* Your assessment is very important for improving the workof artificial intelligence, which forms the content of this project
Download Heat shock proteins
Silencer (genetics) wikipedia , lookup
Biochemical cascade wikipedia , lookup
Gene expression wikipedia , lookup
Metalloprotein wikipedia , lookup
Ancestral sequence reconstruction wikipedia , lookup
Biochemistry wikipedia , lookup
Magnesium transporter wikipedia , lookup
G protein–coupled receptor wikipedia , lookup
Expression vector wikipedia , lookup
Paracrine signalling wikipedia , lookup
Bimolecular fluorescence complementation wikipedia , lookup
Signal transduction wikipedia , lookup
Interactome wikipedia , lookup
Protein structure prediction wikipedia , lookup
Protein purification wikipedia , lookup
Western blot wikipedia , lookup
Two-hybrid screening wikipedia , lookup
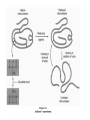
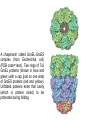
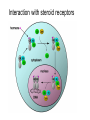
ANFINSEN: AMINO ACID SEQUENCE DETERMINES PROTEIN SHAPE A proportion of all newly-made proteins require assistance to convert from a linear chain of amino acids to a functional three-dimensional entity. This process is called protein folding. Chaperonins are protein complexes that assist the folding of these nascent, non-native polypeptides into their native, functional state. These proteins belong to a large class of molecules that assist protein folding, called molecular chaperones. These molecular machines use chemical energy, in the form of adenosine triphosphate (ATP), to promote protein folding in all cells. A chaperone (or occasionally chaperon) is an adult who accompanies or supervises one or more young, unmarried men or women during social occasions, usually with the specific intent of preventing inappropriate social or sexual interactions or illegal behavior (e.g., underage drinking or illegal drug use). The chaperone is typically accountable to a third party, usually the parents of one of the accompanied young people. The word derives figuratively from the French word chaperon, meaning "hood", and later a kind of hat. This is either from this sense or from falconry, where the same word meant the hood placed over the head of a bird of prey to stop its desire to fly. Traditionally, a chaperone was an older married or widowed woman accompanying a young woman when men would be present. Her presence was a guarantee of the virtue of the young woman in question. A chaperonin called GroEL-GroES complex (from Escherichia coli) (PDB code=1aon). Two rings of 7x2 GroEL proteins (shown in blue and green) with a cap (just on one side) of GroES proteins (red and yellow). Unfolded proteins enter that cavity (which is protein sized) to be protected during folding Heat shock proteins (HSP) are a class of functionally related proteins whose expression is increased when cells are exposed to elevated temperatures or other stress. This increase in expression is transcriptionally regulated. The dramatic upregulation of the heat shock proteins is a key part of the heat shock response and is induced primarily by heat shock factor (HSF).HSPs are found in virtually all living organisms, from bacteria to humans. Heat-shock proteins are named according to their molecular weight. For example, Hsp60, Hsp70 and Hsp90 (the most widely-studied HSPs) refer to families of heat shock proteins on the order of 60, 70 and 90 kilodaltons in size, respectively. Hsp90 (heat shock protein 90) is a molecular chaperone and is one of the most abundant proteins expressed in cells. It is a member of the heat shock protein family which is upregulated in response to stress. Hsp90 is found in bacteria and all branches of eukarya, but it is apparently absent in archaea. Heat shock protein 90 (Hsp90) is one of the most common of the heat related proteins. The protein is named "HSP" for obvious reasons whereas the "90" comes from the fact that it weighs roughly 90 kiloDaltons. A 90 kDa size protein is considered a fairly large for a non-fibrous protein. family subcellular location subfamily HSP90A cytosolic HSP90AA (inducible) gene protein HSP90AA1 Hsp90-α1 HSP90AA2 Hsp90-α2 HSP90AB HSP90AB1 (constitutively expressed) Hsp90-β HSP90B endoplasmicr eticulum HSP90B1 GRP94 TRAP mitochondrial TRAP1 TNF ReceptorAssociated Protein 1 Protein folding and chaperone Hsp90 is known to associate with the non-native structures of many proteins which has lead to the proposal that Hsp90 is involved in protein folding in general. Furthermore Hsp90 has been shown to suppress the aggregation of a wide range of "client" or "substrate" proteins and hence acts as a general protective chaperone. However Hsp90 is somewhat more selective than other chaperones. Interaction with steroid receptors Cancerous cells over express a number of proteins, including growth factor receptors, such as EGFR, or signal transduction proteins such as PI3K and AKT (Inhibition of these proteins may trigger apoptosis). Hsp90 stabilizes various growth factor receptors and some signaling molecules including PI3K and AKT proteins, hence inhibition of Hsp90 may induce apoptosis through inhibition of the PI3K/AKT signaling pathway and growth factor signaling generally. Native disorder explained The majority of water-soluble proteins have structures that are globular and relatively static. However, some proteins have regions that are natively disordered. Disordered regions are flexible, dynamic and can be partially or completely extended in solution. Native disorder also exists in global structures such as extended random coil proteins with negligible secondary structure or molten globules, which have regular secondary structure elements but have not condensed into a stable globular fold. The primary function of disorder appears to be molecular recognition of proteins and nucleic acids. It has been speculated that the multiple metastable conformations, adopted by disordered binding sites, allows recognition of several targets with high specificity and low affinity. Order to disorder transitions also provide a mechanism for controlling protein concentration via proteolytic degradation. Disordered regions are often characterised by low sequence complexity, compositional bias toward aromatic and hydrophilic residues and high flexibility. The absence of a static structure means that disordered residues do not appear in the electron density maps obtained by X-ray crystallography but they can be investigated using other types of spectroscopy such as circular dichroism and NMR.