HonBio Chapter 3 notes
... hydrogens. Examples include beef fat and butter. Solid at room temperature. Trans fat – made by adding hydrogen to unsaturated fats. Associated with health risks. ...
... hydrogens. Examples include beef fat and butter. Solid at room temperature. Trans fat – made by adding hydrogen to unsaturated fats. Associated with health risks. ...
POWERPOINT NOTES SHEET 2.3 Carbon Compounds
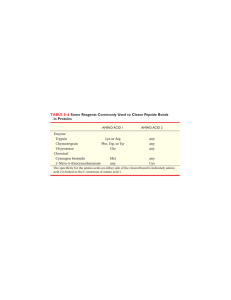
... • _________________________________ is the complete, three-dimensional arrangement of a polypeptide chain. • Proteins with more than ___________________ have a fourth level of structure, which describes the way in which the different polypeptide chains are arranged with respect to each other. • For ...
... • _________________________________ is the complete, three-dimensional arrangement of a polypeptide chain. • Proteins with more than ___________________ have a fourth level of structure, which describes the way in which the different polypeptide chains are arranged with respect to each other. • For ...
Organic Molecules
... carbohydrates are monosaccharides or sugars. • The simplest sugar is glucose, a molecule used to provide fuel for many types of organisms, including humans. ...
... carbohydrates are monosaccharides or sugars. • The simplest sugar is glucose, a molecule used to provide fuel for many types of organisms, including humans. ...
Chapter 3: The Chemistry of Organic Molecules
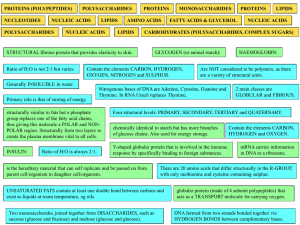
... • Carbohydrates: polymer = polysaccharide monomer= monosaccharide • Proteins: polymer= polypeptide monomer= amino acid • Nucleic acids: polymer= nucleic acid Monomer= nucleotide ...
... • Carbohydrates: polymer = polysaccharide monomer= monosaccharide • Proteins: polymer= polypeptide monomer= amino acid • Nucleic acids: polymer= nucleic acid Monomer= nucleotide ...
Macromolecules - Nolte Science
... Fats = built from glycerol and fatty acids One – fatty acid Glycerol – fatty acid Molecule – fatty acid ...
... Fats = built from glycerol and fatty acids One – fatty acid Glycerol – fatty acid Molecule – fatty acid ...
Chapter 3: The Chemistry of Organic Molecules
... • Carbohydrates: polymer = polysaccharide monomer= monosaccharide • Proteins: polymer= polypeptide monomer= amino acid • Nucleic acids: polymer= nucleic acid Monomer= nucleotide ...
... • Carbohydrates: polymer = polysaccharide monomer= monosaccharide • Proteins: polymer= polypeptide monomer= amino acid • Nucleic acids: polymer= nucleic acid Monomer= nucleotide ...
Biochemistry
... • Types of Lipids (Fats) – Saturated – bonds in molecule are unbendable; tend to clog arteries; typically from animals (fats, butter, lard) – Unsaturated – some bonds in molecule bend; better, but can still clog arteries; typically from plants (oils) – Polyunsaturated – many bonds in molecule bend; ...
... • Types of Lipids (Fats) – Saturated – bonds in molecule are unbendable; tend to clog arteries; typically from animals (fats, butter, lard) – Unsaturated – some bonds in molecule bend; better, but can still clog arteries; typically from plants (oils) – Polyunsaturated – many bonds in molecule bend; ...
Levels of Organization - Bremen High School District 228
... 3. Lipids are generally insoluable in water 4. Formed when a glycerol molecule (C₃H₅O₃) joins a fatty acid a. Saturated fats form when each carbon atom in the fatty acid chain is joined to another carbon atom by a single covalent bond ...
... 3. Lipids are generally insoluable in water 4. Formed when a glycerol molecule (C₃H₅O₃) joins a fatty acid a. Saturated fats form when each carbon atom in the fatty acid chain is joined to another carbon atom by a single covalent bond ...
large molecule consisting of many identical or similar subunits
... Polymer: large molecule consisting of many identical or similar subunits connected together. Monomer: subunit molecules of a polymer. Macromolecule: Large organic polymer. There are four classes of macromolecules: carbohydrates, lipids, proteins and nucleic acid. The structural variation of macromol ...
... Polymer: large molecule consisting of many identical or similar subunits connected together. Monomer: subunit molecules of a polymer. Macromolecule: Large organic polymer. There are four classes of macromolecules: carbohydrates, lipids, proteins and nucleic acid. The structural variation of macromol ...
Ch. 3 Study Guide
... 6. What is the common name for carbohydrates? What suffix is a clue that you are dealing with a carbohydrate? 7. Carbohydrates perform three primary functions for cells. They are: A. B. C. 8. Compare and contrast monosaccharides, disaccharides, and polysaccharides ...
... 6. What is the common name for carbohydrates? What suffix is a clue that you are dealing with a carbohydrate? 7. Carbohydrates perform three primary functions for cells. They are: A. B. C. 8. Compare and contrast monosaccharides, disaccharides, and polysaccharides ...
Biological Molecules
... Two amino acids join together to form a dipeptide. A chain of amino acids is known as a polypeptide. ...
... Two amino acids join together to form a dipeptide. A chain of amino acids is known as a polypeptide. ...
biomolecule
... renders these atoms so appropriate to the chemistry of life? Answer: Their ability to form covalent bonds ...
... renders these atoms so appropriate to the chemistry of life? Answer: Their ability to form covalent bonds ...
Bio-molecule
... • A carbohydrate is a bio-molecule with a ratio of two hydrogen atoms and one oxygen atom for every carbon atom. ...
... • A carbohydrate is a bio-molecule with a ratio of two hydrogen atoms and one oxygen atom for every carbon atom. ...
Macromolecule notes
... - Large molecules containing hundreds of atoms. - Can vary greatly in size. Example: Proteins ...
... - Large molecules containing hundreds of atoms. - Can vary greatly in size. Example: Proteins ...
Structure and Function of Macromolecules
... • Lipids: A group of polymers that have one characteristic in common, they do not mix with water. They are hydrophobic. Some important groups are fats, phospholipids, and steroids. ...
... • Lipids: A group of polymers that have one characteristic in common, they do not mix with water. They are hydrophobic. Some important groups are fats, phospholipids, and steroids. ...
biol 3 biomolecules table activity
... simplest is CHOLESTEROL that consists of a 4 ringed carbon structure and has a structural role in the plasma membrane. Other steroids act as HORMONES, eg testosterone and progesterone. SATURATED FATS contain no double bonds between carbons and exist as solids at room temperature. ...
... simplest is CHOLESTEROL that consists of a 4 ringed carbon structure and has a structural role in the plasma membrane. Other steroids act as HORMONES, eg testosterone and progesterone. SATURATED FATS contain no double bonds between carbons and exist as solids at room temperature. ...
Functional groups - Montgomery County Schools
... large organic molecules also called polymers made of smaller “building blocks” called monomers Monomers link together to form polymers through dehydration reactions, which remove water Polymers are broken apart by hydrolysis, the addition of water ...
... large organic molecules also called polymers made of smaller “building blocks” called monomers Monomers link together to form polymers through dehydration reactions, which remove water Polymers are broken apart by hydrolysis, the addition of water ...
Organic Macromolecules
... Macromolecules are built by linking together smaller molecules (monomers) into long chains (polymers) Monomers combine by disconnecting from some of the hydrogen and oxygen atoms between them After the monomers bond, the excess hydrogen and oxygen atoms form a water molecule. Since this bond ...
... Macromolecules are built by linking together smaller molecules (monomers) into long chains (polymers) Monomers combine by disconnecting from some of the hydrogen and oxygen atoms between them After the monomers bond, the excess hydrogen and oxygen atoms form a water molecule. Since this bond ...
Chapter 3 USU - BEHS Science
... How do you build a cell? Start with water, add lots of small carbon-containing molecules and ……. ...
... How do you build a cell? Start with water, add lots of small carbon-containing molecules and ……. ...
Bio-Macromolecules Worksheet
... Carbohydrates are sugars, starches, and glycogen which are used for short and long term energy storage in cells and structural molecules in cell walls and exoskeletons. Carbohydrates are made of only carbon, hydrogen, and oxygen (CHO). They are found in bread, potatoes, pasta, and fruits. Carbohydra ...
... Carbohydrates are sugars, starches, and glycogen which are used for short and long term energy storage in cells and structural molecules in cell walls and exoskeletons. Carbohydrates are made of only carbon, hydrogen, and oxygen (CHO). They are found in bread, potatoes, pasta, and fruits. Carbohydra ...
Primary Structure - LaurensAPBiology
... How do you build a cell? Start with water, add lots of small carbon-containing molecules and ……. ...
... How do you build a cell? Start with water, add lots of small carbon-containing molecules and ……. ...
study guide section 3-1 carbon compounds
... 2. ______The different shapes and functions of different proteins are determined by a. the R groups of the amino acids they contain. b. the amino groups of the amino acids they contain. c. the carboxyl groups of the amino acids they contain. d. whether or not they contain any amino acids. 3. ______ ...
... 2. ______The different shapes and functions of different proteins are determined by a. the R groups of the amino acids they contain. b. the amino groups of the amino acids they contain. c. the carboxyl groups of the amino acids they contain. d. whether or not they contain any amino acids. 3. ______ ...
Biochemistry
_and_Carl_Ferdinand_Cori.jpg?width=300)
Biochemistry, sometimes called biological chemistry, is the study of chemical processes within and relating to living organisms. By controlling information flow through biochemical signaling and the flow of chemical energy through metabolism, biochemical processes give rise to the complexity of life. Over the last decades of the 20th century, biochemistry has become so successful at explaining living processes that now almost all areas of the life sciences from botany to medicine to genetics are engaged in biochemical research. Today, the main focus of pure biochemistry is in understanding how biological molecules give rise to the processes that occur within living cells, which in turn relates greatly to the study and understanding of whole organisms.Biochemistry is closely related to molecular biology, the study of the molecular mechanisms by which genetic information encoded in DNA is able to result in the processes of life. Depending on the exact definition of the terms used, molecular biology can be thought of as a branch of biochemistry, or biochemistry as a tool with which to investigate and study molecular biology.Much of biochemistry deals with the structures, functions and interactions of biological macromolecules, such as proteins, nucleic acids, carbohydrates and lipids, which provide the structure of cells and perform many of the functions associated with life. The chemistry of the cell also depends on the reactions of smaller molecules and ions. These can be inorganic, for example water and metal ions, or organic, for example the amino acids which are used to synthesize proteins. The mechanisms by which cells harness energy from their environment via chemical reactions are known as metabolism. The findings of biochemistry are applied primarily in medicine, nutrition, and agriculture. In medicine, biochemists investigate the causes and cures of disease. In nutrition, they study how to maintain health and study the effects of nutritional deficiencies. In agriculture, biochemists investigate soil and fertilizers, and try to discover ways to improve crop cultivation, crop storage and pest control.