (Chinese oak silkworm) arylphorin] is an insect hexamerin that consists of 688 amino acids. APA belongs to a growing superfamily of proteins that includes arthropod tyrosinase, arthropod haemocyanin, insect hexamerin and dipteran arylphorin receptor [13]. It was reported that at least two types of hexamerin exist in Lepidoptera: arylphorin and a methionine-rich storage protein [14]. Hexamerins are synthesized in the fat body of a wide range of lepidopteran and dipteran larvae, and they are highly accumulated in the haemolymph. Hexamerins are storage proteins and are required for somatic tissue metamorphosis, because they are reabsorbed into the fat body and then metabolized as energy and amino acid sources during insect maturation, a period during which there is no feeding [15,16]. In addition to their role as a storage protein, Glycosylation is an important cellular modification, and it is related to many human diseases and developmental defects [1]. It introduces diversity into a biological system because of its inherent structural heterogeneity, and thus plays critical roles during a variety of cellular processes, such as protein folding, protein–protein interaction, immune response and responses to pathogens [2–4]. More than half of all eukaryotic proteins have been reported to be glycosylated, and N-glycan is estimated to be present in 90 % of these proteins [5]. The crystal structures and NMR studies of glycoproteins indicate that glycans exist primarily on the protein surface and most of the sugars are not in direct contact with the protein, except for the two proximal GlcNAc residues [6]. Many biochemical and genetic studies have shown that N-glycosylation is important not only for folding, but also for the stability of glycoproteins [3,7,8]. However, there have been rare structural reports showing that the presence of the glycan stabilizes the glycoprotein. NMR studies have shown that the monofucosylation (O-glycosylation of Thr9 ) of the proteinase inhibitor, Pars intercerebralis major peptide C, is critical for its active structure [9]. A purely direct structural role of N-glycan has only been proposed for quercetin 2,3-dioxygenase [10]. However, the N-glycan of the dioxygenase is also located on the surface of the protein, and only two proximal GlcNAc residues appear to form the consistent intersubunit hydrogen bonds. Furthermore, Key words: arylphorin, ecdysone, glycosylation, protein stability, protein structure, X-ray crystallography. Abbreviations used: APA, Antheraea pernyi arylphorin; BC, branched chain; ER, endoplasmic reticulum; GdmCl, guanidinium chloride; HEK, human embryonic kidney; LC, longest chain; MALDI–TOF, matrix-assisted laser-desorption ionization–time-of-flight; MFI, maximum fluorescence intensity; N, native form; PIH, Panulirus interruptus haemocyanin; PNGase F, peptide N-glycosidase F; rAPA, recombinant APA; SASA, solvent-accessible surface area; U, unfolded form. 1 Correspondence may be addressed to either of these authors (email [email protected] or [email protected]). The crystal structure of Antheraea pernyi arylphorin has been deposited in the RCSB Protein Data Bank under code 3GWJ. c The Authors Journal compilation c 2009 Biochemical Society 88 K.-S. Ryu and others hexamerins appear to play other important roles during the lifespan of insects. Hexamerins have been shown to be important for maintaining high proportions of worker caste members in termite societies, where a significant amount of two specific hexamerins have been shown to suppress juvenile hormone-dependent worker differentiation into the soldier caste phenotype [17]. It has also been suggested that hexamerins function as ecdysteroid-carrier proteins in the haemolymph. This conclusion was based on the observation that spider (Eurypelma californicum) haemocyanin and blowfly (Calliphora vicina) calliphorin bind to the insect moulting hormones, ecdysone and 20-hydroxyecdysone [18]. 20-Hydroxyecdysone is the activated form of ecdysone and has been shown to be critical for the maturation of the arylphorin receptor in vivo and in vitro [19]. The structure of APA has attracted considerable interest, because of the presence of monoglucosylated N-glycan and a high content of aromatic amino acids (132 of 688 residues). The presence of two glycosylation sites (Asn196 and Asn344 ) was identified among four possible candidates (additional sites are Asn57 and Asn623 ). The N344-glycan (glycosylation of Asn344 ) appears to be located on the protein surface, because it is easily released from APA through treatment with PNGase F (peptide N-glycosidase F) under non-denaturing conditions. However, the N196-glycan (glycosylation of Asn196 ) remains in an immature form, monoglucosylated oligosaccharide (Glc1 Man9 GlcNAc2 ), and does not dissociate from the protein core after PNGase F treatment [20]. During protein N-glycosylation, the precursor oligosaccharide (Glc3 Man9 GlcNAc2 ) is pre-assembled on the dolichol lipid by many ER (endoplasmic reticulum)-related enzymes, and then is transferred to a specific asparagine residue of a growing polypeptide chain by oligosaccharyltransferase. After the removal of the second glucose by glucosidase II, the monoglucosylated glycoprotein enters the calnexin/calreticulin cycle in the ER, which governs the folding process of glycoproteins. Finally, the removal of the last remaining Glc-a by glucosidase II releases the glycoprotein from these chaperones [4]. The properly folded glycoprotein then moves to the Golgi apparatus, where the attached N-glycan undergoes further trimming and modification processes. Deglucosylation is generally considered to be a major signal, indicating the completion of protein folding [4], and thus monoglucosylated N-glycans are rarely observed in mature glycoproteins. However, this glycan has been detected in avian IgY [21], the egg jelly coat of starfish [22] and human complement component C3 [23]. It has been suggested that the hydrophobicity of large proteins is linked to the presence of glucose-capped oligosaccharides [24]. In the present study, we characterized the specific roles of two N-glycosylations at Asn196 and Asn344 with regard to protein stability and folding. The structural roles of N196-glycan were examined by determining the crystal structure of APA and by employing various biophysical techniques. MATERIALS AND METHODS Protein purifications and identification of N-glycan APA was purified from larval haemolymph by gel-permeation and ion-exchange chromatography using a Superdex-200 column (GE Healthcare) at pH 7.5 and a HiTrap-SP column (GE Healthcare) at pH 5.0 respectively. Autumn APA was used for further experiments, since the profile of N196-glycan was more homogeneous than that of spring APA [16]. Expression vectors containing either the FLAG-tagged wild-type rAPA (recombinant APA) or mutant rAPA-N196Q were transiently transfected into HEK (human embryonic kidney)-293a cells. After 4 days of c The Authors Journal compilation c 2009 Biochemical Society culture, medium was harvested, and the FLAG-tagged rAPA and rAPA-N196Q were purified using an anti-FLAG M2–agarose column (Sigma). To characterize the profiles of N-glycan, the oligosaccharides released from denatured proteins were labelled with 2-aminobenzamide, and then separated using normal-phase HPLC as described previously [20]. The glycans were also permethylated and analysed by MALDI–TOF (matrix-assisted laser-desorption ionization–time-of-flight)-MS as described previously [25]. Determining the binding constants of APA and rAPA-N196Q for ecdysone Ecdysone was purchased from Fluka, and the stock solution (50 mM) was prepared in 100 % ethanol by directly weighing the ecdysone powder. APA (0.2 μM) and rAPA-N196Q (0.1 μM) were prepared in buffer (pH 7.5) containing 50 mM Tris/HCl and 100 mM NaCl. The intrinsic fluorescence of both proteins was measured using the PerkinElmer LS 50 spectrofluorimeter at 25 ◦C. The excitation wavelength was fixed at 280 nm, and the emission spectrum was recorded from 320 to 450 nm. The binding constants were extracted by fitting the changes in maximum intensity to a simple binding equation [18]. Crystallization and acquisition of diffraction data A protein stock solution (10–12 mg/ml) was prepared in buffer (10 mM Tris/HCl containing 100 mM NaCl, pH 7.5), and crystallization conditions were screened using Crystal screen I and II, and Index kits (Hampton Research). The crystals were grown in a buffer [pH 4.6; 100 mM sodium acetate, 14–15 % poly(ethylene glycol) 3350, 150 mM magnesium formate and 20 mM benzamidine chloride] using the hanging-drop method at 20 ◦C. In addition, 30 % poly(ethylene glycol) 400 was added to the crystal growth buffer as a cryoprotectant for freezing the crystal, and the X-ray diffractions were performed under a cold helium gas stream. Crystal screening was performed using the synchrotron beamlines (4A and 6C) at the Pohang Accelerator Laboratory. Diffraction data for the structure determination were obtained using the synchrotron beamline at Spring-8, Japan, and were processed using HKL-2000 package. Crystal structure determination and analysis The initial arylphorin model was generated by the MODELLER program [26], using the structures of homologous haemocyanin (PDB code 1HC1). Molecular replacement was performed using the Molrep program, during which a reasonable solution was obtained only when the trimeric structure of haemocyanin, and not the monomeric structure, was used as an input model. The model building was completed using the Coot program included in the CCP4 package [27]. Structure refinement was performed using the CNSsolve program [28]. A mild non-crystallographic symmetry restraint was applied for each subunit, except for the two GlcNAc sugar moieties of N334-glyan. The default CNSsolve parameter of carbohydrate was corrected to maintain the appropriate geometry of the N-acetyl group in the GlcNAc residue. Figures showing the structure and the electron-density map of arylphorin were generated by using the program Chimera [29]. The cation–π interaction was analysed using the CaPTURE program [30]. The areas of subunit interfaces and the SASAs (solvent-accessible surface areas) of APA were calculated using the NACCESS program (http://www.bioinf.manchester.ac.uk/ naccess/nacwelcome.html). Monoglucosylated N-glycan enhances protein stability Analytical ultracentrifugation The oligomeric status of APA and rAPA-N196Q were analysed by equilibrium sedimentation analysis at 20 ◦C. The experiments were performed using an analytical ultracentrifuge (ProteomeLab XL-A, Beckman Coulter) and An-60Ti analytical rotor. The equilibrium sedimentation distributions of all proteins were obtained after centrifugation for 48 h at 5000 rev./min. The sedimentation coefficients of rAPA-N196Q were also obtained from sedimentation velocity analysis. The SEDFIT program was used for data analysis [31]. GdmCl (guanidinium chloride) equilibrium unfolding experiment All three proteins (1 μM each of APA, rAPA and rAPAN196Q) were pre-equilibrated overnight in buffers (pH 7.5) containing 50 mM Tris/HCl and an appropriate concentration of guanidinium chloride (GdmCl) at 25 ◦C. However, the overnight incubation seemed to be slightly insufficient for the reaction to reach equilibrium for APA and rAPA, and thus a longer incubation time (1 and 2 days) was used for the additional APA unfolding experiments. The intrinsic fluorescence was measured using the PerkinElmer LS 50 spectrofluorimeter at 25 ◦C with 280 nm excitation. The MFIs (maximum fluorescence intensities) of emission spectra (320–450 nm) were used for the analysis of Gunfold . The free energy (Gunfold , kcal · mol− 1 , where 1 kcal = 4.184 kJ) of all proteins was estimated from the ratio of the unfolded form (U) to the native form (N) of the protein at each concentration of GdmCl; Gunfold = −RT · ln[U/N]. The free H2 O energy of the native proteins (G unfold , kcal · mol−1 ) was obtained through linear extrapolation: 2 + m · [GdmCl] G unfold = G unfold H O where m is the number showing the dependence of the free energy on the denaturant concentration. The unfolded fractions of rAPAN196Q were obtained after linear correction of the baselines that were located before and after the denaturant-dependent transition of MFI. However, a gradual pre-unfolding ahead of the main unfolding transition was observed for both APA and rAPA, which may have resulted from the presence of minor heterogeneity in the N196-glycan [16]. The values of −RT · ln[U/N] were obtained again by correction of the tentative population of the minor forms, and the linearity of the plot of Gunfold against [GdmCl] was used as an additional reference for determining the population of the major transition. RESULTS Glycosylation of Asn344 is required for proper folding of APA, but that of Asn196 is not It has been well established that N-glycosylation plays a similar role as protein chaperones, and thus its presence is important for the proper folding of glycoproteins [3,7]. For example, it has been shown that the activity of several ER-related chaperone proteins is dependent on the presence of N-glycosylation in the substrate protein [4,32]. APA has two occupied glycosylation sites, at Asn196 and Asn344 . We therefore tested the effect of glycan occupancy at each glycosylation site on the expression of the APA protein in mammalian cells. APA was expressed in an insoluble form in Escherichia coli (results not shown). Substitution of glutamine for the asparagine residue at the N-glycosylation sites prevented attachment of the glycan in the ER. rAPA and two mutant APA proteins (rAPA-N196Q and rAPA-N344Q) were expressed in HEK-293a cells. The N196-glycan profile of rAPA was determined to be very similar to that of purified APA, although other minor forms were also present. We also confirmed that 89 rAPA-N196Q did not possess any glycan moiety at Gln196 (see Supplementary Figure S1 at http://www.BiochemJ.org/bj/421/ bj4210087add.htm). The profile of the N344-glycans of APA was different from that of rAPA and rAPA-N196Q. The N344glycan existed in a processed form, and thus a differential glycan processing in the Golgi apparatus of Antheraea pernyi and HEK-293a cell is likely to be the cause of the different N344glycan profiles. On the other hand, the N196-glycan is in an immature form, which suggests that further processing in the ER and Golgi apparatus did not occur (see Supplementary Figure S1). Interestingly, rAPA-N196Q was expressed as a soluble protein with a slight decrease in the amount of secreted protein; however, rAPA-N344Q was not secreted into the culture medium, but rather it accumulated inside the cell in an insoluble form (see Supplementary Figure S2A at http://www.BiochemJ.org/bj/421/ bj4210087add.htm). Both rAPA and rAPA-N196Q seemed to be properly folded. We have already found that the presence of intermolecular disulfide bonds stabilizes the trimeric structure of APA (results not shown), and the maintenance of the disulfide bonds of rAPA and rAPA-N196Q was also confirmed by nonreducing SDS/PAGE analysis (Supplementary Figure S2B). The glycosylation of Asn344 seems to be important for the proper folding of rAPA; however, this is not the case for Asn196 . The decreased amount of secreted rAPA-N196Q, compared with rAPA, may have resulted from the loss of the N196-glycan. It has been reported that glucosidase II can efficiently cleave the second glucose when an additional second glycan is present in the protein chain [33]. The resulting monoglucosylated glycoprotein enters the calnexin/calreticulin cycle in the ER, which governs the folding process of glycoproteins [4]. The critical role of the glycosylation of Asn344 during the folding process of APA would be interesting, because the glycosylation motif of Asn344 is not conserved in the arylphorin protein family (results not shown). It is possible that another N-glycosylation could occur at a different site on other arylphorins (e.g. Spodoptera litura arylphorin, Asn56 or Asn455 ), in addition to the common glycosylation site at Asn196 of APA. The lack of glycosylation at Asn196 does not result in a stringent structural change We first investigated the secondary structures of APA, rAPA and rAPA-N196Q using CD. The CD spectra of APA, rAPA and rAPA-N196Q showed that all proteins were properly folded, although the overall α-helical content of rAPA-N196Q seemed to be slightly lower than that of APA or rAPA (results not shown). It has been suggested that some hexamerins and haemocyanin bind to ecdysteroid hormones and thus function as ecdysone-carrier proteins [18]. We therefore evaluated the tertiary folds of APA and rAPA-N196Q by comparing their ecdysone-binding affinities. The protein–ecdysone dissociation constants (K d ) of both proteins were determined using the intrinsic fluorescence of APA and rAPA-N196Q. Their K d values were almost identical at 0.43 mM (Figure 1); thus, rAPA-N196Q exhibited identical folding, at least in terms of ecdysone-binding and disulfide bond formation. There were no clearly separate domains in the crystal structure of APA (see below). The mutation of Asn196 to glutamine did not seem to have a stringent effect on the overall structure of APA and its effect would be localized in the interface of APA hexamer. If the mutation makes a strong structural perturbation, it is likely to propagate in an ecdysone-binding site of rAPA-N196Q, and thus should affect the binding affinity. Although the binding constant of APA to ecdysone was obviously low, a high concentration of APA could facilitate the diffusion of ecdysone. We roughly estimated that APA accumulated c The Authors Journal compilation c 2009 Biochemical Society 90 K.-S. Ryu and others Table 1 Data collection and refinement statistics Parameter Figure 1 Binding of ecdysone to APA or rAPA-N196Q The binding affinities of APA and rAPA-N196Q to ecdysone were estimated using the intrinsic fluorescence of the proteins. Ecdysone was dissolved in absolute ethanol (Et-OH), and fluorescence intensities of the proteins were measured at increasing ecdysone concentrations. The fluorescence of either protein was not significantly changed by the addition + of pure ethanol only. APA (0.43 + − 0.045 mM) and rAPA-N196Q (0.43 − 0.025 mM) have similar binding affinities to ecdysone. in the haemolymph up to a concentration of 0.5 mM at the last stage of pupa (results not shown). Ecdysone is a non-polar steroid hormone produced in the prothoracic gland, and its concentration increases by more than 10 μM at the end of the final larval stage [34]. Ecdysone has been known to mediate a variety of developmental and physiological events in insects, including hexamerin receptor maturation [19,34]. The insect moulting process occurs within a short time, and thus the ecdysteroidcarrier activity of hexamerin may be required for synchronizing the processes related to moulting. Crystal structure of hexameric APA We determined the crystal structure of APA (i) to study the influences of the N196-glycan on the structure of APA at the molecular level, and (ii) to find out why the monoglucosylated oligosaccharide of Asn196 survives the N-glycan trimming process in the ER and Golgi apparatus. Fortunately, molecular replacement using the previously reported PIH [Panulirus interruptus (California spiny lobster) haemocyanin] structure (PDB codes 1HC1) produced a reasonable solution, although the sequence identity between the haemocyanin and APA was somewhat low (∼ 28 %). The statistics of the crystal structure determined are summarized in Table 1. The asymmetric unit of the APA crystal contained one hexamer (Figure 2A). Although several studies have predicted that arylphorins have a hexameric structure on the basis of biochemical analysis [13,16], the crystal structure and the analytical ultracentrifuge investigation (see below) clearly confirm that the oligomeric state of APA is a hexamer. The amino acid sequence alignment of APA and PIH (Figure 3) and their structural comparison (see Supplementary Figure S3 at http://www.BiochemJ.org/bj/421/bj4210087add.htm) indicate that arylphorin is likely to have evolved from haemocyanin, although the population of aromatic amino acid is much higher for arylphorin than for haemocyanin. The overall topology of APA was very similar to that of the previously reported hexamerin c The Authors Journal compilation c 2009 Biochemical Society Data collection Space group a , b , c (Å) α, β, γ (◦) Resolution (Å) R sym (%) I /σ I Completeness (%) Redundancy Refinement Resolution limit (Å) Number of reflections R work /R free (%) RMSD of bond length (Å) RMSD of bond angle (◦) Ramanchandran plot analysis Most favoured region (%) Allowed region (%) Generously allowed region (%) Disallowed region (%) Value P 21 2 1 2 1 121.196, 119.470, 319.878 90.0, 90.0, 90.0 50.0–2.42 (2.51–2.42) 14.1 (31.6) 20.0 (3.3) 95.3 (79.9) 5.9 (4.0) 50.0–2.42 168 198 18.2/23.0 0.0070 1.2367 92.4 7.1 0.5 0.0 structure of PIH [35], and only minor structural differences were identified; APA has an additional β-sheet (residues 12–14 and residues 580–582, β1), helix and one more β-strand (residues 176–182, α9, and residues 184–187, β2), and helix–turn–helix (residues 670–679, α21a and α21b). The APA loop (residues 619–635, coloured red in Supplementary Figure S3B) has a very high B-factor, but the corresponding loop in PIH is stabilized by a disulfide bond. In comparison with PIH, the structure of APA has two distinguishing features. One is the presence of intermolecular disulfide bonds between Cys73 and Cys649 , which form a circular structure within each top (A-B-C) and bottom (D-E-F) trimer unit. The other is the presence of a monoglucosylated oligosaccharide at Asn196 (Figures 2A and 2B). However, the presence of disulfide bonds does not seem to be critical for stabilizing the structure of the arylphorin protein family, because sequence alignment of the insect hexamerin proteins shows that these cysteine residues of APA (Cys73 and Cys649 ) are not conserved even in the arylphorin protein family (results not shown). Analysis of the interface areas between the subunits of the APA hexamer revealed that the interface area between subunit pairs A-D, B-F and C-E was largest. The overall contact surface areas between subunit B and subunit A, C, D and F were 1115.3 (19.8), 1078.3 (17.9), 860.5 (381.4), and 2817.7 (544.7) Å2 (1 Å = 0.1 nm) respectively, where the values in parentheses are the area that is mediated by the N196-glycan. The N196-sugar moieties of subunit A are mainly in contact with subunit D, and those of subunit D are also in major contact with subunit A (Figure 2C). It is likely that the dimeric pair of subunits A and D (similarly, subunits B and F, and subunits C and E) is the core interaction responsible for the formation of the hexameric arylphorin, and the presence of the disulfide bonds of APA that make the circular connection of each top or bottom trimer seems to be incidental. On the other hand, the glycosylation motif of Asn196 is clearly conserved only in the arylphorin protein family (Figure 4). The residue corresponding to Asn196 of APA is not present in very or moderately methionine-rich hexamerin proteins. The N196-glycan of APA is located in the deep cleft of the subunit interface (Figure 2A), and the presence of all sugar moieties was identified in the annealed omit electron-density map (Supplementary Figure S4 at http://www.BiochemJ.org/bj/421/ Monoglucosylated N-glycan enhances protein stability Figure 2 91 Crystal structure of APA (A) The crystal asymmetric unit of APA consisted of six monomers (A, blue; B, yellow; C, aquamarine; D, green; E, cyan; F, brown). The different subunits are indicated using a chain code (.A, .B, .C, .D, .E and .F). All 12 sugar moieties of monoglucosylated N196-glycan (S-N196 ) in each subunit are shown in the crystal structure; however, only two proximal GlcNAc residues of N344-glycan (S-N344 ) were barely detected. The N196-glycan from subunit A are located in the deep cleft that was formed by subunits A, D and E. Two cysteine residues (C649.E and C73.D) forming intersubunit disulfide bonds are indicated using a yellow colour (inset). (B) A different view of the top trimer unit of the APA hexamer (subunits D, E and F) shows that each top and bottom trimer unit were stabilized by the intersubunit disulfide bonds. (C) The orientation and colours for each subunit were the same as in (A). The density of the blue colour represents the variation of the crystal B -factor for subunit A, where a strong colour indicates a high B -factor. Cys649 of subunit E (C649.E) forms intermolecular disulfide bonds with Cys73 of subunit-D (C73.D) (marked with yellow spheres). The LC of subunit A had a lower B -factor in comparison with the BC1 and BC2, and not only had multiple intrasubunit interactions, but also participated in many intersubunit interactions with subunit D (marked with a star). The BC1 of subunit E was spatially close to subunit A, but the BC2s of all subunits were positioned outside the protein core. The regions that were located within 5 Å of the N196-glycan were decorated using the same colours as the contacting sugars. (D) Schematic illustration of the monoglucosylated N196-glycan and the N344-glycan. Sugar chains of the N196-glycan were divided further into LC (GlcNAc-1 to Glc-a), BC1 (Man-A and Man-D2) and BC2 (Man-B and Man-D3). bj4210087add.htm). The longest chain of the N196-glycan (LC in Figure 2C, GlcNAc-1 to Glc-a) is well defined in the electrondensity map in comparison with the first (BC1 in Figure 2C, Man-A to Man-D2) and second (BC2 in Figure 2C, Man-B to Man-D3) branched sugar chains, and thus the B-factors of LC are much lower than that of BC1 and BC2 (see Supplementary Figure S5 at http://www.BiochemJ.org/bj/421/bj4210087add.htm). The sugar moieties of LC form more than 20 direct or watermediated hydrogen bonds with adjacent amino acids, which are located in the same or different subunits (Figure 5). Especially, the sugar moieties (Man-4 to Glc-a) of subunit A form hydrogen bonds with Tyr43 , Lys47 , Phe75 , Leu76 , Lys78 , Val118 , His119 and Tyr182 of subunit D, and the same sugar moieties of subunit D form the reciprocal hydrogen bonds with the same residues of subunit A. The LC region seems to be important for enhancing the interaction between subunits A and D, subunits B and F, and subunits C and E, with regard to the hydrogen-bond network. Therefore this sugar chain could additionally stabilize the overall trimer–trimer interaction (see below) by enhancing the interaction between each top and bottom dimer (Figures 2B and 2C). Moreover, many direct or water-mediated intrasugar hydrogen bonds (∼ 20) were identified in the LC, and not in the BC1 and BC2 regions. This means that the sugar moieties of the LC are well placed to seize adjacent water molecules. The BC1 of subunit A is positioned close to Leu528 , Glu529 , Cys649 and Phe650 of subunit E and Lys392 of subunit D, in which the Man-A moiety c The Authors Journal compilation c 2009 Biochemical Society 92 Figure 3 K.-S. Ryu and others Secondary structures and sequence alignments of arylphorin and haemocyanin Structure-based sequence alignments were performed using the Chimera program (http://www.cgl.ucsf.edu/chimera/). The open and grey-coloured boxes represent the β-sheet and α-helix respectively. Asn196 and Asn344 of APA are indicated with an asterisk (*), and Cys73 and Cys649 are indicated with a plus sign (+), respectively. Two intramolecular disulfide bonds (Cys483 and Cys502 ; Cys562 and Cys609 ) were observed in the structure of PIH (underlined), where the disulfide bond between Cys562 and Cys609 seems to stabilize the loop structure of PIH (see Supplementary Figure S3 at http://www.BiochemJ.org/bj/421/bj4210087add.htm). Figure 4 Sequence alignment of hexamerins in the Asn196 region of APA The identical and conserved residues in the hexamerin protein family are indicated with an asterisk (*) and a plus sign (+) respectively. The N-glycosylation motif is indicated with a box. ApAry, arylphorin from Antheraea pernyi (Chinese oak silkmoth); HcAry, arylphorin from Hyalophora cecropia (cecropia moth); BmSp2, sex-specific storage-protein 2 precursor arylphorin from Bombyx mori (domestic silkworm); MsAry, arylphorin subunit alpha precursor from Manduca sexta (tobacco hornworm); SlAry, arylphorin subunit from Spodoptera litura (common cutworm); HcVmr, very methionine-rich storage protein; HcMmr, moderate methionine-rich storage protein. of subunit A (O3 atom) consistently forms hydrogen bonds with the Cys649 of subunit E (carbonyl O atom). The Man-D2 moiety of subunit A (O4 and O6 atoms) is close to the side chains of Glu529 of subunit E and Lys392 of subunit D (within 3.5–3.9 Å), and thus it might form a transient hydrogen bond due to its dynamic characteristics in comparison with the sugar moieties of the LC. The structural role of BC1 might be important in the other arylphorins that do not contain intermolecular disulfide bonds, because the hydrogen-bond formation between subunit A and subunit E, which is mediated by the sugar moieties of BC1, can mimic the same effect of intersubunit disulfide bonds. The c The Authors Journal compilation c 2009 Biochemical Society sugar moieties of BC2 from all subunits are positioned outside the protein core. The N344-glycan is located on the surface of APA, and two proximal GlcNAc moieties were barely detected in the annealed omit map (see Supplementary Figures S4 and S5). The two proximal GlcNAc moieties on the N344-glycan have a much higher B-factor compared with the corresponding GlcNAc moieties of N196-glycan, and their values vary significantly with the subunit origins. The B-factor of GlcNAc-2 of N344-glycan, which originates from subunit D, is even higher than that of the last sugar moiety of N196-glycan (Man-D3), making the exact model building of the GlcNAc-2 moiety located in subunit D very difficult. The monoglucosylated oligosaccharide of APA can survive the extensive trimming process that occurs in the ER and Golgi apparatus, because the location of N196-glycan in the deep cleft of the subunit interface and the presence of many N196-glycan that mediate the intra- and inter-subunit hydrogen bonds are likely to restrict the access of various glycosidases. Interestingly, the ribbon presentation of the APA monomer indicates that the N196-glycancontacting residues are mainly part of short α-helical structures (Figure 5), and thus the N196-glycan could also stabilize these local α-helices. N196-glycan enhances interaction between top and bottom subunits We compared the oligomeric states of rAPA-N196Q with that of rAPA by analytical ultracentrifugation (Figure 6). The Monoglucosylated N-glycan enhances protein stability Figure 5 93 Stereo view of N196-glycan-mediated intra- and inter-subunit hydrogen bonds Subunits A, D and E are coloured blue, green and cyan respectively, and the observed water molecules are indicated using purple-coloured balls. The density of ribbon colour represents the variation of the crystal B -factor, where a strong colour indicates a higher B -factor. The N196-glycan of subunit A is displayed as a ball-and-stick model. The residues located within 5 Å of the sugar moieties of N196-glycan are displayed as a stick model. The hydrogen bonds satisfying the precise and moderate criteria were indicated using cyan- and orange-coloured lines respectively. The residues that participate in the N196-glycan-mediated hydrogen bonds are listed in the bottom panel. The subunit origination of residues was indicated using a chain code (.A, .B and .E). The names of the atoms that participate in the hydrogen bonds are shown in parentheses (blue, direct hydrogen bond; red, water-mediated hydrogen bond). sedimentation equilibrium analysis clearly showed that rAPA mainly exists as hexamer. However, the equilibrium sedimentation profile of rAPA-N196Q could not be fitted to any single component models, which indicates that rAPA-N196Q undergoes a reversible hexamer–trimer equilibrium, although the hexameric state is still predominant. The hexamer–trimer equilibrium of rAPA-N196Q is likely to be observed, simply due to the presence of intermolecular disulfide bonds that maintains the trimeric state (see Supplementary Figure S2B). The hexameric state of rAPA is more stable than that of rAPA-N196Q (hexamer compared with hexamer–trimer equilibrium). The crystal structure shows that the N196-glycan mediated not only intrasubunit hydrogen bonds, but also many intersubunit hydrogen bonds for each pair of subunits: A and D, B and F, and C and E. The loss of N196-glycan is likely to decrease the overall trimer–trimer interaction and thus shift the equilibrium of APA from the hexameric state to the hexamer– trimer equilibrium. The presence of N196-glycan is also important for the overall protein stability The overall stabilities of APA and rAPA-N196Q were evaluated with a GdmCl equilibrium-unfolding experiment at 25 ◦C. The denaturation midpoint of GdmCl unfolding (Cm ) of rAPA-N196Q was much lower than that of APA. Interestingly, a gradually unfolding ahead of the main transition was observed for APA, but not for rAPA-N196Q (Figure 7). Although it is difficult to explain the molecular basis of this gradual pre-unfolding, it is likely to result from the presence of less populated, more processed N196glycan in the purified APA. The presence of minor heterogeneity in the N196-glycan (20–30 %) has already been characterized and its population also varies with the cultural season of A. pernyi larva [16]. N196-glycans that have a reduced length in sugar moieties are likely less capable of forming intra- and inter-subunit hydrogen bonds. H2 O ) at a The Gibbs free energy of the unfolded state (G unfold zero concentration of GdmCl was obtained following the simple assumption that the Gunfold is linearly dependent on the H2 O concentration of denaturant [36]. Using this method, (G unfold ) −1 of rAPA-N196Q was determined to be 14.8 + 0.6 kcal · mol . − H2 O value for APA was roughly estimated to be 23– The G unfold 24 kcal · mol− 1 for the two tentative populations of the gradual pre-transition (22.9 and 24.0 kcal · mol− 1 for 20 and 25 % H2 O respectively), where the linearity values (R2 ) of the plot of G unfold against [GdmCl] were 0.997 and 0.996 respectively (Figure 7). H2 O The G unfold of APA was 18.2 and 28.1 kcal · mol− 1 when the populations of the gradual pre-transition were assumed to be 15 and 30 % respectively, where the linearity values were 0.989 and H2 O 0.994. Although it is difficult to determine the exact G unfold value of APA, probably due to the presence of minor heterogeneity in the N196-glycan, it is clear that the presence of N196-glycan stabilizes the overall stability of APA by at least ∼ 10 kcal · mol− 1 . The overall GdmCl-unfolding profile of rAPA was similar to that of APA (results not shown). We cannot completely rule out the possibility that decreased stability of rAPA-N196Q could result from a structural perturbation by the mutation of Asn196 into c The Authors Journal compilation c 2009 Biochemical Society 94 K.-S. Ryu and others Figure 7 Figure 6 Differential oligomeric states of APA and rAPA-N196Q The oligomeric states of rAPA (A) and rAPA-N196Q (B) were resolved using an equilibrium sedimentation analysis at 20 ◦C. Experimental data are shown with small open circles and the fitting results are shown as lines. The rAPA exists as an almost pure hexamer, because the fit residuals of the hexamer and hexamer–trimer equilibrium models were almost identical. However, a hexamer–trimer equilibrium was observed for rAPA-N196Q. tri, trimer; hexa, hexamer; octa, octamer. glutamine. However, the side chain of Asn196 is exposed to the protein surface and there are many hydrogen bonds mediated by N196-glycan (Figure 5), where even GlcNAc-1 moiety forms six hydrogen bonds with the protein core. Therefore the results from the GdmCl-unfolding experiments of APA and rAPA-N196Q are likely to show that the presence of N196-glycan is important not only for stabilizing the hexameric state, but also for increasing the overall stability of APA. The presence of N196-glycan-mediated intra- and inter-subunit hydrogen bonds seems to be the primary reason for the decreased stability of rAPA-N196Q in comparison with APA and rAPA. The presence of N196-glycan decreases the hydrophobic SASA of APA APA has a high proportion of aromatic residues compared with PIH (19.2 and 11.2 % respectively), and thus its overall proportion of hydrophobic residues is also higher than that of PIH (47.7 and 39.7 % respectively). Although the increased number of aromatic c The Authors Journal compilation c 2009 Biochemical Society GdmCl unfolding of APA and rAPA-N196Q The fluorescence intensity maxima of rAPA-N196Q were obtained with 280 nm excitation after overnight incubation. However, two additional incubation times (1 and 2 days) were used to test whether complete equilibrium was reached during the GdmCl-unfolding of APA. Fractions of the unfolded form (U) of APA and rAPA-N196Q were obtained from the baseline correction of the measured intrinsic fluorescence. A gradual unfolding before the main transition was observed for APA, but not for rAPA-N196Q. This probably resulted from the presence of a minor population of more processed N196-glycans (20–30 %) [16,20]. The unfolded fraction at the main transition was recalculated after subtraction of the population from the minor transition. Two representative populations of the minor transition (a, 20 %; b, 25 %) were used to show the influence of the H2 O ). Inset, The G unfold of all prominor transition on the overall stability of APA (G unfold teins was estimated from the ratio of the unfolded form (U) to the native form (N) of the protein: H2 O , kcal · mol−1 ) was G unfold = −RT × ln[U/N]. The free energy of the native proteins (G unfold H2 O + m · [GdmCl]. APA (22.9 + obtained through linear extrapolation; G unfold = G unfold − 0.5 −1 was more stable than rAPA-N196Q and 24.0 + − 0.7 kcal · mol for a and b respectively) H2 O −1 (14.8 + − 0.6 kcal · mol ). The errors of G unfold were obtained from the errors of the linear fitting. residues for APA compared with PIH increases the number of the cation–π interactions between positively charged and aromatic residues (137 in APA compared with 78 in PIH) [30,37], the higher population of hydrophobic residues possibly decreases the overall stability via increasing the hydrophobic SASA. The hydrophobic SASAs of APA (without glycans) and PIH were 76752 and 73278 Å2 respectively, and the presence of glycans in APA reduced the hydrophobic SASA from 76752 to 73738 Å2 , and thus probably decreased the overall unfavourable exposure of hydrophobic surfaces to the aqueous phase. It is also possible that the N196-glycan additionally stabilizes the structure of APA by decreasing the Cp (heat capacity of unfolding) of APA. Recently, chemical glycosylation studies have shown that increasing the degree of glycosylation by lactose or dextran linearly enhances the thermal stability of proteins by decreasing the Cp , where the effect is intensified by increasing the size of the modified glycan, and by increasing the protein melting temperature [38]. The decreased Cp supposedly result from the association of the exposed non-polar residues of the unfolded protein with the glycan moieties [39]. DISCUSSION Although the crystal structure of APA and the biophysical results indicate that the N196-glycan plays an important role in Monoglucosylated N-glycan enhances protein stability stabilizing the APA structure, more detailed studies to determine its structural roles must be conducted. The inner region of APA is much more hydrophobic than that of the hexamerin homologue (PIH), and thus the Cp of APA would be higher [40,41]. The hydrophobic SASAs of unfolded and folded APA (without glycans) were 347 399.4 and 76 752.2 Å2 respectively, and those of the unfolded and folded PIH were 310 556.4 and 73 277.9 Å2 respectively. Monoglucosylated N-glycan (Glc1 Man9 GlcNAc2 ), which corresponds to the N196-glycan of APA, seems to be present mainly in the arylphorin protein family and this is related to the high population of hydrophobic residues since members contain many aromatic residues. Therefore characterizing more H2 O H2 O and Sunfold ), detailed thermodynamic parameters (Cp , Hunfold H2 O in addition to G unfold , for both APA and rAPA-N196Q is still required for achieving a better understanding of the complete mechanism by which N196-glycan stabilizes the structure of APA. It has been reported that the presence of glycans stabilize RNase A and B (∼ 1.2 kcal · mol− 1 ) [42], and α 1 -antitrypsin (2.1 kcal · mol− 1 ) [43]. Moreover, the increased Gunfold of αchymotrypsin even after multiple dextran chemical modifications is also less than 10 kcal · mol− 1 [38]. However, the glycosylation of Asn196 seems to be critical for enhancing the stability of APA, because its stabilizing effect is very large. This may be due to the location of N196-glycan (the deep cleft of subunit interface) and also due to the presence of N196-glycan mediated intra- and intersubunit hydrogen bonds. The backbone of the glycan is known to be very flexible, and thus it is implausible that a structurally pre-assembled N196-gycan forms hydrogen bonds with a protein partner. Paradoxically, this high flexibility could provide a sufficient degree of freedom for glycan moieties to adapt to its surrounding protein environment, and to form hydrogen bonds via their many hydroxy groups, although this is a somewhat unfavourable process in terms of entropy. The effect of the N196glycan on the overall stability may originate from its location in APA. It would be interesting to study the effect of glycosylation sites on the overall stability of the glycoprotein (surface compared with the deep cleft) and to see whether the glycan could adapt to the deep cleft of the subunit interface and generate additional hydrogen bonds. The crystal structure of APA also explains why the N196-glycan can survive the intensive trimming processes that occur in the ER and Golgi apparatus. The remaining Glc-a residue of the mature APA suggests that at least a dimer formation (subunits A and D, or B and F, or C and E pairs) occurs before the completion of folding. The largest interface area was observed for these dimer pairs, and each pair was additionally stabilized by reciprocal intersubunit hydrogen bonds that involved the sugar moieties of Asn196 , including Glc-a. Therefore the accessibility of Glc-a to glucosidase II, which exists in N196-glycan, may be greatly reduced. Monoglucosylated N-glycans that exist in other proteins, such as avian IgY, the egg jelly coat of starfish and human complement component C3, probably face a similar environment as the monoglucosylated N-glycan of APA. The complete hexamer and interdisulfide bridge formation of APA would also be cleaved during the calnexin/calreticulin cycle, because it includes ERp57 (ER protein 57), a thiol-disulfide oxidoreductase [4]. The chaperone-like activity of N-glycosylation may also be dependent on the location of N-glycosylation in the partially folded protein, which could influence the accessibility of the calnexin/calreticulin chaperon complex to the monoglucosylated N-glycan. The arylphorin protein family, which appears to have been evolved from haemocyanin, has an exceptionally high proportion of aromatic amino acids. These proteins may have acquired glycosylation during evolution to prevent hydrophobic aggregation during the folding process and/or to enhance their stability. 95 It is expected that the high stability of APA contributes to its long half-life during the insect lifespan, even in the absence of protease activity in the haemolymph, because (i) the insect larva seems to face a hostile environment involving large deviations in temperature, and (ii) APA should be accumulated and remained in large amounts before being used for insect maturation. Finally, ecdysone activates the maturation of the arylphorin receptor, and the receptor incorporates APA into the larval fat body for utilization as energy and amino acid sources. These factors could constitute a reason to generate a unique trafficking pathway for arylphorin in the class Insecta, in which arylphorin is secreted from the fat body to the haemolymph and is then reabsorbed into the fat body. AUTHOR CONTRIBUTION Kyoung-Seok Ryu mainly performed the structural determination, biophysical studies and the writing of the paper. Jie-Oh Lee helped with the data collection and the crystal symmetry determination. Taek Hun Kwon, Young Hyan Han, Yun-Seok Choi and Young Ho Jeon assisted with the X-ray data analysis and the biochemical experiments. Han-Ho Choi and Hong-Seog Park purified the natural APA protein. Soo Kyung Hwang, Zee-Won Lee and Kyung-Bok Lee conducted the purification and characterization of recombinant arylphorin proteins from HEK-293a cells and the analysis of glycosylation. Chaejoon Cheong and Soohyun Kim outlined experimental direction, performed data analysis and writing of the paper. ACKNOWLEDGEMENTS We thank S. M. Lee (Pusan National University, Busan, South Korea) and O. K. Cho (Korea Basic Science Institute) for the generous supply of insect haemolymph and mutant generation respectively. We also thank S. J. Park and N. Y. Oh (both at the Korea Basic Science Institute) for glycan analysis. FUNDING This study was supported by a Korea Basic Science Institute (KBSI) grant [grant number T27010] to S. K. REFERENCES 1 Dennis, J. W., Granovsky, M. and Warren, C. E. (1999) Protein glycosylation in development and disease. BioEssays 21, 412–421 2 Varki, A. (1993) Biological roles of oligosaccharides: all of the theories are correct. Glycobiology 3, 97–130 3 Mitra, N., Sinha, S., Ramya, T. N. and Surolia, A. (2006) N-linked oligosaccharides as outfitters for glycoprotein folding, form and function. Trends Biochem. Sci. 31, 156–163 4 Helenius, A. and Aebi, M. (2004) Roles of N-linked glycans in the endoplasmic reticulum. Annu. Rev. Biochem. 73, 1019–1049 5 Apweiler, R., Hermjakob, H. and Sharon, N. (1999) On the frequency of protein glycosylation, as deduced from analysis of the SWISS-PROT database. Biochim. Biophys. Acta 1473, 4–8 6 Wormald, M. R., Petrescu, A. J., Pao, Y. L., Glithero, A., Elliott, T. and Dwek, R. A. (2002) Conformational studies of oligosaccharides and glycopeptides: complementarity of NMR, X-ray crystallography, and molecular modelling. Chem. Rev. 102, 371–386 7 Mitra, N., Sharon, N. and Surolia, A. (2003) Role of N-linked glycan in the unfolding pathway of Erythrina corallodendron lectin. Biochemistry 42, 12208–12216 8 Buck, T. M., Eledge, J. and Skach, W. R. (2004) Evidence for stabilization of aquaporin-2 folding mutants by N-linked glycosylation in endoplasmic reticulum. Am. J. Physiol. Cell Physiol. 287, C1292–C1299 9 Mer, G., Hietter, H. and Lefevre, J. F. (1996) Stabilization of proteins by glycosylation examined by NMR analysis of a fucosylated proteinase inhibitor. Nat. Struct. Biol. 3, 45–53 10 Fusetti, F., Schroter, K. H., Steiner, R. A., van Noort, P. I., Pijning, T., Rozeboom, H. J., Kalk, K. H., Egmond, M. R. and Dijkstra, B. W. (2002) Crystal structure of the copper-containing quercetin 2,3-dioxygenase from Aspergillus japonicus . Structure 10, 259–268 11 Shental-Bechor, D. and Levy, Y. (2008) Effect of glycosylation on protein folding: a close look at thermodynamic stabilization. Proc. Natl. Acad. Sci. U.S.A. 105, 8256–8261 12 Petrescu, A. J., Milac, A. L., Petrescu, S. M., Dwek, R. A. and Wormald, M. R. (2004) Statistical analysis of the protein environment of N-glycosylation sites: implications for occupancy, structure, and folding. Glycobiology 14, 103–114 c The Authors Journal compilation c 2009 Biochemical Society 96 K.-S. Ryu and others 13 Burmester, T. and Scheller, K. (1996) Common origin of arthropod tyrosinase, arthropod hemocyanin, insect hexamerin, and dipteran arylphorin receptor. J. Mol. Evol. 42, 713–728 14 Telfer, W. H. and Kunkel, J. G. (1991) The function and evolution of insect storage hexamers. Annu. Rev. Entomol. 36, 205–228 15 Telfer, W. H. and Pan, M. L. (2003) Storage hexamer utilization in Manduca sexta . J. Insect Sci. 3, 26 16 Jung, H. I., Kim, Y. H. and Kim, S. (2005) Structural basis for the presence of a monoglucosylated oligosaccharide in mature glycoproteins. Biochem. Biophys. Res. Commun. 331, 100–106 17 Zhou, X., Oi, F. M. and Scharf, M. E. (2006) Social exploitation of hexamerin: RNAi reveals a major caste-regulatory factor in termites. Proc. Natl. Acad. Sci. U.S.A. 103, 4499–4504 18 Jaenicke, E., Foll, R. and Decker, H. (1999) Spider hemocyanin binds ecdysone and 20-OH-ecdysone. J. Biol. Chem. 274, 34267–34271 19 Burmester, T. and Scheller, K. (1997) Developmentally controlled cleavage of the Calliphora arylphorin receptor and posttranslational action of the steroid hormone 20-hydroxyecdysone. Eur. J. Biochem. 247, 695–702 20 Kim, S., Hwang, S. K., Dwek, R. A., Rudd, P. M., Ahn, Y. H., Kim, E. H., Cheong, C., Kim, S. I., Park, N. S. and Lee, S. M. (2003) Structural determination of the N-glycans of a lepidopteran arylphorin reveals the presence of a monoglucosylated oligosaccharide in the storage protein. Glycobiology 13, 147–157 21 Ohta, M., Hamako, J., Yamamoto, S., Hatta, H., Kim, M., Yamamoto, T., Oka, S., Mizuochi, T. and Matsuura, F. (1991) Structures of asparagine-linked oligosaccharides from hen egg-yolk antibody (IgY): occurrence of unusual glucosylated oligo-mannose type oligosaccharides in a mature glycoprotein. Glycoconjugate J. 8, 400–413 22 Endo, T., Hoshi, M., Endo, S., Arata, Y. and Kobata, A. (1987) Structures of the sugar chains of a major glycoprotein present in the egg jelly coat of a starfish, Asterias amurensis . Arch. Biochem. Biophys. 252, 105–112 23 Crispin, M. D., Ritchie, G. E., Critchley, A. J., Morgan, B. P., Wilson, I. A., Dwek, R. A., Sim, R. B. and Rudd, P. M. (2004) Monoglucosylated glycans in the secreted human complement component C3: implications for protein biosynthesis and structure. FEBS Lett. 566, 270–274 24 Khalaila, I., Peter-Katalinic, J., Tsang, C., Radcliffe, C. M., Aflalo, E. D., Harvey, D. J., Dwek, R. A., Rudd, P. M. and Sagi, A. (2004) Structural characterization of the N-glycan moiety and site of glycosylation in vitellogenin from the decapod crustacean Cherax quadricarinatus . Glycobiology 14, 767–774 25 Kang, P., Mechref, Y., Klouckova, I. and Novotny, M. V. (2005) Solid-phase permethylation of glycans for mass spectrometric analysis. Rapid Commun. Mass Spectrom. 19, 3421–3428 26 Marti-Renom, M. A., Stuart, A. C., Fiser, A., Sanchez, R., Melo, F. and Sali, A. (2000) Comparative protein structure modeling of genes and genomes. Annu. Rev. Biophys. Biomol. Struct. 29, 291–325 27 Collaborative Computational Project, Number 4 (1994) The CCP4 suite: programs for protein crystallography. Acta Crystallogr. Sect. D Biol. Crystallogr. 50, 760–763 Received 3 November 2008/3 April 2009; accepted 9 April 2009 Published as BJ Immediate Publication 9 April 2009, doi:10.1042/BJ20082170 c The Authors Journal compilation c 2009 Biochemical Society 28 Brunger, A. T., Adams, P. D., Clore, G. M., DeLano, W. L., Gros, P., Grosse-Kunstleve, R. W., Jiang, J. S., Kuszewski, J., Nilges, M., Pannu, N. S. et al. (1998) Crystallography & NMR system: a new software suite for macromolecular structure determination. Acta Crystallogr. Sect. D Biol. Crystallogr. 54, 905–921 29 Pettersen, E. F., Goddard, T. D., Huang, C. C., Couch, G. S., Greenblatt, D. M., Meng, E. C. and Ferrin, T. E. (2004) UCSF Chimera: a visualization system for exploratory research and analysis. J. Comput. Chem. 25, 1605–1612 30 Gallivan, J. P. and Dougherty, D. A. (1999) Cation–π interactions in structural biology. Proc. Natl. Acad. Sci. U.S.A. 96, 9459–9464 31 Brown, P. H. and Schuck, P. (2006) Macromolecular size-and-shape distributions by sedimentation velocity analytical ultracentrifugation. Biophys. J. 90, 4651–4661 32 Vandenbroeck, K., Martens, E. and Alloza, I. (2006) Multi-chaperone complexes regulate the folding of interferon-γ in the endoplasmic reticulum. Cytokine 33, 264–273 33 Deprez, P., Gautschi, M. and Helenius, A. (2005) More than one glycan is needed for ER glucosidase II to allow entry of glycoproteins into the calnexin/calreticulin cycle. Mol. Cell 19, 183–195 34 Burmester, T., Antoniewski, C. and Lepesant, J. A. (1999) Ecdysone-regulation of synthesis and processing of fat body protein 1, the larval serum protein receptor of Drosophila melanogaster . Eur. J. Biochem. 262, 49–55 35 Volbeda, A. and Hol, W. G. (1989) Crystal structure of hexameric haemocyanin from Panulirus interruptus refined at 3.2 Å resolution. J. Mol. Biol. 209, 249–279 36 Creighton, T. E. (1993) Stability of the folded conformation. In Proteins: Structures and Molecular Properties, 2nd edn, pp. 287–291, W. H. Freeman, New York 37 Pack, S. P. and Yoo, Y. J. (2004) Protein thermostability: structure-based difference of amino acid between thermophilic and mesophilic proteins. J. Biotechnol. 111, 269–277 38 Sola, R. J., Rodriguez-Martinez, J. A. and Griebenow, K. (2007) Modulation of protein biophysical properties by chemical glycosylation: biochemical insights and biomedical implications. Cell. Mol. Life Sci. 64, 2133–2152 39 van Teeffelen, A. M., Broersen, K. and de Jongh, H. H. (2005) Glucosylation of β-lactoglobulin lowers the heat capacity change of unfolding; a unique way to affect protein thermodynamics. Protein Sci. 14, 2187–2194 40 Makhatadze, G. I. and Privalov, P. L. (1990) Heat capacity of proteins. I. Partial molar heat capacity of individual amino acid residues in aqueous solution: hydration effect. J. Mol. Biol. 213, 375–384 41 Privalov, P. L. and Makhatadze, G. I. (1990) Heat capacity of proteins. II. Partial molar heat capacity of the unfolded polypeptide chain of proteins: protein unfolding effects. J. Mol. Biol. 213, 385–391 42 Joao, H. C. and Dwek, R. A. (1993) Effects of glycosylation on protein structure and dynamics in ribonuclease B and some of its individual glycoforms. Eur. J. Biochem. 218, 239–244 43 Kwon, K. S. and Yu, M. H. (1997) Effect of glycosylation on the stability of α 1 -antitrypsin toward urea denaturation and thermal deactivation. Biochim. Biophys. Acta 1335, 265–272 Biochem. J. (2009) 421, 87–96 (Printed in Great Britain) doi:10.1042/BJ20082170 SUPPLEMENTARY ONLINE DATA The presence of monoglucosylated N196-glycan is important for the structural stability of storage protein, arylphorin Kyoung-Seok RYU*, Jie-Oh LEE†, Taek Hun KWON*, Han-Ho CHOI‡, Hong-Seog PARK‡, Soo Kyung HWANG§, Zee-Won LEE§, Kyung-Bok LEE§, Young Hyun HAN*, Yun-Seok CHOI*, Young Ho JEON*, Chaejoon CHEONG*1 and Soohyun KIM§1 *Magnetic Resonance Team, Korea Basic Science Institute, Gwahangno 113, Daejeon 305-333, South Korea, †Department of Chemistry, Korea Advanced Institute of Science and Technology, Gwahangno 335, Daejeon 305-701, South Korea, ‡Genome Research Center, Korea Research Institute of Bioscience and Biotechnology, Gwahangno 111, Daejeon 305-806, South Korea, and §Division of Life Science, Korea Basic Science Institute, Gwahangno 113, Daejeon 305-333, South Korea Figure S2 Characterizations of the wild-type and mutant APA proteins that were expressed in HEK-293a cells Figure S1 Identification of N-glycans attached to Asn196 and Asn344 using normal-phase HPLC and MALDI–TOF-MS (A) Oligosaccharides released from denatured proteins were labelled with 2-aminobenzamide, and then separated by normal-phase HPLC. APA, rAPA and rAPA-N196Q were expressed in HEK-293a cells. The monoglucosylated N196-glycan is indicated with an asterisk (*). The N196-glycan profile of rAPA was very similar to that of APA. However, the N344-glycan profile of rAPA was different from that of APA, possibly due to the differential glycan processing between the Golgi apparatus of insect and mammalian cells. GU, glucose units. (B) The same glycans were permethylated and analysed by MALDI–TOF-MS to confirm the HPLC results. The monoglucosylated glycan is indicated with an asterisk (*). (A) Top panel: Western blot analysis of the total cells using anti-FLAG antibody confirmed that wild-type rAPA and two mutant APA proteins (rAPA-N196Q and rAPA-N344Q) were expressed in HEK-293a cells. Middle panel: the same Western blot analysis of the culture medium shows that the rAPA and rAPA-N196Q proteins were secreted from the cell, but the rAPA-N344Q was not. The amount of secreted rAPA-N196Q was less than that of rAPA. Bottom panel: Western blot analysis with anti-actin antibodies was used to confirm that the same amount of cells was used as in the top panel. (B) rAPA and rAPA-N196Q were purified using anti-FLAG affinity column, and then the presence of intermolecular disulfide bonds in rAPA and rAPA-N196Q was determined from non-reducing SDS-PAGE analysis [ETSH (ethanethiol), 2-mercaptoethanol], which stabilizes the trimeric structure of APA. The monomeric, dimeric and trimeric bands are indicated by the arrows. 1 Correspondence may be addressed to either of these authors (email [email protected] or [email protected]). The crystal structure of Antheraea pernyi arylphorin has been deposited in the RCSB Protein Data Bank under code 3GWJ. c The Authors Journal compilation c 2009 Biochemical Society K.-S. Ryu and others Figure S3 Structure comparison of arylphorin and haemocyanin (A) The overall hexameric structure of APS (left-hand panel) was very similar to that of PIH (right-hand panel). The subunit origination of residues was indicated using a chain code. The cysteine residues that form disulfide bonds are shown as yellow spheres. (B) Superimposed ribbon presentation of APA (brown) and PIH (cyan) monomers. The overall structures of both proteins were very similar, except for some additional local regions. The additional secondary structures of APA are labelled. The APA loop (residues 619–635, coloured red) has a very high B -factor; the corresponding loop in PIH was stabilized by a disulfide bond between Cys562 and Cys609 . c The Authors Journal compilation c 2009 Biochemical Society Monoglucosylated N-glycan enhances protein stability Figure S4 Stereo view annealed omit map of Asn196 monoglucosylated oligosaccharide and N344-glycan (A) All the sugar moieties of N196-glycan can be observed in the electron-density map. We clearly determined the positions of most sugar moieties from GlcNAc-1 to Man-B, and moderately for those of Man-D2 and Man-D3, which were located in the terminus. (B) Only two proximal sugar moieties of N344-glycan were observed in the electron-density map. Figure S5 B -factor analysis of N196- and N344-glycans The B -factors of N196-glycans did not vary with their subunit origins, but those of N344-glycans had diverse distributions according to their subunit origins. The position of the sugar moieties of BC2 (Man-B and Man-D3) were outside of the protein core and thus had the highest B -factors. On the other hand, the sugar moieties of LC (GlcNAc-1 to Glc-a) were involved in many inter- and intra-subunit hydrogen bonds, and thus their B -factors were lower than those of BC1 (Man-A and Man-D2) and BC2. Received 3 November 2008/3 April 2009; accepted 9 April 2009 Published as BJ Immediate Publication 9 April 2009, doi:10.1042/BJ20082170 c The Authors Journal compilation c 2009 Biochemical Society