* Your assessment is very important for improving the workof artificial intelligence, which forms the content of this project
Download PowerPoint 演示文稿
Mitochondrion wikipedia , lookup
Basal metabolic rate wikipedia , lookup
Biochemical cascade wikipedia , lookup
Metabolic network modelling wikipedia , lookup
Metalloprotein wikipedia , lookup
Adenosine triphosphate wikipedia , lookup
Light-dependent reactions wikipedia , lookup
Photosynthetic reaction centre wikipedia , lookup
Photosynthesis wikipedia , lookup
Biosynthesis wikipedia , lookup
Lactate dehydrogenase wikipedia , lookup
Nicotinamide adenine dinucleotide wikipedia , lookup
Evolution of metal ions in biological systems wikipedia , lookup
Biochemistry wikipedia , lookup
Electron transport chain wikipedia , lookup
Fatty acid metabolism wikipedia , lookup
Amino acid synthesis wikipedia , lookup
Fatty acid synthesis wikipedia , lookup
Glyceroneogenesis wikipedia , lookup
Microbial metabolism wikipedia , lookup
NADH:ubiquinone oxidoreductase (H+-translocating) wikipedia , lookup
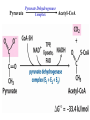
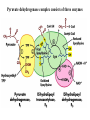
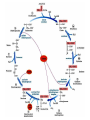
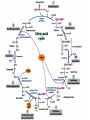
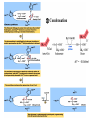
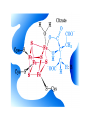
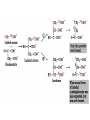
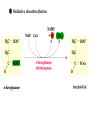
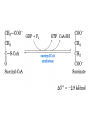
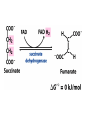
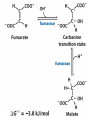
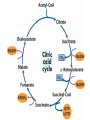
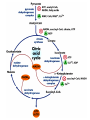
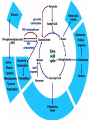
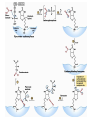
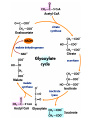
The TCA Cycle A common metabolic pathway for glucose, aa and fatty acid • Citric Acid Cycle, Krebs Cycle • Pyruvate (actually acetate) from glycolysis is degraded to CO2 • Some ATP is produced • More NADH is made • NADH goes on to make more ATP in electron transport and oxidative phosphorylation Entry into the TCA Cycle • Pyruvate Dehydrogenase Complex • ☻Pyruvate is oxidatively decarboxylated to form acetylCoA ☻Pyruvate dehydrogenase uses TPP, CoASH, lipoic acid, FAD and NAD+ ☻Pyruvate dehydrogenase (E1) (丙酮酸脱氢酶) ☻Dihydrolipoyl transacetylase (E2) (二氢硫辛酰转乙酰基酶) ☻Dihydrolipoyl dehydrogenase (E3) (二氢硫辛酰脱氢酶) Pyruvate Dehydrogenase Pyruvate Acetyl-CoA Complex Pyruvate dehydrogenase complex consists of three enzymes Arsenic Compounds Are Poisonous in part because They Sequester Lipoamide ① Condensation Citrate Synthase • Formation of citrate ☻Another example for the induced fit model ☻OAA, the first substrate to bind to the enzyme, induce a large conformational change, creating a binding site for the second substrate, acetyl-CoA. When citroyl-CoA forms on the enzyme surface, another conformational change brings the side of a crucial Asp residue into position to cleavage the thioester. ☻This mechanism decreases the likelihood of premature and unproductive cleavage of the thioester bond of acetyl-CoA Aconitase • Isomerization of Citrate to Isocitrate ☻Citrate is a poor substrate for oxidation ☻So aconitase isomerizes citrate to yield isocitrate which has a secondary -OH, which can be oxidized ☻Note the stereochemistry of the Rxn: aconitase removes the pro-R H of the pro-R arm of citrate! ☻Aconitase uses an iron-sulfur cluster ② Dehydration and hygration Isocitrate Dehydrogenase • Oxidative decarboxylation of isocitrate to yield -ketoglutarate ☻Classic NAD+ chemistry (hydride removal) followed by a decarboxylation ☻Isocitrate dehydrogenase is a link to the electron transport pathway because it makes NADH ☻Know the mechanism! ③ Oxidative decarboxylation ③ Oxidative decarboxylation -Ketoglutarate Dehydrogenase Complex • A second oxidative decarboxylation ☻This enzyme is nearly identical to pyruvate dehydrogenase - structurally and mechanistically ☻Five coenzymes used - TPP, CoASH, Lipoic acid, NAD+, FAD ☻You know the mechanism if you remember pyruvate dehydrogenase ☻Another target for arsenic compounds ④ Oxidative decarboxylation Succinyl-CoA Synthetase • A substrate-level phosphorylation ☻A nucleoside triphosphate is made ☻Its synthesis is driven by hydrolysis of a CoA ester ☻The mechanism involves a phosphohistidine Succinate Dehydrogenase • An oxidation involving FAD ☻This enzyme is actually part of the electron transport pathway in the inner mitochondrial membrane ☻The electrons transferred from succinate to FAD (to form FADH2) are passed directly to ubiquinone (UQ) in the electron transport pathway Fumarase • Hydration across the double bond ☻trans-addition of the elements of water across the double bond ☻The actual mechanism is not known for certain Malate Dehydrogenase • An NAD+-dependent oxidation ☻The carbon that gets oxidized is the one that received the -OH in the previous reaction ☻This reaction is energetically expensive Go' = +30 kJ/mol ☻This and the previous two reactions form a reaction triad that we will see over and over! The Fate of Carbon in TCA ☻Carboxyl C of acetate turns to CO2 only in the second turn of the cycle (following entry of acetate) ☻Methyl C of acetate survives two cycles completely, but half of what's left exits the cycle on each turn after that. TCA Cycle Summary • Total rxn: • Acetyl-CoA+3NAD+ +FAD+GDP+Pi+2H2O→2CO2+3NADH+FADH2+G TP+2H++CoA • One Acetyl-CoA through the cycle produces two CO2, one ATP, four reduced coenzymes • Two H2Os are used as substrates • Absolutely depends on O2 Function of the TCA Cycle Produces More ATPs As a source of biosynthetic precursors A common final metabolic pathway for glucose, aas and fatty acids Some Immediates act as effectors to regulate other metabolic pathways Produces CO2 The Glyoxylate Cycle • A variant of TCA for plants and bacteria Acetate-based growth - net synthesis of carbohydrates and other intermediates from acetate - is not possible with TCA Glyoxylate cycle offers a solution for plants and some bacteria and algae The CO2-evolving steps are bypassed and an extra acetate is utilized Isocitrate lyase and malate synthase are the short-circuiting enzymes Glyoxylate Cycle II Isocitrate lyase produces glyoxylate and succinate Malate synthase does a Claisen condensation of acetyl-CoA and the aldehyde group of glyoxylate - classic CoA chemistry! The glyoxylate cycle helps plants grow in the dark! Glyoxysomes borrow three reactions from mitochondria: succinate to oxaloacetate