* Your assessment is very important for improving the workof artificial intelligence, which forms the content of this project
Download Discussion Worksheet #10 Formation of Alcohols Skill 1: Functional
Physical organic chemistry wikipedia , lookup
George S. Hammond wikipedia , lookup
Ring-closing metathesis wikipedia , lookup
Diels–Alder reaction wikipedia , lookup
Elias James Corey wikipedia , lookup
Ene reaction wikipedia , lookup
Baylis–Hillman reaction wikipedia , lookup
Wolff rearrangement wikipedia , lookup
Kinetic resolution wikipedia , lookup
Tiffeneau–Demjanov rearrangement wikipedia , lookup
Wolff–Kishner reduction wikipedia , lookup
Asymmetric induction wikipedia , lookup
Hydroformylation wikipedia , lookup
Stille reaction wikipedia , lookup
Petasis reaction wikipedia , lookup
Strychnine total synthesis wikipedia , lookup
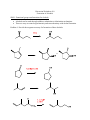
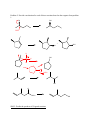
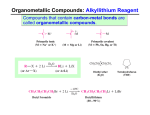
Discussion Worksheet #10 Formation of Alcohols Skill 1: Functional group transformations for alcohols Alcohols can be made through addition, substitution or elimination mechanisms There are ways to control regiochemistry and stereochemistry with alcohol formation Problem 1: Provide the reagents necessary for formation of these alcohols. Problem 2. Provide a mechanism for each of these reactions based on the reagents from problem 1. Br OH HO- + OH OH H O H B OH H H OH OCH3 OH O OCH3 Skill 2: Predict the products of Grignard reactions OH Reaction of Grignard with an aldehyde (except formaldehyde) leads to a secondary alcohol Reaction of a Grignard with a ketone gives a tertiary alcohol Grignard reagents react with esters twice to form tertiary alcohols Problem 3: Predict the products of each Grignard reaction. Include stereochemistry where necessary. OH 1. CH3MgBr 2. acid quench O 1. CH3MgBr O 2. acid quench OCH3 1. CH3MgBr O 2. acid quench OH 1. PhMgCl 2. H+ O I 1. Mg, ether 2. CH3CH2CHO 3. H+ OH Skill 2: Grignard in retrosynthesis Plan a Grignard retrosynthesis if you need to make a carbon-carbon bond next to a hydroxyl group Problem 4: Provide the necessary reagents Problem 5. Plan two alternative syntheses involving Grignard reagents for each of these compounds. If two syntheses are not possible, explain. In which cases could an ester be used as the starting material? 1. xs CH3Ch2MgI 2. H3O+ MeO 1. CH3CH2MgBr 2. H3O+ O O OH O OH 1. CH3MgBr 2. H3O+