* Your assessment is very important for improving the workof artificial intelligence, which forms the content of this project
Download Biology Unit 2
Protein moonlighting wikipedia , lookup
Gaseous signaling molecules wikipedia , lookup
Western blot wikipedia , lookup
Deoxyribozyme wikipedia , lookup
Genetic code wikipedia , lookup
Expanded genetic code wikipedia , lookup
Intrinsically disordered proteins wikipedia , lookup
Endomembrane system wikipedia , lookup
Photosynthesis wikipedia , lookup
Evolution of metal ions in biological systems wikipedia , lookup
Protein adsorption wikipedia , lookup
Fatty acid metabolism wikipedia , lookup
Protein structure prediction wikipedia , lookup
Cell-penetrating peptide wikipedia , lookup
Biosynthesis wikipedia , lookup
Nucleic acid analogue wikipedia , lookup
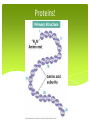
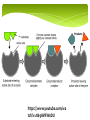
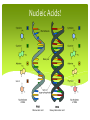
Biology Unit 2 Macromolecules Chapter 6.3 Macromolecules MACROMOLECULES: Large molecules in groups containing carbon atoms. Made up of MONOMERS (small, singular molecules, or a subunit) Polymers are multiple monomers Organic vs Inorganic Macromolecules Organic – Contains Carbon For example: Carbs, Proteins, Lipids, Nucleic Acids Inorganic – Doesn’t contain carbon For example: Water Organic or Inorganic? Organic or Inorganic? Carbohydrates Contain 1 Carbon, 2 Hydrogen, and 1 Oxygen ratio (1:2:1 CHO) Simple Sugars aka. Monosaccharides (single sugar) aka. Glucose C6H12O6 The more sugar, the larger the carbohydrate. We call that Complex Carbs or Polysaccharides. Carbohydrates QUICK ENERGY SOURCE FOR ALL CELLS Ring-shaped structure (either hexagon or pentagon) Examples of Carbs Starch – found in plants to store food/energy in seeds and bulbs Example – potatoes, rice Glycogen – polysaccharide found in animals to store energy Housed in the liver in mammals Cellulose – found in plants that provides support and structure Carbohydrates! Lipids Contain Carbon, Hydrogen, and VERY LITTLE oxygen Made up of two parts (subunits) Glycerol – 3 carbon chain that acts as a backbone Fatty Acid Chain – 3 long chains that are attached to each carbon separately. Lipids INSOLUBLE! (doesn’t dissolve in water). This is what allows them to form cell membranes. LIPIDS STORE MORE ENERGY THAN CARBS! Saturated fats only contain single bonds where as unsaturated fats contain double bonds. Examples of Lipids Fats – found in animals to store energy, provide protection and insulation, and build cell membranes Oils – found in plants to store energy for later use Waxes – found in BOTH plants and animals for protection and structure Steroids – found in animals as a chemical messenger Cholesterol – found in animals lubricates cell membranes. Lipids! Proteins! Proteins! Made of Carbon, Hydrogen, Oxygen, and Nitrogen (sometimes Sulfur) Made up of 20 different monomers called Amino Acids Peptide Bonds hold together long chains (polypeptide) of amino acids CODED FOR BY DNA! Structure of Protein The number and sequence of amino acids determine the shape of a protein Protein Examples Structural Proteins Hair, fingernails, feathers, muscle fibers, spider webs Functional Proteins - have a specific role to carry out in a cell Hemoglobin – transports oxygen in your blood Insulin – transports glucose to the cells for energy Antibodies – fight off disease Enzymes Enzymes – also called biological catalysts. Speeds up chemical reaction. Dependent upon temperature, and pH 1. Enzymes start with active sites 2. Each substrate fits into the active site 3. The bond releases the new product which can now be processed easier 4. The active site can be used again https://www.youtube.com/wa tch?v=tI69AVRW0DU Proteins! Nucleic Acids Contains the elements Carbon, Hydrogen, Oxygen, Nitrogen, and Phosphorous Subunits are called nucleotides and contain 3 parts Sugar group (pentagon or hexagon shape) Phosphate group (circle) Nitrogenous base (square/rectangle) Nucleic Acids Two examples are DNA (deoxyribonucleic acid) and RNA (ribonucleic acid) DNA stores all genetic information and provides coded instructions RNA contains the sugar ribose and decodes the information from the DNA to make proteins in the cell Nucleic Acids!