* Your assessment is very important for improving the workof artificial intelligence, which forms the content of this project
Download Chapter 3: The Chemistry of Organic Molecules
Evolution of metal ions in biological systems wikipedia , lookup
Artificial gene synthesis wikipedia , lookup
Vectors in gene therapy wikipedia , lookup
Signal transduction wikipedia , lookup
Citric acid cycle wikipedia , lookup
Photosynthesis wikipedia , lookup
Peptide synthesis wikipedia , lookup
Basal metabolic rate wikipedia , lookup
Western blot wikipedia , lookup
Fatty acid synthesis wikipedia , lookup
Point mutation wikipedia , lookup
Photosynthetic reaction centre wikipedia , lookup
Amino acid synthesis wikipedia , lookup
Genetic code wikipedia , lookup
Protein–protein interaction wikipedia , lookup
Two-hybrid screening wikipedia , lookup
Metalloprotein wikipedia , lookup
Nuclear magnetic resonance spectroscopy of proteins wikipedia , lookup
Fatty acid metabolism wikipedia , lookup
Nucleic acid analogue wikipedia , lookup
Protein structure prediction wikipedia , lookup
Biosynthesis wikipedia , lookup
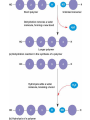
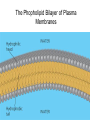
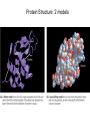
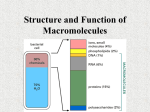
Chapter 3: The Chemistry of Organic Molecules 3.1 Organic Molecules • Organic Molecule= A molecule that contains carbon and hydrogen; it may also have O, N, P, or S. Carbon • Has four electrons in outer shell, therefore make up to four covalent bonds with four other atoms. • It can also bond itself to form both chains and rings. Carbon chains make up the skeleton or “backbone” of organic molecules. Functional Groups • Functional groups= clusters of certain atoms, attached to the carbon back bone (see figure 3.2). • Differences in the carbon backbone and attached functional groups cause an organic molecule to have different chemical properties. – (ex) molecules composed of only carbon and hydrogen are hydrophobic (not attracted to water). But the addition of a functional group like – OH makes the molecule polar, or hydrophilic (attracted to water). • 4 classes of organic molecules: carbohydrates, lipids, proteins, and nucleic acids. • Each of these 4 types of macromolecules is a polymer, which is a long chain of covalently bonded unit molecules called monomers. Monomers and Polymers • Carbohydrates: polymer = polysaccharide monomer= monosaccharide • Proteins: polymer= polypeptide monomer= amino acid • Nucleic acids: polymer= nucleic acid Monomer= nucleotide • Condensation synthesis: when a water molecule is removed in order to form a bond between two monomers. (an –OH group is removed from one molecule and a hydrogen (H) is removed from the other.) • Hyrdolysis: The means by which polymers are broken down. A water molecule is added to break the bond between monomers. 3.2 Carbohydrates • Energy storage compounds & building materials. • Monosaccharides: simple sugars, carbon back has 3-7 atoms. – Glucose (C6H12O6) and fructose are both hexoses: 6-carbon sugars; glucose found in blood of animals, fructose found in fruits. (isomers) – Ribose and deoxyribose are both pentoses: 5-carbon sugars; found in DNA and RNA. Disaccharides • Two monosaccharides joined by condensation. • Lactose: contains galactose and glucose; found in milk. • Maltose: two glucose molecules; result in starch digestion. Polysaccharides • Long polymers of monosaccharides • Glycogen- have many branches of Glucose. Storage vessel for animals. Liver and Muscles. Allows for breakdown to happen at many points. • Starch- have many branches of glucose. Storage vessel of carbohydrates for plants. Seeds and roots. Allows for breakdown to happen at many points. Polysaccharides • Cellulose: Straight and Fibrous (structure support) glucose. – Hydrogen bonds. – Plant cell walls (cotton) (wood) Polysaccharides Cont’d Chitin: Exoskeletons, amino group attached to each glucose. – Sutures 3.2 Lipids • Lipids: organic molecules that are generally insoluble in water; used as longterm energy storage compounds in plants and animals. Fats and Oils Glycerol: compound with 3 hydroxyl groups (OH); hydroxyl groups are polar which makes glycerol soluble in water. Fatty Acids: Long hydrocarbon chain with carboxyl group (COOH)at one end Formation of a fat/oil: Condensation synthesis involving 3 fatty acids and one glycerol, forming a triglyceride. Fatty acids: carboxyl group is polar which makes fatty acid soluble in water. Types: » Saturated: no double bonds between carbon atoms, causes molecule to be more rigid. » Unsaturated: have double bonds in carbon chain, causes molecule to be more fluid. Waxes • Waxes are long-chain fatty acid bonded to a long-chain alcohol. • Solid at room temp., hydrophobic, usually act as a protective coating in plants and animals. (ex.: ear wax in humans for trapping dirt and dust particles, preventing them from reaching the eardrum.) Phospholipids • Soluble in water; contains a glycerol molecule, 2 fatty acids, and one phosphate group. • Phosphate group is the “polar head” of molecule. • Fatty acid chains are “nonpolar tails” of molecule. • Plasma membrane in eukaryotic cells is a phospholipid bilayer. The Phopholipid Bilayer of Plasma Membranes Steriods • Backbone of 4 fused carbon rings, vary according to the types of functional groups bonded to the rings. • Cholesterol- component of animal cell membrane, precursor for other types of steroids (estrogen, testosterone). Cholesterol is the molecule from which other steroids, including the sex hormones, are synthesized. 3.4 Proteins • Polymers of amino acids • Functions: – Support / structure: Keratin in hair and nails, collagen in ligaments, skin, tendons. – Regulation: Enzymes that speed up reactions (catalyze), Hormones like insulin regulates levels of glucose in blood Protein Functions contd • Defense: Antibodies, and antigens • Motion: contractile proteins (actin and Myosin) in muscles. • Transport: Channel and carrier proteins in plasma membrane, hemoglobin (O2) Monomers Of Proteins • Amino Acids: Carbon Atom bonded to 3 functional groups. – Animo group (-NH2) – Carboxyl group (COOH) (acidic) – R group = “remainder” of molecule; determines the 20 different amino acids found in life; unique properties. Polymers of proteins • Polypeptides: two or more amino joined by condensation – Peptide bond: between Carbon of one amino acid and the nitrogen of another. – Most proteins are at least 150 A.A. long. – Some proteins can contain more than one polypeptide chain Protein Structure • Primary Structure: The sequence of amino acids joined by peptide bonds. • Secondary Structure: Coiling or folding of polypeptide chain due to properties of A.A. w/in primary structure. (H-bonds b/w different A.A.) – Beta sheets – Alpha helix Protein Structure Contd • Tertiary Structure: The folding and twisting that results in the 3-D shape of polypeptide. – H-bonds, disulfide links (fxnl group?), Ionic bonds, and other molecular interactions between R groups. • Quaternary Structure: arrangement of more than one polypeptide chain – Hemoglobin: globular protein with 4 peptide chains – Some proteins Protein Structure: 2 models Denaturation • When a protein loses its 40/30/20 structure. • Renders a protein inactive. • Caused when the environment that protein is in changes (temperature, pH) – Causes interactions/bonds to break – Proteins have optimal environments. • Renaturation: when protein is place back into optimal environment it will reform into proper structure. High temperatures or various chemical treatments will denature a protein, causing it to lose its conformation and hence its ability to function. If the denatured protein remains dissolved, it can often renature when the chemical and physical aspects of its environment are restored to normal. Importance of Denaturation / Renaturation • Regulation: This is a way for cells to regulate which chemical reactions will happen and when they will occur. • Not very efficient if cells are undergoing all reaction all at once. Nucleic Acids Three types of nucleic acids: 1) DNA 1. DNA (deoxyribonucleic acid) – the genetic material that store information for replicating itself and the sequence of amino acids to make all of an organisms proteins • Monomers = nucleotide • There are three molecules that make up a nucleotide: 1. Phosphate functional group 2. Nitrogen containing base 3. Pentose monosaccharide = deoxyribose • There are four types of nucleotides that are determined by the type of base each has: Cytosine, Thymine, Adenine, and Guanine DNA Continued The different nucleotides are joined together by a bond between the phosphate of one nucleotide and the sugar of another. (Sugar–Phosphate Backbone) Process? DNA has two strands of nucleotides that are joined together by hydrogen bond between different bases (A-T, T-A, C-G, G-C) This results in the double-helix 2) RNA • RNA (ribonucleic acid) – involved in the process of making proteins form DNA. – Similar to DNA except: 1. Single Stranded 2. Ribose instead of Deoxyribose 3. Uracil instead of Thymine 3) ATP • ATP (Adenosine Triphosphate) – supplies energy for synthetic reactions and for all energy requiring processes in cells. (a cell’s energy currency) – Adenosine = ribose + adenine – Triphosphate = three phosphates bonded together ATP Contd High energy molecule due to the instability of phosphates. ATP → ADP + P + H20 (energy) Reversible reaction constantly recycled in cells