* Your assessment is very important for improving the workof artificial intelligence, which forms the content of this project
Download Acetyl CoA - WordPress.com
Ultrasensitivity wikipedia , lookup
Metalloprotein wikipedia , lookup
Photosynthesis wikipedia , lookup
Biochemical cascade wikipedia , lookup
Lipid signaling wikipedia , lookup
Mitogen-activated protein kinase wikipedia , lookup
Light-dependent reactions wikipedia , lookup
Biosynthesis wikipedia , lookup
Basal metabolic rate wikipedia , lookup
Phosphorylation wikipedia , lookup
Butyric acid wikipedia , lookup
Photosynthetic reaction centre wikipedia , lookup
Mitochondrion wikipedia , lookup
Evolution of metal ions in biological systems wikipedia , lookup
Adenosine triphosphate wikipedia , lookup
Microbial metabolism wikipedia , lookup
Electron transport chain wikipedia , lookup
Amino acid synthesis wikipedia , lookup
Fatty acid synthesis wikipedia , lookup
Glyceroneogenesis wikipedia , lookup
Nicotinamide adenine dinucleotide wikipedia , lookup
Fatty acid metabolism wikipedia , lookup
Biochemistry wikipedia , lookup
Oxidative phosphorylation wikipedia , lookup
Lactate dehydrogenase wikipedia , lookup
NADH:ubiquinone oxidoreductase (H+-translocating) wikipedia , lookup
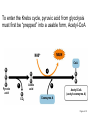
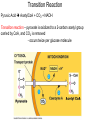
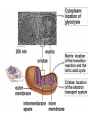
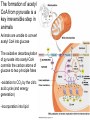
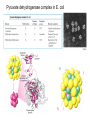
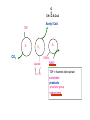
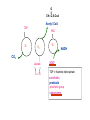
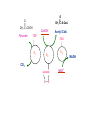
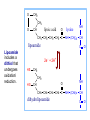
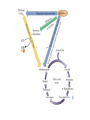
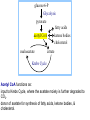
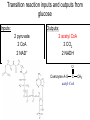
PDH 17.1 What Are the Metabolic Fates of NADH and Pyruvate Produced in Glycolysis? NADH is energy - two possible fates: If O2 is available: NADH is re-oxidized in the electron transport pathway, making ATP in oxidative phosphorylation If O2 is not available (anaerobic conditions): NADH is re-oxidized by lactate dehydrogenase (LDH), providing additional NAD+ for more glycolysis What Are the Metabolic Fates of NADH and Pyruvate Produced in Glycolysis? Pyruvate is also energy - two possible fates: If O2 is available pyruvate enters the mitochondria, where it undergoes further breakdown If O2 is not available (anaerobic conditions) fermentation occurs and pyruvate undergoes reduction Fermentation is an anaeorbic process and does not require oxygen. In humans, pyruvate is reduced to lactic acid during fermentation. To enter the Krebs cycle, pyruvic acid from glycolysis must first be “prepped” into a usable form, Acetyl-CoA CoA 2 Acetic acid 1 Pyruvic acid CO2 3 Acetyl-CoA (acetyl-coenzyme A) Coenzyme A Figure 6.10 Transition Reaction Pyruvic Acid AcetylCoA + CO2 + NADH Transition reaction – pyruvate is oxidized to a 2-carbon acetyl group carried by CoA, and CO2 is removed - occurs twice per glucose molecule Mitochondria: powerhouse A mitochondrion is a cellular organelle that has a double membrane, with an intermembrane space between the two layers. The transition reaction and citric acid cycle occur in the mitochondrial matrix. The electron transport system is located in the cristae of the mitochondria. The formation of acetyl CoA from pyruvate is a key irreversible step in animals Animals are unable to convert acetyl CoA into glucose The oxidative decarboxylation of pyruvate into acetyl-CoA commits the carbon atoms of glucose to two principle fates -oxidation to CO2 by the citric acid cycle (and energy generation) -incorporation into lipid Entry of Pyruvate into the Mitochondrion Pyruvate translocase transports pyruvate into the mitochondria in symport with H+ Symport: is used to denote an integral membrane protein that simultaneouly transports two substances across membrane in the same direction. Pyruvate Dehydrogenase H3C O O C C pyruvate HSCoA O O H3C NAD+ NADH C S CoA + CO2 acetyl-CoA Pyruvate Dehydrogenase, catalyzes oxidative decarboxylation of pyruvate, to form acetyl-CoA. Pyruvate dehydrogenase complex consists of three distinct enzymes (in addition to the five coenzymes) This reaction involves --generation of a reduced electron carrier (NADH) --decarboxylation of pyruvate --metabolic activation of the remaining two carbons of pyruvate the three enzymes involved are assembled into a highly organized multienzyme assembly called the pyruvate dehydrogenase complex Conversion of Pyruvate to Acetyl CoA Pyruvate dehydrogenase complex (PDH complex) is a multienzyme complex containing: 3 enzymes + 5 coenzymes + other proteins (+ ATP coenzyme as a regulator) E1 = pyruvate dehydrogenase E2 = dihydrolipoamide acetyltransferase E3 = dihydrolipoamide dehydrogenase Structure of the PDH complex (a) Core of the complex (24 E chains) (b) Model of the entire complex: 12 E1 dimers (blue), 12 E2 dimers (yellow), 6 E3 dimers (green) surround the core Pyruvate dehydrogenase complex in E. coli Pyruvate Dehydrogenase Subunits Enzyme Abbreviated Prosthetic Group Pyruvate Dehydrogenase E1 Thiamine pyrophosphate (TPP) Dihydrolipoyl Transacetylase E2 Lipoamide Dihydrolipoyl Dehydrogenase E3 FAD Pyruvate dehydrogenase complex - mechanism 1. E1 accepts a two-carbon aldehyde group from pyruvate and binds it to TPP forming hydroxyethyl TPP 2. Aldehyde group on TPP is transferred by E1 to the first lipoamide swinging arm on E2 and simultaneously oxidized to an acetyl group 6. Reduced FADH2 is oxidized by NAD+ 5. E3 oxidizes the reduced lipoamide by transferring two hydrogens to FAD 3. Acetyl group is tranferred to the second swinging arm of lipoamide which positions it for transfer to CoA 4. Acetyl group is transferred to CoA producing acetyl-CoA http://www.uwsp.edu/chemistry/tzamis/ch260/pyrdh2aseanim.gif O OH OH CH3 -C CH3 -C -COOHOH CH3 -C CoASH CoASH CH3 -C TDP Pyruvate CO2 O CH3 -C-S-CoASH Acetyl CoA FAD CO2 CO2 E1 E2 E3 CO2 Lipoate NAD+ HS S TDP = thiamine diphosphate SH S substrates products prosthetic group coenzymes O CH3 -C-S-CoA Acetyl CoA TDP E1 E3 E2 CO2 FADH2 Lipoate S S NAD+ TDP = thiamine diphosphate substrates products prosthetic group coenzymes O CH3 -C-S-CoA Acetyl CoA TDP FAD E1 E2 E3 NADH CO2 Lipoate S S NAD+ TDP = thiamine diphosphate substrates products prosthetic group coenzymes O O CH3 -C-S-CoA CH3 -C -COOH Pyruvate CoASH TDP Acetyl CoA FAD E1 E2 E3 NADH CO2 Lipoate S S NAD+ Inhibition of lipoamide by arsenic and mercury Arsenic poisoning Arsenite complexes with lipoamide – inactivating it Some sulfhydral reagents relieve the inhibition by forming a complex with the arsenite that can be excreted (competitive inhibition) Mercury poisons in the same way Thiamine deficiency (B1) What would be the results of a diet deficient in thiamine? Thiamine pyrophosphate is a prosthetic group of three important enzymes • pyruvate dehydrogenase • a-ketoglutarate dehydrogenase (CAC) • transketolase (pentose phosphate pathway) If the pyruvate dehydrogenase complex were unable to catalyze the formation of acetyl-CoA from pyruvate – what would accumulate? In cells that rely on glucose for fuel (do not use fats) – the energy that is provided when pyruvate is converted to acetyl-CoA is not generated Which cells in the body rely primarily on glucose for energy? Cells of the nervous system and heart, therefore neurologic and cardiac symptoms are associated with a thiamine deficiency Formation of Acetyl Coenzyme A from pyruvate: Summary this occurs in the mitochondrial matrix and is the link between glycolysis and the citric acid cycle under aerobic conditions, acetyl coenzyme A is formed in the mitochondria by the oxidative decarboxylation of pyruvate pyruvate + NAD+ + CoA acetyl CoA + CO2 + NADH this reaction is catalyzed by pyruvate dehydrogenase the NAD+ required for this reaction and for the oxidation of glyceraldehyde 3 phosphate is regenerated when NADH ultimately transfers its electrons to O2 through the electron transport chain in mitochondria In the overall reaction, the carboxyl group of pyruvate is lost as CO2 while the remaining two carbons form the acetyl moiety of acetyl-CoA generation of a reduced electron carrier (NADH) this reaction is highly exergonic and is essentially irreversible in vivo ∆G0’ = - 33.5 kJ/mol S CH2 CH2 S lipoic acid CH O CH2 CH2 CH2 CH2 C lipoamide Lipoamide includes a dithiol that undergoes oxidation/ reduction. lysine NH NH (CH2)4 CH C O 2e + 2H+ HS CH2 CH2 HS O CH CH2 CH2 CH2 CH2 C dihydrolipoamide NH NH (CH2)4 CH C O dimethylisoalloxazine O H C C N O H3C C C C NH H3C C C C C C H N H C + 2e +2H O N H N H3C C C C NH H3C C C C C C H CH2 FAD N O N H CH2 HC OH HC OH HC OH O H2C C O P O- Adenine O O P O- O Ribose FADH2 HC OH HC OH HC OH O H2C O P O- Adenine O O P O Ribose O- FAD (Flavin Adenine Dinucleotide is derived from the vitamin riboflavin. The dimethylisoalloxazine ring system undergoes oxidation/reduction. FAD is a prosthetic group, permanently part of E3. Reaction: FAD + 2 e- + 2 H+ FADH2 phosphorylation by pyruvate dehydrogenase kinase, (on E1) switches off the activity of the complex dephosphorylation by pyruvate dehydrogenase phosphatase restores activity of the complex Both the kinase and the phosphatase are bound to E2 Kinase activation involves interaction with E2 subunits to sense changes in oxidation state & acetylation of lipoamide caused by NADH & acetyl-CoA. Phosphorylation by pyruvate dehydrogenase kinase, (on E1) switches off the activity of the complex dephosphorylation (by a pyruvate dehydrogenase phosphatase) restores activity of the complex The phosphatase is activated by Ca2+ and Mg2+ Since ATP binds Mg2+ tighter than ADP does, the concentration of free Mg2+ reflects the ATP/ADP ratio within the mitochondrion A Ca++-sensitive isoform of the phosphatase that removes Pi from E1 is expressed in muscle. Pyruvate Dehydrogenase in matrix mitochondrion Ca++ The increased cytosolic Ca++ that occurs during activation of muscle contraction can lead to Ca++ uptake by mitochondria. The higher Ca++ stimulates the phosphatase, & dephosphorylation activates Pyruvate Dehydrogenase. Thus mitochondrial metabolism may be stimulated during exercise. Phosphorylation by pyruvate dehydrogenase kinase, (on E1) switches off the activity of the complex dephosphorylation (by a pyruvate dehydrogenase phosphatase) restores activity of the complex Insulin accelerates the conversion of pyruvate into acetyl-CoA by stimulating the phosphatase and thereby the dephosphorylation and resultant activation of the complex)why? to get glucose out of circulation pyruvate also stimulates the enzyme (but by inhibiting the kinase) Pyruvate Dehydrogenase Kinase are activated by NADH & acetyl-CoA, Kinase activation involves interaction with E2 subunits to sense changes in oxidation state & acetylation of lipoamide caused by NADH & acetyl-CoA. During starvation: inhibition of Pyruvate Dehydrogenase: this prevents muscle and other tissues from catabolizing glucose precursors. Metabolism shifts toward fat utilization. Available glucose is spared for use by the brain. No Krebs, NAD+ plenty, glycolysis only stimulation of Pyruvate Dehydrogenase Complex: ADP/ pyruvate ATP activates... kinase ADP activates... phosphatase ADP/ pyruvate inhibit kinase that inactivates PDH inhibition of Pyruvate Dehydrogenase Complex: ATP, NADH Acetyl-CoA (means fats catabolized) ATP activates... kinase NADH competes with NAD+ for binding to E3. Acetyl CoA competes with CoA for binding to E2 stimulation of Pyruvate Dehydrogenase: ADP PDH phosphorylation: by PDH kinase removal of Pi phosphoprotein phosphatase both part of PDH complex! ATP activates... kinase ADP activates... Phosphatase..... inhibition of Pyruvate Dehydrogenase: ATP, NADH Acetyl-CoA (means fats catabolized) A Ca++-sensitive isoform of the phosphatase that removes Pi from E1 is expressed in muscle. Pyruvate Dehydrogenase in matrix mitochondrion Ca++ The increased cytosolic Ca++ that occurs during activation of muscle contraction can lead to Ca++ uptake by mitochondria. The higher Ca++ stimulates the phosphatase, & dephosphorylation activates Pyruvate Dehydrogenase. Thus mitochondrial metabolism may be stimulated during exercise. Entry into cycle and rate of cycle strictly controlled Pyruvate Acetly-CoA irreversible in animals Figure 17.6 Stryer O H3C C S CoA acetyl-coenzyme A Acetyl CoA, a product of the Pyruvate Dehydrogenase reaction, is a central compound in metabolism. The "high energy" thioester linkage makes it an excellent donor of the acetate moiety. glucose-6-P Glycolysis pyruvate fatty acids acetyl CoA oxaloacetate ketone bodies cholesterol citrate Krebs Cycle Acetyl CoA functions as: input to Krebs Cycle, where the acetate moiety is further degraded to CO2. donor of acetate for synthesis of fatty acids, ketone bodies, & cholesterol. Transition reaction inputs and outputs from glucose Inputs: 2 pyruvate 2 CoA 2 NAD+ Outputs: 2 acetyl CoA O 2 CO+2 HO C CH Coenzyme A-SH 2 NADH acetic acid O Coenzyme A-S C acetyl-CoA CH3 + H2O Citric Acid Cycle/Krebs Cycle citric acid cycle is used to harvest high energy electrons from carbon fuel. the central metabolic hub of the cell produces intermediates which are precursors for fatty acids, amino acids, nucleotide bases, and cholesterol named after Hans Krebs who was largely responsible for elucidating its pathways in the 1930s. What Are the Metabolic Fates of NADH and Pyruvate Produced in Glycolysis? NADH is energy - two possible fates: If O2 is available: NADH is re-oxidized in the electron transport pathway, making ATP in oxidative phosphorylation If O2 is not available (anaerobic conditions): NADH is re-oxidized by lactate dehydrogenase (LDH), providing additional NAD+ for more glycolysis What Are the Metabolic Fates of NADH and Pyruvate Produced in Glycolysis? Pyruvate is also energy - two possible fates: If O2 is available pyruvate enters the mitochondria, where it undergoes further breakdown If O2 is not available (anaerobic conditions) fermentation occurs and pyruvate undergoes reduction Fermentation is an anaeorbic process and does not require oxygen. In humans, pyruvate is reduced to lactic acid during fermentation.