* Your assessment is very important for improving the work of artificial intelligence, which forms the content of this project
Download Organic Compounds
Evolution of metal ions in biological systems wikipedia , lookup
Vectors in gene therapy wikipedia , lookup
Protein–protein interaction wikipedia , lookup
Two-hybrid screening wikipedia , lookup
Basal metabolic rate wikipedia , lookup
Point mutation wikipedia , lookup
Western blot wikipedia , lookup
Peptide synthesis wikipedia , lookup
Gene expression wikipedia , lookup
Artificial gene synthesis wikipedia , lookup
Metalloprotein wikipedia , lookup
Deoxyribozyme wikipedia , lookup
Genetic code wikipedia , lookup
Fatty acid synthesis wikipedia , lookup
Amino acid synthesis wikipedia , lookup
Protein structure prediction wikipedia , lookup
Fatty acid metabolism wikipedia , lookup
Proteolysis wikipedia , lookup
Nucleic acid analogue wikipedia , lookup
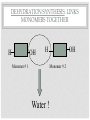
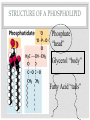
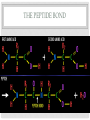
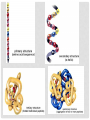
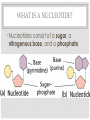
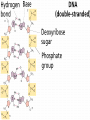
ORGANIC COMPOUNDS AKA. MACROMOLECULES MRS. TAKTAK / MRS. STOREY ORGANIC CHEMISTRY = CARBON CHEMISTRY • Carbon is the most important element to living things! CHNOPS (these are the 6 most common). • Carbon has an Atomic # of 6 (sketch out a carbon atom) • How many bonds can it readily form? Carbon Compounds AND FUNCTIONAL GROUPS • Carbon compounds are what makes up most of our bodies….(we’ll get to that later). What makes them unique are the functional groups at the ends. • -OH (hydroxyl group) makes alcohols • -COOH (carboxyl group) makes proteins • -NH3 (Amine group) makes proteins • -PO4 (phosphate group) makes phospholipids WORD-STEMS TO MEMORIZE Mono = one synth = to make - mer = unit -sis = process of Poly = many lysis = to break De = remove Hydra = water - tion = process of MAKING AND BREAKING POLYMERS SEE POP BEAD DEMO! THE MACROMOLECULES ARE… • 1. Carbohydrates (polysaccharides) • 2. Lipids (Triglycerides, phospholipids) • 3. Proteins (polypeptides) • 4. Nucleic Acids (DNA/RNA) MAKING MACROMOLECULES • Macromolecules are made by covalently bonding monomers by dehydration synthesis: where water is removed from functional groups on the monomers. • Example) “pop-beads” DEHYDRATION SYNTHESIS: LINKS MONOMERS TOGETHER H OH H Monomer # 1 OH Monomer # 2 Water ! HYDROLYSIS: BREAKING DOWN COMPOUNDS BY ADDING WATER. Water IN H OH THE MACROMOLECULES ARE… • 1. Carbohydrates (polysaccharides) • 2. Lipids (Triglycerides, phospholipids) • 3. Proteins (polypeptides) • 4. Nucleic Acids (DNA/RNA) CARBOHYDRATES (SUGARS AND STARCHES) • Carbohydrates have the general formula [CH2O]n where n is a variable. • Function in short-term energy storage (such as sugar); as intermediate-term energy storage (starch for plants and glycogen for animals) MORE WORD-STEMS • Mono = one • Sacchar = sugar • -ose = sugar • Di = two • Poly = many THE MONOMERS OF CARBS • Sugars are the simplest carbohydrates and are used to make energy (metabolism). • Monosaccharides are single (mono=one) sugars. • Ex) glucose (C6H12O6), and fructose (same formula but different structure than glucose). STRUCTURES OF GLUCOSE • Diagram: DISSACCHARIDES • Disaccharides are formed when two monosaccharides are chemically bonded together. • Ex. Sucrose = table sugar (glucose + fructose) POLYSACCHARIDES • Polysaccharides are large molecules composed of individual monosaccharide units. • Ex) starch, glycogen, cellulose CELLULOSE UP CLOSE LIPIDS • Lipids are involved mainly with long-term energy storage. They are generally insoluble in water. • Mostly contain C and H atoms. • Secondary functions of lipids are as structural components (the major building block in cell membranes) and as "messengers" (hormones) that play roles in communications within and between cells. MONOMERS OF LIPIDS • Fatty Acids! • Long Hydrocarbon chains (nonpolar) DIFFERENT FATTY ACIDS 1. Unsaturated = may be double bonds between carbons. • Less stable, liquid at room temp 2. Saturated = all carbons have single covalent bonds with Hydrogen. • More stable, solid at room temp. WHY AREN’T PLANTS FAT??? • Fats and oils function for energy storage. • Animals convert excess sugars (beyond their glycogen storage capacities) into fats ( saturated ). • Most plants store excess sugars as starch, although some seeds and fruits have energy stored as oils (e.g. corn oil, peanut oil, palm oil, and sunflower oil). POLYMERS OF LIPIDS • Triglycerides • Can be saturated or unsaturated. • Made of 1 glycerol and 3 fatty acid chains. G L Y C E R O L Fatty Acid Fatty Acid Fatty Acid PHOSPHOLIPIDS • Phospholipids are important structural components of cell membranes. Structure: • a phosphate group (PO4-) is added to a glycerol body. • Next are 2 non-polar tails. STRUCTURE OF A PHOSPHOLIPID Phosphate “head” Glycerol “body” Fatty Acid “tails” PROTEINS • Proteins important in biological systems as control and structural elements. • Contain C,H,O, and Nitrogen • Example) enzymes, some hormones, transport “bridges” across the cell membrane, hair, hemoglobin (in blood), meats, …… THE MONOMERS OF PROTEINS • The building block of any protein is the amino acid. • There are 20 A.A’s and we only make 12 of them. (where do we get the rest?) • has an amino end (NH2) and a carboxyl end (COOH). STRUCTURE OF AN AMINO ACID EXAMPLE OF AMINO ACIDS HOW ARE AMINO ACIDS LINKED? • Dehydration Synthesis? Yes, but for proteins there is a special name for the bonds that are formed…. • Amino acids are linked together by joining the amino end of one molecule to the carboxyl end of another. • Thus, the formation of a type of covalent bond known as a peptide bond. THE PEPTIDE BOND TIME FOR YOUR OWN RESEARCH! • Use your textbook to research the 4(5) types of Proteins. • Divide your paper into 4 sections. • Use “curly ribbon” to create an example for each structure. (2 for the secondary structure) • Write an explanation for each structure. POLYPEPTIDES 4 TYPES!!! Amino acids are linked together into a polypeptide. 1. Primary Structure (single strand) 2. Secondary Structure (alpha helix or pleated sheet) 3. Tertiary Structure (folded helixes, sheets) 4. Quartenary Structure (2 or more polypeptides together) ENZYMES: SPECIAL PROTEINS • Catalysts = anything that will speed up a reaction • enzymes Protein molecules that act as catalysts in biochemical reactions. • Enzymes will only work under • Specific temperatures • Specific pH HOW DO THEY WORK??? • Each enzyme has a very specific structure! • It will only bond to a specific molecule. • Once it bonds to the molecule (called a substrate), it breaks it into 2 pieces. • Then, it binds to another and repeats the process until there are no more substrates left to break. PICTURES… • CLARIFICATION • The substrate fits into the enzyme like a key fits into a lock. • The enzyme then breaks or builds the substrate into 2 usable molecules called the products. • Example…..Can we easily digest sucrose (table sugar) into our cells? How about lactose??? How might enzymes play a role in this digestive process????? NUCLEIC ACIDS (A SHORT REVIEW) • Nucleic acids are polymers composed of monomer units known as nucleotides. Ex) DNA and RNA • The functions of the nucleotides are mainly to: 1.store genetic information 2. To play an integral part of protein synthesis WHAT IS A NUCLEOTIDE? • Nucleotides consist of a sugar, a nitrogenous base, and a phosphate. WHAT SUGARS? • In DNA, the sugar is called: Deoxyribose. • In RNA, the sugar is called: Ribose. • These are both (5 carbon) sugars which is why the shape is a pentagon! • The sugars and phosphates link together to form the “backbone” of the ladders for DNA and RNA. WHAT ARE NITROGENOUS BASES? • There are 5 bases that contain nitrogen, and make up the “rungs” of the ladders. • For DNA: These bases are; Adenine = Thymine Guanine = Cytosine • In RNA: The Thymine is replaced with Uracil