* Your assessment is very important for improving the workof artificial intelligence, which forms the content of this project
Download Regulation on Cellular respiration
Peptide synthesis wikipedia , lookup
Photosynthetic reaction centre wikipedia , lookup
Mitochondrion wikipedia , lookup
Paracrine signalling wikipedia , lookup
Nicotinamide adenine dinucleotide wikipedia , lookup
Mitogen-activated protein kinase wikipedia , lookup
Butyric acid wikipedia , lookup
Genetic code wikipedia , lookup
Signal transduction wikipedia , lookup
Microbial metabolism wikipedia , lookup
Oxidative phosphorylation wikipedia , lookup
Evolution of metal ions in biological systems wikipedia , lookup
Biochemical cascade wikipedia , lookup
Proteolysis wikipedia , lookup
Adenosine triphosphate wikipedia , lookup
Basal metabolic rate wikipedia , lookup
Biosynthesis wikipedia , lookup
Fatty acid synthesis wikipedia , lookup
Amino acid synthesis wikipedia , lookup
Glyceroneogenesis wikipedia , lookup
Fatty acid metabolism wikipedia , lookup
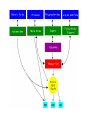
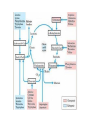
Regulation of Cellular respiration and Related pathways Allosteric regulation: Hexokinase is inhibited by glucose-6-P. If glucose 6 phosphate accumulates because the rate of glycolysis is low, then hexokinase is inhibited and the conversion of glucose to G6P slows. This is a very important regulatory step, since it prevents the consumption of too much cellular ATP to form G6P PFK is the ‘valve’ controlling the rate of glycolysis. -ATP inhibits the phosphofructokinase reaction. AMP activates the reaction. Thus, when energy is required, glycolysis is activated. When energy is plentiful, the reaction is slowed down. • Phosphofructokinase is inhibited by citrate. A large number of compounds— for example, fatty acids and amino acids— can be metabolized to TCA cycle intermediates. High concentrations of citrate indicate a plentiful supply of intermediates for energy production; therefore, high activity of the glycolytic pathway is not required. • This allows regulation between glycolysis and the Kreb’s cycle. -ATP inhibits pyruvate kinase; similar to the inhibition of PFK. -Pyruvate kinase is also inhibited by acetylCoenzyme A. -Fatty acids also allosterically inhibit pyruvate kinase, serving as an indicator that alternative energy sources are available for the cell. • Pyruvate kinase is also activated by fructose-1,6-bisphosphate. This is an example of feed-forward activation. If glycolysis is activated, then the activity of pyruvate kinase must also be increased in order to allow overall carbon flow through the pathway. Feed-forward activation ensures that the enzymes act together. Krebs cycle Pyruvate oxidation -Pyruvate dehydrogenase is allosterically inhibited by NADH and activated by high concentrations of NAD+. -A high concentration of NADH in the cell means that the Electron Transport Chain is full of electrons and that ATP production is high. -Inhibition of this enzyme reduces the amount of Acetyl Co-A that enters into the Kreb’s cycle. • • • Citrate synthase is inhibited by ATP and NADH. Isocitrate dehydrogenase (ICDH)*: allosterically activated by ADP and NAD+; inhibited by ATP and NADH. Also, citrate accumulation and transport into the cytosol leads to activation of fatty acid biosynthesis (storage of acetyl-CoA as fat). Metabolic Pathways Glucose is not the only fuel on which cells depend. Other carbohydrates, fats, even proteins may in certain cells or at certain times be used as a source of ATP. One of the great advantages of the step-by-step oxidation of glucose into CO2 and H2O is that several of the intermediate compounds formed in the process link glucose metabolism to the metabolism of other food molecules. • Fats are stored in adipose tissue. • When needed as an energy source, the fat reserves are moved out of adipose tissue, and broken down into glycerol and fatty acids in the liver • The glycerol portion of the molecule may be converted into DHAP and then to G3P and enters the glycolytic pathway. • The glycerol may also be converted into glucose. This process is called gluconeogenesis. • Fatty acids are converted into molecules of acetyl-CoA , in a process called b-oxidation, and are oxidized in the Kreb’s cycle of the mitochondria. • B oxidation involves the successive removal of two-carbon acetyl groups from the fatty acid. Each cleavage requires one ATP, but produces 1 NADH and 1 FADH2. • The result is that Palmitic Acid, a 16-carbon fatty acid, produces 131 ATP molecules, whereas 2 glucose molecules produce 73 ATPs. • By mass, lipids produce about twice the energy yield of carbohydrates • When fats are being used as the primary energy source such as in starvation, fasting or untreated diabetes, an excess amount of acetyl CoA is produced, and is converted into acetone and ketone bodies. This produces the sweet smell of acetone on the breath, noticeable in a diabetic state. • The amino acids liberated by the hydrolysis of proteins can also serve as fuel. First, the nitrogen is removed, a process called deamination. The remaining fragments then enter the respiratory pathway at several points. • For example: the amino acids Gly, Ser, Ala, and Cys are converted into pyruvic acid and enter the mitochondria to be respired. • Acetyl-CoA and several intermediates in the Kreb’s cycle serve as entry points for most of the other amino acids. • These links permit the respiration of excess fats and proteins in the diet. • No special mechanism of cellular respiration is needed by those animals that depend largely on ingested fats (e.g., many birds) or proteins (e.g., carnivores) for their energy supply. • Many of the points that connect carbohydrate metabolism to the catabolism of fats and proteins serve as two-way valves. They provide points of entry not only for the catabolism (cellular respiration) of fatty acids, glycerol, and amino acids, but for their synthesis (anabolism) as well. Thus the catabolic breakdown of starches can lead (through acetyl-CoA and PGAL) to the synthesis of fat.