* Your assessment is very important for improving the work of artificial intelligence, which forms the content of this project
Download Structure and Function of Macromolecules
Signal transduction wikipedia , lookup
Western blot wikipedia , lookup
Citric acid cycle wikipedia , lookup
Photosynthesis wikipedia , lookup
Two-hybrid screening wikipedia , lookup
Point mutation wikipedia , lookup
Vectors in gene therapy wikipedia , lookup
Size-exclusion chromatography wikipedia , lookup
Photosynthetic reaction centre wikipedia , lookup
Basal metabolic rate wikipedia , lookup
Metalloprotein wikipedia , lookup
Genetic code wikipedia , lookup
Protein structure prediction wikipedia , lookup
Proteolysis wikipedia , lookup
Nucleic acid analogue wikipedia , lookup
Amino acid synthesis wikipedia , lookup
Fatty acid synthesis wikipedia , lookup
Fatty acid metabolism wikipedia , lookup
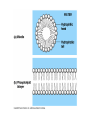
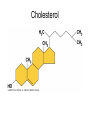
Structure and Function of Macromolecules Four Main Types of Macromolecules Macromolecules are constructed of smaller units repeating units called monomers Carbohydrates Lipids Proteins Nucleic Acids Making and breaking of polymers: 1. Making polymers Dehydration Synthesis Losing a water molecule to join two monomers together. Anabolic reaction Building polymers 2. Breaking Polymers Hydrolysis Water molecules break polymers apart. Catabolic reaction Breaking down polymers Carbohydrates monomer monosaccharides known All as simple sugars Disaccharide have a ratio of 1:2:1 ratio of C:O:H CH2O Monosaccharides: Examples: pentose sugars. 5 carbons. hexose sugars 6 carbons Ribose Deoxyribose Ribulose Glucose, Galactose Fructose. Typically ring shaped in aqueous environments like the cell. Disaccharides: These are double sugars with the formula C11H22O11 Polysaccharides The basic formula is ( C6H10O5)n . These are macromolecules capable of acting as structural or storage molecules. Polysaccharides Composed of repeating glucose molecules. Storage Starch Found in plants roots Bulky molecule Glycogen Found in liver of animals More compact Structural Polysaccharides Cellulose Found in plant walls Cannot be digested by animals Chitin Only carbohydrate containing nitrogen. Found in insect shells Structural Polysaccharides: Fats are composed of: Glycerol 3 3 carbon alcohol fatty acid molecules Saturated Verses Non-Saturated Saturated or Unsaturated? Saturated fatty acids Single bond between carbons. Found in animals Typically solids at room temperature: Lard, butter Unsaturated fatty acids Contain one or more double bonds between the carbons. Double reduces the number of hydrogens that that can be attached to the carbon in the molecule. This causes the molecule to bend or kink at each of the double bond sites. Found in plants Typically liquids at room temperature: vegetable oil Saturated vs UnSaturated Lipids • Lipids: A group of polymers that have one characteristic in common, they do not mix with water. They are hydrophobic. Some important groups are fats, phospholipids, and steroids. • Lipids Provide insulation Serves as an energy storage source 1g.=9 Kcal of energy Shock absorber for internal organs. Steroids and sex hormones are made from fats. Phospholipids: Structurally related to fats but contain 2 fatty acids and one molecule of phosphate. These molecules are found making up the plasma membrane of cells. Amphipathic They exhibit a polar and non polar quality. The phosphate group is hydrophilic while the fatty acid area is hydrophobic. Steroids: Lipids characterized by a carbon skeleton of 4 fused rings. Cholesterol is an important steroid found in all animal tissue. Plants do not contain cholesterol. Cholesterol is a precursor to steroids Cholesterol adds fluidity to membranes. Animals that live in cold climates have more cholesterol and unsaturated fatty acids in their membranes Cholesterol Proteins: macromolecules that make up 50% of the dry weight of most cells. Types of proteins: 1. Structural ( support) 2. Storage food source, examples: examples: actin and myosin 6. Antibodies defense, example insulin, 5. Contractile movement, examples: hemoglobin and cell membrane proteins Hormonal coordinates bodily activities, ovalbumin and casein 3. Transport moves other substances, examples: elastin, collagen, and keratin examples: Ig.E, IgA, and Ig.G 7. Enzymes aid in chemical reactions, examples: amylase and proteases Amino Acids: Most amino acids consist of an asymmetrical carbon bonded to an, amino group, hydrogen, an R group,and a carboxyl group. Amino Acids: Most amino acids consist of an asymmetrical carbon bonded to an, amino group, hydrogen, an R group,and a carboxyl group. Protein conformation: refers to the three dimensional shape of a protein molecule. This shape is important to its function. If the conformation is changed, even slightly , then the function of the protein changes. Nucleic Acids ( DNA and RNA) Nucleotides: monomers that come together to form a nucleic acid. They contain either a ribose or deoxyribose sugar ( ribose has one more oxygen in its molecule), a phosphate and a nitrogen base DNA Base pairing rules. DNA