Chemistry notes 2013
... move molecules from one place to another around the body. Examples include hemoglobin and cytochromes. Hemoglobin transports oxygen through the blood. Cytochromes operate in the electron transport chain as electron carrier proteins ...
... move molecules from one place to another around the body. Examples include hemoglobin and cytochromes. Hemoglobin transports oxygen through the blood. Cytochromes operate in the electron transport chain as electron carrier proteins ...
Molecules of Life
... – A large molecule that contains many molecules – A large molecule made of smaller, molecules of the same type (monomers) linked together. • A protein (the polymer) is made of many amino acids (monomers) ...
... – A large molecule that contains many molecules – A large molecule made of smaller, molecules of the same type (monomers) linked together. • A protein (the polymer) is made of many amino acids (monomers) ...
Organic Molecules
... • Usually H, O, N, or another C • Single, double, or triple bonds • Can also form chains or rings which allows for many different arrangements ...
... • Usually H, O, N, or another C • Single, double, or triple bonds • Can also form chains or rings which allows for many different arrangements ...
The six elements that make up 99.9% of all living things include
... most body chemicals 3. they can only be used once 4. they usually slow down reactions and prevent overheating of the cells 5. they usually speed up chemical reactions ...
... most body chemicals 3. they can only be used once 4. they usually slow down reactions and prevent overheating of the cells 5. they usually speed up chemical reactions ...
1 - edl.io
... 18. Define the following terms: solution, solvent, solute, concentration 19. Describe what the pH scale tells us about H+ concentration & how to use it. 20. What is an acid? What is a base? 21. What is an organic compound? 22. What are the four main organic molecules found in organism? 23. List the ...
... 18. Define the following terms: solution, solvent, solute, concentration 19. Describe what the pH scale tells us about H+ concentration & how to use it. 20. What is an acid? What is a base? 21. What is an organic compound? 22. What are the four main organic molecules found in organism? 23. List the ...
File
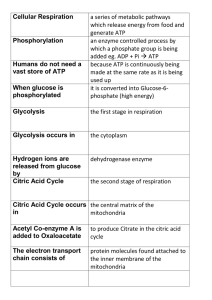
... generate ATP an enzyme controlled process by which a phosphate group is being added eg. ADP + Pi ATP because ATP is continuously being made at the same rate as it is being used up it is converted into Glucose-6phosphate (high energy) ...
... generate ATP an enzyme controlled process by which a phosphate group is being added eg. ADP + Pi ATP because ATP is continuously being made at the same rate as it is being used up it is converted into Glucose-6phosphate (high energy) ...
AP Biology 042 – Biological Molecules Video
... 7. Protein monomers are: 8. What differentiates one amino acid from another? 9. Carbohydrate monomers are 10. The significance of “directionality” of the monomers in a polymer is that when you put the monomers together in a certain sequence/order they have a. The process of “putting monomers togethe ...
... 7. Protein monomers are: 8. What differentiates one amino acid from another? 9. Carbohydrate monomers are 10. The significance of “directionality” of the monomers in a polymer is that when you put the monomers together in a certain sequence/order they have a. The process of “putting monomers togethe ...
Macromolecules Worksheet - High School Science Help
... Organic - compounds that contain both carbon and hydrogen atoms Inorganic - compounds that DO NOT contain both carbon and hydrogen There are four classes of organic compounds that are central to life on earth. 1. Carbohydrates 2. Lipids 3. Proteins 4. Nucleic Acids ...
... Organic - compounds that contain both carbon and hydrogen atoms Inorganic - compounds that DO NOT contain both carbon and hydrogen There are four classes of organic compounds that are central to life on earth. 1. Carbohydrates 2. Lipids 3. Proteins 4. Nucleic Acids ...
Macromolecules
... composed of carbon, hydrogen and oxygen molecules Monosaccharides typically have five or six carbon atoms. Monosaccharides can, such as the ribose and deoxyribose of RNA and DNA, can serve very important functions in cells. ...
... composed of carbon, hydrogen and oxygen molecules Monosaccharides typically have five or six carbon atoms. Monosaccharides can, such as the ribose and deoxyribose of RNA and DNA, can serve very important functions in cells. ...
Biomolecules are organic molecules built and used inside of cells
... ______________ for a short term • Monosaccharides are broken down in cellular ________________ into carbon dioxide and water • The energy released from the broken bonds is used to form molecules of ______ (the energy currency of the cell) • Examples of monosaccharides are ___________, fructose, dext ...
... ______________ for a short term • Monosaccharides are broken down in cellular ________________ into carbon dioxide and water • The energy released from the broken bonds is used to form molecules of ______ (the energy currency of the cell) • Examples of monosaccharides are ___________, fructose, dext ...
Macromolecules Notes
... Organic - compounds that contain both carbon and hydrogen atoms Inorganic - compounds that DO NOT contain both carbon and hydrogen There are four classes of organic compounds that are central to life on earth. 1. Carbohydrates 2. Lipids 3. Proteins 4. Nucleic Acids ...
... Organic - compounds that contain both carbon and hydrogen atoms Inorganic - compounds that DO NOT contain both carbon and hydrogen There are four classes of organic compounds that are central to life on earth. 1. Carbohydrates 2. Lipids 3. Proteins 4. Nucleic Acids ...
Chapter 2 - (www.ramsey.k12.nj.us).
... Draw a molecule that has a 3-C (carbon) skeleton and a hydroxyl group on the middle carbon. (Hint: formula is C3H8O) Explain the connection between monomers and polymers. What molecule is released during the construction of a polymer? What is this process called? Draw at least three ways in which fi ...
... Draw a molecule that has a 3-C (carbon) skeleton and a hydroxyl group on the middle carbon. (Hint: formula is C3H8O) Explain the connection between monomers and polymers. What molecule is released during the construction of a polymer? What is this process called? Draw at least three ways in which fi ...
Chemistry of Life: The Four Macromolecules
... • Proteins contain nitrogen as well as carbon, hydrogen, and oxygen. • Proteins are polymers of molecules called Amino Acids (monomers). ...
... • Proteins contain nitrogen as well as carbon, hydrogen, and oxygen. • Proteins are polymers of molecules called Amino Acids (monomers). ...
Macromolecules Worksheet
... Organic - compounds that contain both carbon and hydrogen atoms Inorganic - compounds that DO NOT contain both carbon and hydrogen There are four classes of organic compounds that are central to life on earth. 1. Carbohydrates 2. Lipids 3. Proteins 4. Nucleic Acids ...
... Organic - compounds that contain both carbon and hydrogen atoms Inorganic - compounds that DO NOT contain both carbon and hydrogen There are four classes of organic compounds that are central to life on earth. 1. Carbohydrates 2. Lipids 3. Proteins 4. Nucleic Acids ...
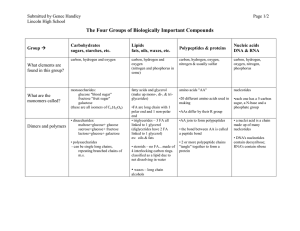
The Four Groups of Biologically Important Compounds
... polar end and 1 non-polar end • triglycerides – 3 FA all linked to 1 glycerol (diglycerides have 2 FA linked to 1 glycerol) ex: oils & fats • steroids – no FA…made of 4 interlocking carbon rings. classified as a lipid due to not dissolving in water ...
... polar end and 1 non-polar end • triglycerides – 3 FA all linked to 1 glycerol (diglycerides have 2 FA linked to 1 glycerol) ex: oils & fats • steroids – no FA…made of 4 interlocking carbon rings. classified as a lipid due to not dissolving in water ...
Biological Molecules wHelp Sheet
... From the smallest single-celled organism to the tallest tree, all life depends on the properties and reactions of four classes of organic (carbon-based) compounds—carbohydrates, lipids, proteins, and nudeic acids. These organic molecules are the building blocks of all living things, and are responsi ...
... From the smallest single-celled organism to the tallest tree, all life depends on the properties and reactions of four classes of organic (carbon-based) compounds—carbohydrates, lipids, proteins, and nudeic acids. These organic molecules are the building blocks of all living things, and are responsi ...
Name - Humble ISD
... 8. ________ Substance that increases OH – concentration when added to water ...
... 8. ________ Substance that increases OH – concentration when added to water ...
Describe in simple terms the chemical nature of sugars, proteins
... Describe in simple terms the chemical nature of sugars, proteins, lipids, nucleotides and enzymes: Sugars – a simple sugar, known as a monosaccharide, is made up of 3 to 7 carbon atoms arranged in a ring. A disaccharide is two monosaccharides, such as glucose and fructose equals sucrose. A polysacch ...
... Describe in simple terms the chemical nature of sugars, proteins, lipids, nucleotides and enzymes: Sugars – a simple sugar, known as a monosaccharide, is made up of 3 to 7 carbon atoms arranged in a ring. A disaccharide is two monosaccharides, such as glucose and fructose equals sucrose. A polysacch ...
Biochemistry
_and_Carl_Ferdinand_Cori.jpg?width=300)
Biochemistry, sometimes called biological chemistry, is the study of chemical processes within and relating to living organisms. By controlling information flow through biochemical signaling and the flow of chemical energy through metabolism, biochemical processes give rise to the complexity of life. Over the last decades of the 20th century, biochemistry has become so successful at explaining living processes that now almost all areas of the life sciences from botany to medicine to genetics are engaged in biochemical research. Today, the main focus of pure biochemistry is in understanding how biological molecules give rise to the processes that occur within living cells, which in turn relates greatly to the study and understanding of whole organisms.Biochemistry is closely related to molecular biology, the study of the molecular mechanisms by which genetic information encoded in DNA is able to result in the processes of life. Depending on the exact definition of the terms used, molecular biology can be thought of as a branch of biochemistry, or biochemistry as a tool with which to investigate and study molecular biology.Much of biochemistry deals with the structures, functions and interactions of biological macromolecules, such as proteins, nucleic acids, carbohydrates and lipids, which provide the structure of cells and perform many of the functions associated with life. The chemistry of the cell also depends on the reactions of smaller molecules and ions. These can be inorganic, for example water and metal ions, or organic, for example the amino acids which are used to synthesize proteins. The mechanisms by which cells harness energy from their environment via chemical reactions are known as metabolism. The findings of biochemistry are applied primarily in medicine, nutrition, and agriculture. In medicine, biochemists investigate the causes and cures of disease. In nutrition, they study how to maintain health and study the effects of nutritional deficiencies. In agriculture, biochemists investigate soil and fertilizers, and try to discover ways to improve crop cultivation, crop storage and pest control.