* Your assessment is very important for improving the work of artificial intelligence, which forms the content of this project
Download Epigenetics
List of types of proteins wikipedia , lookup
Nucleic acid analogue wikipedia , lookup
Molecular cloning wikipedia , lookup
Eukaryotic transcription wikipedia , lookup
Genomic imprinting wikipedia , lookup
Cre-Lox recombination wikipedia , lookup
Gene expression profiling wikipedia , lookup
Genome evolution wikipedia , lookup
Deoxyribozyme wikipedia , lookup
Community fingerprinting wikipedia , lookup
Molecular evolution wikipedia , lookup
Epitranscriptome wikipedia , lookup
Non-coding DNA wikipedia , lookup
Gene regulatory network wikipedia , lookup
Vectors in gene therapy wikipedia , lookup
Secreted frizzled-related protein 1 wikipedia , lookup
Gene expression wikipedia , lookup
Endogenous retrovirus wikipedia , lookup
Promoter (genetics) wikipedia , lookup
Bisulfite sequencing wikipedia , lookup
Silencer (genetics) wikipedia , lookup
Artificial gene synthesis wikipedia , lookup
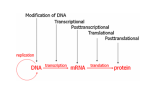
Epigenetics Epigenetics Epi- (Greek: over, above, outer) The study of mitotically (separating chromasomes in a cell) and/or meiotically heritable changes in gene function that cannot be explained by changes in DNA sequence (96 Russo) (classical) 11_Graff (www.cs.uml.edu/~kim/580/11_Graff.pdf) Structural adaptation of chromosomal regions so as to register, signal, or perpetuate altered activity states (recent) Study of changes in gene expression or cellular phenotype (simple) Meiosis Chromatin A complex of macromolecules in cells Consists of DNA, protein, RNA Primary functions Package DNA Reinforce DNA macromolecule for mitosis Prevent DNA damage Control gene expression and DNA replication Primary protein component of chromatin histones Chromosome Length • • • 3.4A per base 3 Billion bases • 1.8 meters of DNA • 0.09 nm of chromatin after being wound on histones Five families of histones • H1/H5, H2A, H2B, H3, and H4 A nucleosome is the basic unit of DNA packaging in eukaryotes, consisting of a segment of DNA wound in sequence around eight histone protein cores. This structure is often compared to thread wrapped around a spool. Nucleosomes form the fundamental repeating units of eukaryotic chromatin, which is used to pack the large eukaryotic genomes into the nucleus while still ensuring appropriate access to it (in mammalian cells approximately 2 m of linear DNA have to be packed into a nucleus of roughly 10 µm diameter). A nucleosome core has about 146 bp of DNA Typical exon of around 140 nt Three Major Levels of Epigenetic Changes 1. 2. 3. Chemical modifications at the level of nucleotides • Including DNA methylation and RNA interference Modifications at the level of histones that encompass posttranslational modifications (PTMs) of histone proteins and the incorporation of histone variants Nucleosome remodeling • ATP (Adenosine Triphosphate)-dependent processes that regulate the accessibility of nucleosomal DNA (ATP: stores energy) => Regulation of the accessibility of the chromatin structure to the transcription machinery 1. a. DNA Modification: Methylation Covalent addition of a methyl group from methyl donor SAM (Sadenosylmethionine) to a cytosine base Occurs mainly at 5’ end of cytosine in CpG, CpHpG and CpHpH, where H is A,T, or C This reaction is catalyzed by a family of DNMT (DNA methyltransferase) DNMT1 is the main enzyme in mammals Methylation patterns change over evolution In invertebrate animals, mosaic methylation, with stable methylated domains interspersed with methylation-free regions In vertebrate genomes, globally methylated, with exception of CpG islands Methylation dynamically change among different cells, and even in a single cell The reaction catalyzed by DNA methyltransferases (DNMTs). DNMTs are the key enzymes for DNA methylation and catalyze the transfer of a methyl group from SAM to cytosine, thus forming 5-methyl-cytosine and SAH. Methylation of CpG sequences might induce chromatin conformational modifications and inhibit the access of the transcriptional machinery to gene promoter regions, thus altering gene expression levels. Therefore, promoter rmethylation of CpG islands is commonly associated with gene silencing and promoter demethylation with gene expression, though several exceptions to this rule are known. Methylation Inheritance Both C & its complementary G are methylated (fully methylated) After replication, rapidly acted on by DNMT1 to regenerate two identical fully methylated double helices Epigenetic info is inherited in the form of DNA methylation patterns DNA Methylation Binding Methyl-binding proteins (MBPs) bind to methylated DNA, typically in promoters (e.g. MeCP2– Methyl CpG binding Protein 2) Binding recruits other protein complexes that lead to transcription repression 1.b. RNAi Epigenetic alterations of DNA can also be produced by double-stranded RNA (dsRNA) and protein components of RNAi machinery Small RNAs produced by cleavage of dsRNA are thought to serve as sequence-specific facilitators to guide other enzymes of epigenetic machinery into place D.melanogaster (fruit fly) Members of RNAi machinery such as genes piwi and homeless are mutated, centromeric heterochromatin formation is inhibited Fission yeast (Schizosaccharomyces pombe) Deletions of genes involved in RNAi machinery, such as argonaute, result in reduced hetrochromatin formation and reduced methylation on H3K9 (marker of gene repression) Mammals Short-interfering RNA (siRNA) induce methylation alongside H3 methylation, resulting in decreased gene expression 2. a. Histone Modification Histone Basic proteins regulating compaction of chromatin Consist of a loosely structure NH2-terminal tail out acting as regulatory substrates for nucleosomal stability These substrates establish condensed/uncondensed states of the chromatin a globular histone core (nucleosome) Octamer of four core histones H2A, H2B, H3, and H4 in duplicates Around the core, 147 bp wrap around in 10-nm-thick primary structure 2. a. Histone Modification Histone Nucleosomes are linked together by linker histone H1 Post Translational Modifications (PTMs) can occur on all histones Majority occurs on NH2-terminal tail PTM types Acetylation on lysine (K) Methylation Phosporylation Ubiquitnation sumoylation DNA-gray H2A – blue H2B – yellow H3 – green H4 - red PTM Their accessibility of DNA will change Current research emphasis is on Role of modification in the transformation process from normal to cancer cells Study imbalances in net expression of tumor suppressor vs. oncogenes or overall genomic imbalances Needs substrate specificity and residue-specific alteration Acetylation Positively charged AA (lysine(K) and arginine (R) are neutralized by acetyl group, leading to decreased affinity between histone tail and negatively charged DNA HAT (Histone Acetyltransferase) regulates acetylation of histones In most cancers, HAT genes are muted and HAT includes chromosomal translocation of respective HAT But HDACs (Histone deacetylase) are frequently overexpressed Histones prefer being methylated or phosphorylated at R, at acetylated at K Acetylation of K initiates active gene expression Acetylation plays a role in nucleosome assembly and maintenance of chromatin state affecting DNA repair, etc. The unmodified side chain of posttranslationally modifiable residues is first presented, followed by representations of the residue on which the posttranslational modification has occurred at respective sites. Abbreviations are as follows R, arginine; Y, tyrosine; S, serine; T, threonine; -ac, acetylation; -me, monomethylation; -me2, dimethylation; -me3, trimethylation; -ph The color code is as follows: yellow, carbon; blue, nitrogen; pink, polar hydrogen; red, oxygen; orange, phosphorus; green, methyl gro of posttranslational modifications. Red – acetyl group Yellow - methylation Green – methylation of R HAT (Histone Acetyltransferease) HDAC (Histone Deacetylase) HMT (Histone Methyltransferase) HDM (Histone Demethylase) Effects of acetylation on protein functions. Acetylation of proteins affects many different functions, some of which are listed. The double up-arrows indicate increase and the double downarrows indicate decrease with respect to the particular function. Some of the genes affected by acetylation under specific protein functions are listed [60]. Methylation K and R can be mono-, di- or tri-methylated forms Monomethylated H3K4 is found in expressed and repressed genes Trimethylated H3K4 is exclusively in silenced genes Location – H3K9me in coding region for expression, in promoter, repression Some times, same K is acetylated or methylated example: K4 and K9 residues of histone H3 Methylation is regulated by HMT (histone methyltransferase), specific to K and R And HDM (Histone Demethylase) Methylation 2 Common pattern in many cancers Loss of H4K16 acetylation and H4K20 tri-methylation When tumor suppressor genes are down-regulated by hypermethylation, oncogenes may be stimulated by acetylation or hypomethylation Example: hypermethylation of H3K79 promotes leukemogenesis Tumor-specific epigenetic abnormalities can stem from altered modifications of the histone residues, and/or altered expression of the enzymes that catalyze the modifications Methylation 3 Histone lysine resideus are methylated by methyltransferases and utilizes S-adnosyl methionine (SAM) in catalyzing the transfer of methyl group to specific histone residues Methyltransferases are specific based on target residues PKMT (Protein lysine methyltransferase) for lysine PRMT (Protein Arginine MT) for R PRMT primarily catalyze mono- and di-methylation of histone R 2,8,17, 26 of H3, and R 3 of H4 H3K27 methylation is mediated by a PKMT called EZH2, which is over-expressed in many tumors and considered to be responsible for cancer aggressiveness Leukemogenesis is promoted by aberrant recruitment of H3K79 Role of Methylation Gene silencing (exceptions are found) Maintaining cellular functions and development of autoimmunity and aging Aberrant methylation may be associated with disorder of gene expression Irregular memory function in development by heritability Reversible process Demethylation by enzymes such as DNA glycolaes 2.b. Histone Variants Results from sequential and structural variation of core histones Replacement of large groups of AAs in histone tails and globular central domains Only a few AA substitutions Four core Histones are incorporated into nucleosomal structure exclusively during replication Histone variants can be integrated into specific regions of genome throughout cell cycle 3. Nucleosomal Remodeling Chromatin structure is changed from net energy input Nucleosome remodeling is carried out by enzymes that are catalytically dependent on ATP as energy source Gene regulation J.Su, et al., “Revealing epigenetic patterns in gene regulation …,” Mol. Biol Rep, 2012 Chromatin components mainly include Histone modifications Histone variants DNA-binding proteins and associate complexes In mammalian genomes, chromatin components and DNA methylation are associated with chromatin regulation, and influence gene transcription How they regulate chromatin structure and gene expression has implications for understanding development, aging and disease Most histone modifications occur at the flexible N-terminal tails Gene regulation Histone acetylation – gene activation Histone methylation – gene activation and repression example. Enrichment of H3K9ac and H3K4me3 in promoters and CpG islands are associated with gene repression Histone variant H2A.Z and RNA pol-II are preferentially deposited in promoters, yielding gene activation Gene Expression How chromatin components and DNA methylation affect gene expression? Independently or synergistically ? 41 chromatin components 16,003 promoters are examined Genes divided into high- and low-expression according to 50% present and absent – 7,911 high- and 8,092 low-expression genes Modification intensities of HGP (High-expression gene promoter) & LGP are plotted Gene Expression Modification intensities except H3K9me2 and H4K20me3 are significantly distinct between HGPs and LGPs Chromatin Structure Alteration Alteration of chromatin fiber structure is critical to control cellular processes and regulate the expression fidelity of genes in particular cell types Such regulation is carried out by a combination of several factors, posttranslational histone tail modification, chromatin remodeling enzymes. One factor contributing this process comes from architectural proteins such as H1 and members of HMG (high-mobility-group) superfamily HMG superfamily – HMGA, HMGB, HMGN HMGN – unique in its ability to bind directly to the nucleosome core particles HMGN – associated with generation and maintenance of open chromatin regions HMGN1 is enriched in transcriptionally active domains. Transcriptionally inactive chromatin is marked by H3K27me3. Active chromatin marks such as H3K4me3 and H3K9ac, is seen close to the gene Actrively transcribed with RNA Pol II across promoter Expression Level BT Wilhelm, et al., “Differential patterns of intronic and exonic DNA regions …,” Genome Bio, 2011 Splicing is initiated together with transcription in a chromatin Maybe possible to have a functional relationship between splicing and local chromatin environment Different markings in introns and exons may influence splicing Highly transcribed genes tend to be efficiently spliced Data FAIRE (Formaldehyde-assisted isolation of regulatory elements) – histone H3 occupancy and protein free area FAIRE (red) – genefree Histone H3 (blue) H3K36me3 (green) Pol II (black) Alzheimer’s Disease AD Neurodegeneration in brain regions including temporal and paretal lobes and restricted regions in frontal oortes and cingulate gyrus Exracelluar amyloid deposits (senile plques, SP) and the presence of neurofibrilliary tangels (NFT) composed of intraneuronal aggregates of hyperphosphorylated tau protein Primary component of SP is about 40 bp amyloid β (Aβ), resulting from proteolytic processing of its precursor, amyloid precursor protein (APP) APP is processed by β- and γ-secretase (presenilin and other protein complex) to produce Aβ: Aβ40, Aβ42 A high Aβ42/Aβ40 => AD AD 1% early AD 50% due to mutations in APP, PSEN1, PSEN2 50% may involve other LOAD (Late-onset AD) over age 65 ALZGene database (alzgene.org) lists over 1000 genes Most like genes APOE (apolipoprotein E) BIN1 (bridging integrator 1) CLU (clusterin) Found to have decreased folate values and increased plasma homocysteine levels (hyperhomocysteinemia) One-carbon metabolism Folate Essential nutrients required for one-carbon biosynthetic and epigenetic process Derived entirely from dietary sources, mainly from green vegetables, fruits, cereals, and meat After intestinal absorption, folate metabolism requires reduction and methylation into the liver to form 5-methylterahydrofolate (5MTHF), release into blood and cellular uptake 5-MTHF is used for synthesis of DNA and RNA precursors or for conversion of homcyctein (Hcy) to methionine, which is used to form S-adenoylmethionine (SAM) B6 and B12 participate in one-carbon metabolism Folic acid is used for DNA methylation process Synthesis of nucleic acid precursors One-carbon (Folate) Metabolism MTR (methionine synthase) transfers a methyl group from 5methulTHF to methionine and tetrahydrofolate (THF). Met is converted to SAM (S-adenosylmethionine). SAM transfers mythyl group and is converted to SAH (Sadenosylhomocycteine) One-carbon (Folate) Metabolism DNMT (DNA methyltransferase) is the key enzyme for DNA methylation DNA methylation is dependent on its potential measured by SAM/SAH level High SAH level inhibit DNMTs (DNA methyltransferases) High Hcy levels are found in AD patients Integrative Genomics RD Hawkins, et al., “Next-generation genomics: an integrative approach,” Nature, 2010 Genomic Data Sets Available Sequence variation data from individual genomes Transcriptome Epigenomic data – methylation in MethDB Interactome – RNA-protein, protein-protein Integrative Genomics Can address questions related to fundamental mechanisms of genome function and disease How might particular risk-associated SNPs affect cellualr function, leading to a disease ? What functional sequences exist in human genome ? How are key development pathways regulated by epigenetic mechanisms ? Annotating functional features of genome Regulation elements are not fully understood Enhancers, insulators From characteristics of known Res, identify novel elements Chromatin signature of enhancers are used to find new enhancers Inferring function of genetic variants SNPs in non-coding regions are still poorly defined SNVs (single-nucleotide variants) in transcription factor binding sites or chromatin-marked regulatory elements may be used to determine regulatory SNPs SNP at Pol II bonding regions cause variability of gene expression levels