* Your assessment is very important for improving the workof artificial intelligence, which forms the content of this project
Download Mathematics Semester 1 Study Guide
Magnesium in biology wikipedia , lookup
Paracrine signalling wikipedia , lookup
Gene regulatory network wikipedia , lookup
Biochemical cascade wikipedia , lookup
Western blot wikipedia , lookup
Metalloprotein wikipedia , lookup
Lipid signaling wikipedia , lookup
Proteolysis wikipedia , lookup
Polyclonal B cell response wikipedia , lookup
Microbial metabolism wikipedia , lookup
Electron transport chain wikipedia , lookup
Vectors in gene therapy wikipedia , lookup
Signal transduction wikipedia , lookup
Evolution of metal ions in biological systems wikipedia , lookup
Oxidative phosphorylation wikipedia , lookup
Light-dependent reactions wikipedia , lookup
Photosynthesis wikipedia , lookup
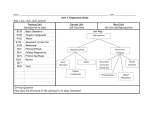
Honors Biology Semester 1 Study Guide A note about studying for Biology: This course contains a large amount of information. You cannot possibly understand the concepts without first understanding the terminology. However, this does not mean that you should just study definitions. This will not be enough. You have to fully understand the content and the big picture in order to be successful on the final exam. Materials: Your primary source materials are in order of importance: 1. 2. 3. 4. 5. Quizes and Tests Diagrams Notes Labs and Assignments Textbook A Basic Overview of the Concepts Biochemistry Properties of water and importance for life Ionic, non-polar covalent and covalent bonding Structure and function of carbohydrates Structure and function of lipids Structure and function of proteins Structure and function of nucleic acids Qualitative identification of the four primary biological compounds Cells Cell theory Light microscopes and electron microscopes Similarities and differences of prokaryotic and eukaryotic cells Internal cellular structures and their functions Excretion of proteins Similarities and differences of plant and animal cells Passive transport and active transport Osmosis Endocytosis and exocytosis Photosynthesis and cellular respiration Structure and function of mitochondrion and chloroplasts Chemical equations of photosynthesis and cellular respiration Photosynthesis and cellular respiration in balance Light independent and light dependent reactions of photosynthesis Glycolysis, Krebs cycle and the electron transport chain of cellular respiration Anaerobic respiration Toxins of cellular respiration Biochemistry Questions 1. 2. 3. 4. 5. 6. What did Rutherford reason about the position of protons and neutrons in the atom? Where are electrons found in the atom? What are atoms? What are the six most important elements for organisms? What is a covalent bond? What are organic compounds? Which two elements must be contained in all organic compounds? 7. What is metabolism? 8. What are polymers and how are they made? 9. What is a condensation or dehydration synthesis reaction? 10. What is a hydrolysis reaction? How is water involved in this type of reaction? 11. What are the four major classes of organic compounds? 12. What organic compound class includes the sugars and starches? 13. What elements form carbohydrates? How does the number of hydrogen compare to the number of oxygen atoms in a carbohydrate? 14. What are some main functions of carbohydrates in living things? 15. What are monosaccharides? How many carbon atoms are in a monosaccharide? 16. What is a disaccharide? 17. List two examples of disaccharides and where they are found? 19. What are polysaccharides? 18. Glycogen is a polysaccharide found in humans. Where is it stored and what is its function? 19. Starch is a polysaccharide found in plants. What is its function? 20. Cellulose is a polysaccharide found in plants. What is its function? 21. Oils and fats are examples of lipids. What is their chief function? 22. What is the structural difference between a saturated fat and an unsaturated fat? 23. Which kind of lipid or fat is most likely to cause heart disease? 24. List several compounds, which are made from the steroid cholesterol. 25. What can high levels of blood cholesterol lead to? 26. Why does every individual have his or her own unique characteristics? a. List five functions of proteins and examples of specific proteins, which perform those functions. 27. What are the subunits of protein molecules? How many of those subunits are there? 28. What determines the characteristics of the protein molecule? 29. What is meant by the conformation of a protein molecule? 30. What is the name (full name and abbreviation) of the two major nucleic acids? What are they responsible for? Biochemistry Review Continued Instructions: This sheet is designed to help you review topics in biochemistry. By no means does this review sheet contain everything that you need to know about Biochemistry. If you don’t know something on this sheet prior to your exam you should do the following things: 1) look in your notes and diagrams 2) look in your textbook 3) call a friend or send an e-mail to Mr. Balog 1. What is the ratio of C: H: O in a carbohydrate? What is the difference in the CHO ratio when comparing carbohydrates to lipids? 2. There are four groupings of biochemical compounds: carbohydrates, lipids, proteins and nucleic acids. Complete the following table regarding the structural properties of each compound. Write YES or NO. carbohydrates lipids proteins nucleic acids Always contains phosphorous (in the form of phosphate) May contain phosphorous (in the form of phosphate) Always contains nitrogen Frequently contains Sulfur 3. Functional groups (portion of a molecule) can modify the properties of organic molecules. In the table below, indicate whether each functional group is polar or nonpolar and hydrophobic or hydrophilic. Which of these functional groups are found in proteins and lipids? Functional Polar or Hydrophobic Found in Found in many Found in many group Nonpolar? or Hydrophilic all proteins proteins lipids —OH —CH3 —COOH —NH2 —SH —PO4 4. Identify the following structures as CARBOHYDRATE, LIPID, PROTEIN or NUCLEIC ACID. 5. Identify the following polymers as CARBOHYDRATE, PROTEIN or NUCLEIC ACID and write the name of the monomer next to it. 6. Name three different monosaccharides from class and where you would find them 7. Name the three different disaccharides from class and where you would find them. 8. Which of the following diagrams represents starch, which represents glycogen? 9. What carbohydrate is not easily digested by humans and creates high levels of flatulence? 10. What chemical reaction learned about in class is ANABOLIC (it builds things), which is CATABOLIC (breaks things down)? 11. What are some of the functions of lipids within living organisms? 12. What are the names of the following lipids? 13. What chemical reaction builds both molecule A and molecule B? (Hint: You have only learned about two chemical reactions…choose one) 14. What chemical reaction could be used to break down molecules A and B? 15. Create a list of differences between saturated and unsaturated fatty acids. Which of the two is healthier and why? 16. The following is a diagram of a phospholipid. What does hydrophilic mean? Hydrophobic? Which part of the phospholipids is POLAR, NON POLAR? 17. Steroids are derived (constructed from which lipid)? 18. Label the three parts of this nucleotide. 19. The following diagrams represent an analysis of protein structure. Label each diagram as representing primary, secondary, tertiary or quaternary structure. 20. There are 20 different types of proteins. This means that there are many different combinations of amino acids that can be constructed. Why is this important for living things? 21. Each of the following is a test that is used to qualitatively determine the biochemical nature of compounds. Which compound is being tested for? A) Benedict’s Reagent B) Lugol’s Iodine C) Translucent test D) Sudan IV E) Biuret Reagent Cell Review 1. Describe the location, structure and function of the following cellular components. Be sure to relate the structure to the function. Place this information in chart form. Leave space in the chart for additional information to be added during class discussion. a. nucleus b. Golgi complex c. nucleolus d. vacuole e. cytoplasm f. lysosome g. chloroplast h. centrioles i. mitochondrion j. cell wall k. ribosome l. cell membrane m. endoplasmic reticulum n. microvillus (microvilli) 2. Differentiate between a plant and an animal cell on the basis of four structural differences. 3. Label the diagrams of plant and animal cells provided. 4. Summarize the new cell technologies explained on pages 134, 138 (clip) and 141. 5. Using examples, identify and explain the following levels of organization within multicellular organisms: organelle, cell, tissue, organ, organ system. B. Cell Membrane and Cell Transport 6. Draw and label a diagram of the cell membrane. 7. Describe in detail the structure and function of the membrane. 8. Define diffusion and explain how it occurs useing the kinetic molecular energy theory. 9. Illustrate and justify the effects on diffusion of concentration, temperature and pressure. 10. Identify three processes in the human body that occur as a result of diffusion. 11. Define and provide an example of each of the following terms: a) osmosis b) equilibrium c) selectively permeable d) semipermeable e) solute f) solvent g) isotonic h) hypotonic i) hypertonic j) osmotic pressure k) dialysis l) endocytosis m) phagocytosis n) pinocytosis o) exocytosis 12. Explain what would happen to a body cell when placed in an isotonic, a hypotonic and a hypertonic solution. 13. Explain how active transport occurs and why energy is required. 14. Illustrate the following processes: a) active transport b) endocytosis c) phagocytosis d) pinocytosis e) exocytosis 15. Differentiate between a plant and an animal cell on the basis of four structural differences. Plant Cells Animal Cells 16. Using examples, identify and explain the following levels of organization within multicellular organisms: organelle, cell, tissue, organ, organ system. 17. Label a diagram of the cell membrane 18. Describe in detail the structure and function of the membrane. 19. Define and provide an example of each of the following terms: a) Osmosis b) Equilibrium c) selectively permeable d) semipermeable e) Solute f) solvent g) Isotonic h) hypotonic i) hypertonic j) osmotic pressure k) dialysis l) endocytosis m) Phagocytosis n) Pinocytosis o) Exocytosis . 20. Explain what would happen to a body cell when placed in an isotonic, a hypotonic and a hypertonic solution. 21. Explain how active transport occurs and why energy is required. Cell Review Continued Match the following terms to the correct definition. _____ 1. Chloroplast _____ 2. Ribosomes _____ 3. Nucleus _____ 4. Mitochondria _____ 5. Golgi Apparatus _____ 6. Lysosomes _____ 7. Endoplasmic Reticulum _____ 8. Chromosomes _____ 9. Nucleolus _____ 10. Cell Wall _____ 11. Vacuoles _____ 12. Organelles _____ 13. Cell Membrane A. Site where most of the ATP is made. B. An intracellular highway. Used to move materials from one part of the cell to another. C. Enclose hydrolytic enzymes. D. Sunlight is converted to glucose here. E. Controls what enters or leaves the cell. F. Stores wastes and enzymes G. Storage site of genetic information. H. Site of protein manufacture. I. Small organs. J. The processing, packaging and secreting structure of the cell. K. Contain the genes L. Site of ribosome synthesis. M. Outer covering of plant cells. 14. Draw a diagram of the cell membrane including the two primary components. Label the diagram according to its reactions with water. 15. Why is the cell membrane said to be selectively permeable? 16. Distinguish between the structure of rough ER and that of smooth ER. 17. What is the difference between chromatin and chromosomes? Cell Review Continued 1. When would cell biologists use an electron microscope? 2. Describe how an electron microscope works. 3. What advantages does light microscopy have over the use of electron microscopes? 4. How does the nucleus control protein synthesis in the cytoplasm? 5. What is the function of the following components of the endomembrane system? a. Smooth ER – b. Nuclear envelope – c. Rough ER – d. Transport vesicle – e. Golgi Apparatus – f. Plasma membrane – g. Vacuole – h. Lysosome – 6. Why are there pores in the nuclear envelope? 7. Fill in the blanks with the appropriate cellular organelle or structure ______________________A. Transports membranes and products to various locations ______________________B. Infoldings of mitochondrial membrane with attached enzymes. ______________________C. On the outside of the plasma membrane this allows cells to attach to each other. ______________________D. Small sacs with specific enzymes for a particular metabolic pathway. ______________________E. Responsible for the conversion of light energy into chemical energy. ______________________F. Structures located in sperm that aid in movement. ______________________G. Semifluid medium between nucleus and plasma membrane. ______________________H. System of protein fibers that maintain cell shape and anchors organelles. ______________________I. Holes in the cell walls of plants that aid in the movement of molecules. ______________________J. Responsible for producing usable cellular energy in the form of ATP. 8. Starting with the DNA, create a flow chart showing all of the steps in the secretion of a protein from the cell. 9. Create a table comparing all of the differences between plant and animal cells. 10. Create a table comparing all of the differences between eukaryotic and prokaryotic cells. 11. Describe differences and similarities between cilia and flagella. 12. List functions of the cytoskeleton. Cell Review Continued 1. Label the components in this diagram of the fluid mosaic model of membrane structure. Indicate the regions that are hydrophilic and those that are hydrophobic. D.______________ A._____________ B.______________ C.________________ E.______________ 2. What type of molecules have difficulty crossing the plasma membrane? Why? 3. What is diffusion? 4. What is osmosis? How is it different from diffusion? 5. What is a concentration gradient? Why do molecules move down a concentration gradient? 6. Solution A has a concentration of 6 glucose molecules per mL, while solution B has a concentration of 13 glucose molecules per mL. Which concentration is hypertonic? Hypotonic? Show the direction of osmosis on the diagram. 7. What problems might occur for a cell living in a low solute concentration pond? What structural adaptations to organisms have to prevent them from lysing in these solutions. 8. The ideal osmotic environment for animal cells is ___________ (hypertonic, isotonic, hypotonic) and the ideal osmotic environment for plant cells is ________________. 9. Why do plant cells behave differently than animal cells in the same concentration solutions? 10. Why is facilitated diffusion considered passive transport? 11. What is the difference between passive and active transport? Provide an example of an instance in which a cell would want to engage in active transport. Draw a diagram to support your explanation. ______12. Which is of the following is not true about osmosis? a) It increases free energy in a system. b) Water moves from a hypotonic to a hypertonic solution. c) Solute molecules bind to water and decrease the water available to move. d) It increases the entropy in a system e) There is no net osmosis between isotonic solutions. ______13. Ions (such as Na+) diffuse across membranes down their __?__. a) electrochemical gradient b) electrogenic gradient c) electrical gradient d) concentration gradient e) osmotic gradient. ______14. A plant cell placed in a hypotonic environment will __?__. a) plasmolyze b) shrivel c) become turgid d) become flaccid e) lyse ______15. The fluid mosaic model describes biological membranes consisting of: a) a phospholipids bilayer with proteins sandwiched between the layers. b) a lipid bilayer with proteins coating the outside of this hydrophobic structure. c) a phospholipid bilayer with proteins embedded in and attached to it. d) a protein bilayer with phospholipids embedded in it. e) a cholesterol bilayer with proteins embedded in the hydrophobic center. Photosynthesis Review 1. Label Stroma, inner and outer membranes, granum and thylakoid of a chloroplast. 2. In the following diagram label the Calvin Cycle, CO2, Light Reactions, NADP+, NADPH, Light, O2, Sugar, ATP, ADP, H2O and the chloroplast 3. A photon of which color of light would contain more energy: orange (620 nm) or blue (480nm)? 4. An action spectrum shows the relative rates of photosynthesis under different wavelengths of light. Label the absorption and action areas of the pigments on these graph. Why are these lines different? 5. What is a photosystem? 6. In the diagram of noncyclic electron flow below, label the following: Photosystem II, P680, Water, Oxygen, electrons, primary electron acceptor, electron transport chain, photophosphorylation by chemiosmosis, ATP, Photosystem I, P700, Primary electron acceptor, NADP reductase, NADPH. Circle the important products that will be used to provide chemical energy and reducing power to the Calvin Cycle. 8. In the light, the proton gradient across the thylakoid membrane is as great as 3 pH units. On which side is the pH the lowest? 9. What two factors contribute to the formation of this large difference in H+ concentration between the thylakoid space and the stroma? 10. Use the following diagram to answer the following question. Translate the diagram into words. Use a number system to help. _____ 17. The chlorophyll known as P680 has its electron “holes” filled by electrons from __?__. a. photosystem I b. photosystem II c. water d. NADPH e. accessory pigments _____ 18. Electrons that flow through the two photosystems have their highest potential energy in __?__. a. water b. P680 c. NADPH d. the electron transport chain e. photo-excited P700 _____ 19. Chloroplasts can make carbohydrate in the dark if provided with __?__. a. ATP and NADPH and CO2 b. an artificially induced proton gradient c. organic acids or four-carbon compounds d. a source of hydrogen e. photons and CO2 _____ 21. How many turns of the Calvin cycle are required to produce one molecule of glucose? a. 1 b. 2 c. 3 d. 6 e. 12 _____ 23. What are the final electron acceptors for the electron transport chain in the light reactions of photosynthesis and in cellular respiration? a. O2 in both. B. CO2 in both c. H2O in the light reactions and O2 in respiration d. NADP+ in the light reactions and NAD+ or FAD in respiration e. NADP+ in the light reactions and O2 in respiration Indicate if the following events occur during: a. Respiration b. Photosynthesis c. Both respiration and photosynthesis d. Neither respiration nor photosynthesis _____ 24. Chemiosmotic synthesis of ATP _____ 25. Reduction of oxygen _____ 26. Reduction of CO2 _____ 27. Reduction of NAD+ _____ 28. Oxidation of NADP+ _____ 29. Oxidative Phosphorylation 13. Label the three phases (Carbon fixation, reduction, and regeneration of CO2) and key molecules (3CO2, ribulose biphosphate, 3-phosphpglycerate, 6ATP →6ADP) in the diagram of the Calvin cycle on the next page. Cellular Respiration Review 1. Fill in this summary for cellular respiration: (3 pts) _____________ + 6 O2 → ___________ + 6 H2O + ___________ 2. What is the difference between fermentation and cell respiration? (2 pts) 3. What do we call the reactions where there is a complete transfer of electrons from one reactant to another? (1 pt) 4. Fill in the appropriate terms in this equation (3 pts) X is the reducing agent; it becomes _______________ Oxidation Y is the __________________; it becomes ________________. Xe- + Y X + Reduction 5. In the conversion of glucose and oxygen to carbon dioxide and water, which molecule is reduced? (1 pt) 6. In the conversion of glucose and oxygen to carbon dioxide and water, which molecule is oxidized? (1 pt) 7. What happens to the energy that is released in this Redox reaction? (1 pt) 8. NAD+ is called an ____________________________. Its reduced form is __________. (2 pts) Ye- 9. Fill in the three stages of respiration. Indicate whether ATP is produced by substrate-level (direct addition of phosphate) or oxidative Phosphorylation. Label the arrows indicating electrons carried by NADH. (8 pts) 10. Fill in the blanks in this summary diagram of Glycolysis. Where does this part take place? (7 pts) Glucose a. __________ 2 ADP b. ____________________________ c. _____________ d. ___________ 4 ADP e. ___________ f. ___________________ 11. Fill in the blanks in this summary figure of the Krebs cycle. Balls represent carbon atoms. (11 pts) A. _____________ A. B. _____________ B. C. _____________ D. D. _____________ E. _____________ C. F. _____________ E. G. _____________ H. _____________ I. _____________ J. _____________ K. _____________ F. K. G. J. H. I. 12. Label the following on the diagram of the electron transport chain and chemiosmosis in a mitochondrial membrane: (12 pts) a. Intermembrane space b. Innermembrane space c. Mitochondrial matrix d. Electron transport chain e. NADH + H+ f. NAD+ g. H+ h. 1H+ + ½ O2 i. H2O j. ATP Synthase k. ADP + phosphate l. ATP 13. Fill in the table on the next page to summarize the major inputs and outputs of Glycolysis, the Krebs cycle, the electron transport chain, chemiosmosis, and fermentation. Base outputs on one glucose molecule. (30 pts) Process Glycolysis Pyruvate to Acetyl CoA Krebs cycle Electron transport chain and chemiosmosis Fermentation Main Function Inputs Outputs