* Your assessment is very important for improving the work of artificial intelligence, which forms the content of this project
Download The Membrane: Achieving Balance
Cell membrane wikipedia , lookup
Extracellular matrix wikipedia , lookup
Tissue engineering wikipedia , lookup
Cell growth wikipedia , lookup
Endomembrane system wikipedia , lookup
Cellular differentiation wikipedia , lookup
Cytokinesis wikipedia , lookup
Cell culture wikipedia , lookup
Cell encapsulation wikipedia , lookup
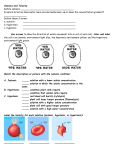
Osmosis: Striking a Balance Maintaining A Balance Cells are surrounded by watery solutions and are filled by watery solutions. A solution is a mixture in which one or more substances (Solutes) are dissolved in another substance (Solvent) The concentration of a solute is important to organisms. Organisms cannot live unless the concentration of dissolved substances stays within a narrow range. Balance and Homeostasis This balance of conditions is called Homeostasis The movement of water across the membrane helps to balance the concentrations of dissolved substances in a cell The diffusion of water across a selectively permeable membrane is called osmosis Osmosis Like other molecules the movement of water is controlled by differences in concentration (a concentration gradient) Water will move in the direction where there is a high concentration of solute (hence a lower concentration of water) A simple rule to remember is SALT SUCKS Salt is a solute, when it is concentrated inside or outside a cell, it will draw water in its direction Tonicity The measurement of solute concentration on either side of a membrane is known as Tonicity The tonicity of a cell’s environment affects its rate of osmosis. Isotonic In an isotonic solution, the concentration of solute is the same on both sides of the membrane (inside the cell and outside). A cell placed in an isotonic solution neither gains or loses water. Most cells in the body are in an isotonic solution. Why? Red Blood Cells Plant Cells Hypertonicity A hypertonic solution is one that has a high solute (Less water) concentration. Cells in a hypertonic solution will lose water. Hypertonicity As a result of a difference in structure, plant and animal cells respond differently Animal Cells shrink crenate Plant cells, maintain shape because of their cell wall, the cell membrane breaks away from the cell wall resulting in Plasmolyis Red Blood Cells Plant Cells Hypotonicty A hypotonic solution is one that has less solute (more water). When placed in a hypotonic solution, cells tend to gain water and swell Hypotonicty If an animal cell swells too much it will burst. This is called Lysis. Plant cells rarely burst because of their cell wall. As water fills the cell the membrane is pushed up against the wall (turgor pressure) making the plant cell turgid. Red Blood Cells Plant Cells Hypotonicity Many single cell organisms live in hypotonic solution so they may be in danger of constantly bursting. To help these organisms they have a contractile vacuole to pump water out of the cell. Questions How can we determine which solution is isotonic to a given set of cells? Is the concentration the same for all cells?