Molecules of life
... of glucose Galactose is a stereoisomer of glucose Enzymes that act on different sugars can distinguish structural and stereoisomers of this basic six-carbon skeleton ...
... of glucose Galactose is a stereoisomer of glucose Enzymes that act on different sugars can distinguish structural and stereoisomers of this basic six-carbon skeleton ...
Nucleic Acids - faculty at Chemeketa
... What will be the composition of the DNA strand complementary to –AGCCA– ? a. b. c. d. ...
... What will be the composition of the DNA strand complementary to –AGCCA– ? a. b. c. d. ...
Unit Topic: Chemistry of Life
... Broad Concept: All life is built out of four essential molecules: proteins, carbohydrates, lipids, and nucleic acids. Carbon compounds: 1. Organic means made from carbon - CHONPS (carbon, hydrogen, oxygen, nitrogen, phosphorus, and sulfur) 2. Protein, lipids, carbohydrates, and nucleic acids 3. Draw ...
... Broad Concept: All life is built out of four essential molecules: proteins, carbohydrates, lipids, and nucleic acids. Carbon compounds: 1. Organic means made from carbon - CHONPS (carbon, hydrogen, oxygen, nitrogen, phosphorus, and sulfur) 2. Protein, lipids, carbohydrates, and nucleic acids 3. Draw ...
How Did Life Begin? Unit Objectives Vocabulary: Miller
... By the end of this unit students will be able to: o Describe what it means to be alive using no less than six criteria. o List the two components of cell theory and explain how they apply to the fossil record explored in unit 1 and the origin of life itself. o Explain the origin of organic molecules ...
... By the end of this unit students will be able to: o Describe what it means to be alive using no less than six criteria. o List the two components of cell theory and explain how they apply to the fossil record explored in unit 1 and the origin of life itself. o Explain the origin of organic molecules ...
8/27 Organic Chemistry
... • Chemical formula = [CH2O]N (multiples of CH2O) • e.g., C6H12O6 = glucose • Carbohydrates have many functions • Structural components of molecules (e.g., DNA, RNA), cells and tissues – cellulose is the most abundant organic substance on earth – we cannot digest it, but it is an important part of ou ...
... • Chemical formula = [CH2O]N (multiples of CH2O) • e.g., C6H12O6 = glucose • Carbohydrates have many functions • Structural components of molecules (e.g., DNA, RNA), cells and tissues – cellulose is the most abundant organic substance on earth – we cannot digest it, but it is an important part of ou ...
Macromolecules Test Review Test Date: 1. What does the term
... 5. How many times larger is the number of hydrogen atoms than the number of oxygen atoms in carbohydrates? 6. Draw the chemical structure of an amino acid. ...
... 5. How many times larger is the number of hydrogen atoms than the number of oxygen atoms in carbohydrates? 6. Draw the chemical structure of an amino acid. ...
Organic Compounds
... • In DNA, the sugar is called: Deoxyribose. • In RNA, the sugar is called: Ribose. • These are both (5 carbon) sugars which is why the shape is a pentagon! • The sugars and phosphates link together to form the “backbone” of the ladders for DNA and RNA. ...
... • In DNA, the sugar is called: Deoxyribose. • In RNA, the sugar is called: Ribose. • These are both (5 carbon) sugars which is why the shape is a pentagon! • The sugars and phosphates link together to form the “backbone” of the ladders for DNA and RNA. ...
1. Which substances are inorganic compounds?
... 4. Molecule C belongs in the general class of substances known as (1.) vitamins (2.) minerals (3.) inorganic acids (4.) organic compounds ...
... 4. Molecule C belongs in the general class of substances known as (1.) vitamins (2.) minerals (3.) inorganic acids (4.) organic compounds ...
Unit 3: Chemistry of Life
... amino (NH2) group of one amino acid and the carboxylic group (COOH) of another >Dipeptide – two amino acids bonded together >Polypeptide – 3+ amino acids bonded together ...
... amino (NH2) group of one amino acid and the carboxylic group (COOH) of another >Dipeptide – two amino acids bonded together >Polypeptide – 3+ amino acids bonded together ...
Biology 2.3 Carbon Compounds
... chemistry is devoted to studying carbon Carbon atoms form 4 bonds ...
... chemistry is devoted to studying carbon Carbon atoms form 4 bonds ...
Chemical Principles
... part of bacterial cell wall part of DNA and RNA (deoxyribose and ribose) ...
... part of bacterial cell wall part of DNA and RNA (deoxyribose and ribose) ...
amino acids - 11 College Biology
... Very large organic compounds are called MACROMOLECULES Macromolecules are composed of smaller subunits ...
... Very large organic compounds are called MACROMOLECULES Macromolecules are composed of smaller subunits ...
Molecules of Life Review Topics
... Lipids – elements CHO – no ratio, functions – concentrated energy 9 cal/gram Monomers: glycerol and fatty acid, structure of each Triglyceride – which monomers, how many of each Saturated and unsaturated – how different, where found o Hydrogenated and trans fat – what are they? Why important? ...
... Lipids – elements CHO – no ratio, functions – concentrated energy 9 cal/gram Monomers: glycerol and fatty acid, structure of each Triglyceride – which monomers, how many of each Saturated and unsaturated – how different, where found o Hydrogenated and trans fat – what are they? Why important? ...
Covalent Reactions Atoms SHARE electrons
... Carbohydrates (CH2O)n Functions: a) quick & short term energy storage b) structural role in plants, bacteria, insects Types: Monosaccharide= simple sugar, 3-7 carbon atoms ex. Glucose, fructose, galactose Disaccharide= two monosaccharides joined by dehydration ex. Maltose, sucrose, lactose Polysacc ...
... Carbohydrates (CH2O)n Functions: a) quick & short term energy storage b) structural role in plants, bacteria, insects Types: Monosaccharide= simple sugar, 3-7 carbon atoms ex. Glucose, fructose, galactose Disaccharide= two monosaccharides joined by dehydration ex. Maltose, sucrose, lactose Polysacc ...
3.2 Carbohydrates, Lipids, and Proteins
... Role in Plants or Animals Chemical fuel for cell respiration in both plants and animals ...
... Role in Plants or Animals Chemical fuel for cell respiration in both plants and animals ...
Notes handout for Basic Biochemistry
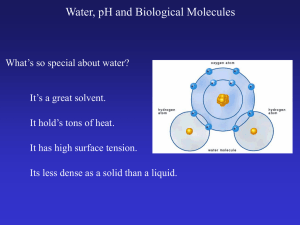
... Carbon is special because it has ____ valence electrons. It forms strong, stable ___________________ bonds with up to 4 other atoms (usually H, O, N, S, P, or another C). Carbon can form long chains, branched structures, or rings. Adjacent carbon atoms can also form Double and Triple bonds. There ar ...
... Carbon is special because it has ____ valence electrons. It forms strong, stable ___________________ bonds with up to 4 other atoms (usually H, O, N, S, P, or another C). Carbon can form long chains, branched structures, or rings. Adjacent carbon atoms can also form Double and Triple bonds. There ar ...
Document
... 27. A reactant being catalyzed is knows as the __________________________________________________. 28. The monomers that make up nucleic acids are called __________________________. 29. The four main classes of organic compounds are _____________________________________, ____________________________ ...
... 27. A reactant being catalyzed is knows as the __________________________________________________. 28. The monomers that make up nucleic acids are called __________________________. 29. The four main classes of organic compounds are _____________________________________, ____________________________ ...
I - Decatur ISD
... Proteins are building blocks of structures called _______________________. Proteins are what your DNA codes to make A peptide bond forms between amino acids by dehydration synthesis. ____________________________= the building up of large molecules by removing water molecules Enzymes A. Speci ...
... Proteins are building blocks of structures called _______________________. Proteins are what your DNA codes to make A peptide bond forms between amino acids by dehydration synthesis. ____________________________= the building up of large molecules by removing water molecules Enzymes A. Speci ...
g. ¶I - wwphs
... Comes in two slightly different forms, alpha and beta; two of each form make up one hemoglobin molecule in humans Airoteins that transport attached cholesterol, triglycerides, and phospholipids The type of covalent bond linking one amino acid to another ‘-tC Fourth level of protein organization; glo ...
... Comes in two slightly different forms, alpha and beta; two of each form make up one hemoglobin molecule in humans Airoteins that transport attached cholesterol, triglycerides, and phospholipids The type of covalent bond linking one amino acid to another ‘-tC Fourth level of protein organization; glo ...
1. Identify the structural formula. Use these choices - burgess
... _C_ 3. It is a disaccharide with the formula C12H22O11 _A_ 4. It is an isomer of fructose and galactose [each have 6 carbons]. _B_ 5. There are twenty-some different types of these. ...
... _C_ 3. It is a disaccharide with the formula C12H22O11 _A_ 4. It is an isomer of fructose and galactose [each have 6 carbons]. _B_ 5. There are twenty-some different types of these. ...
Biochemistry
_and_Carl_Ferdinand_Cori.jpg?width=300)
Biochemistry, sometimes called biological chemistry, is the study of chemical processes within and relating to living organisms. By controlling information flow through biochemical signaling and the flow of chemical energy through metabolism, biochemical processes give rise to the complexity of life. Over the last decades of the 20th century, biochemistry has become so successful at explaining living processes that now almost all areas of the life sciences from botany to medicine to genetics are engaged in biochemical research. Today, the main focus of pure biochemistry is in understanding how biological molecules give rise to the processes that occur within living cells, which in turn relates greatly to the study and understanding of whole organisms.Biochemistry is closely related to molecular biology, the study of the molecular mechanisms by which genetic information encoded in DNA is able to result in the processes of life. Depending on the exact definition of the terms used, molecular biology can be thought of as a branch of biochemistry, or biochemistry as a tool with which to investigate and study molecular biology.Much of biochemistry deals with the structures, functions and interactions of biological macromolecules, such as proteins, nucleic acids, carbohydrates and lipids, which provide the structure of cells and perform many of the functions associated with life. The chemistry of the cell also depends on the reactions of smaller molecules and ions. These can be inorganic, for example water and metal ions, or organic, for example the amino acids which are used to synthesize proteins. The mechanisms by which cells harness energy from their environment via chemical reactions are known as metabolism. The findings of biochemistry are applied primarily in medicine, nutrition, and agriculture. In medicine, biochemists investigate the causes and cures of disease. In nutrition, they study how to maintain health and study the effects of nutritional deficiencies. In agriculture, biochemists investigate soil and fertilizers, and try to discover ways to improve crop cultivation, crop storage and pest control.