* Your assessment is very important for improving the workof artificial intelligence, which forms the content of this project
Download Positive Regulation of IκB Kinase Signaling by Protein
Survey
Document related concepts
Extracellular matrix wikipedia , lookup
Cell culture wikipedia , lookup
Phosphorylation wikipedia , lookup
Cell encapsulation wikipedia , lookup
Hedgehog signaling pathway wikipedia , lookup
Cellular differentiation wikipedia , lookup
Protein phosphorylation wikipedia , lookup
Biochemical switches in the cell cycle wikipedia , lookup
Organ-on-a-chip wikipedia , lookup
List of types of proteins wikipedia , lookup
Signal transduction wikipedia , lookup
Transcript
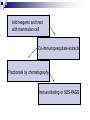
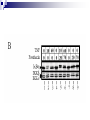
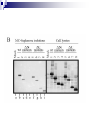
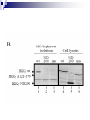
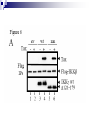
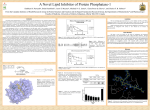
Positive Regulation of IκB Kinase Signaling by Protein Serine/Threonine Phosphatase 2A Arlene E. Kray1, Robert S. Carter2, etc. From the 1Department of Pharmacology and the 2Department of Microbiology and Immunology,Vanderbilt University Medical Center, Nashville, TN 37232 RUNNING TITLE: Regulation of IKK by associated PP2A 系級:銘傳大學生物科技系4年級 學生:劉永偉 日期:2005.10.11 Introduction Transcription factor NF-κB plays a key regulatory role in the cellular response to proinflammatory cytokines such as tumor necrosis factor-α (TNF-α). http://www-ermm.cbcu.cam.ac.uk/ 03006756h.htm http://www.devbio.com/ image.php?id=125 NF-κB, nuclear factor-kappa B; IκB, inhibitor of kappa B; IKK, IκB kinase; PP2A,protein serine/threonine phosphatase 2A; TNF, tumor necrosis factor α; TNF-R1, TNF-receptor type 1; MEFs, murine embryonic fibroblasts. Prior in vitro mixing experiments indicate that protein serine/threonine phosphatase 2A (PP2A) can dephosphorylate these T loop serines and inactivate IKK,suggesting a negative regulatory role for PP2A in IKK signaling. First, TNF-induced degradation of IκB okadaic acid , fostriecin Second, PP2A forms stable complexes with IKK in untransfected mammalian cells. amino acid residue 121-179 of the IKKγ Third, deletion of the PP2A binding site in IKKγ. attenuates T loop phosporylation and catalytic activation Materials and Methods B lymphocytes, cell culture and transfections Generation of T7-tagged IKK γ internal deletion mutant of IKKγ lacking amino acids 121-179 Generation of MEF cell lines stably expressing T7-tagged IKK γ constructs Immunoblotting Fast Performance Liquid Chromatography ATP-Sepharose and microcystin-Sepharose affinity purifications Immunoprecipitations In vitro kinase assays Microcystin-Sepharose affinity isolations Add reagents and treat with mammalian cell Co-immunoprecipitate extracts Fractionate by chomatography Immunoblotting or SDS-PAGE Results and Discussion Effects of PP2A inhibitors on I κB degradation PP2A associates with IKK complexes Identification of a putative PP2A binding site in IKK γ Role of PP2A in IKK signaling Rescue studies of IKK γ-deficient cells Effects of PP2A inhibitors on I κB degradation Conclusion: Despite the capacity of PP2A to inactivate IKK in vitro, exposure of TNFtreated cells to an antagonist of PP2A impairs rather than enhances the degradation of IκBα. PP2A associates with IKK complexes JTCE: Jurkat T cell extracts ATP→MC: ATP eluate was then incubated with microcystin-Sepharose Conclusion: The PP2A catalytic subunit (PP2AC) co-eluted with these IKK subunits in the presence of ATP. Conclusion: These data provide multiple independent lines of evidence indicating that PP2A forms stable complexes with IKK in untransfected mammalian cells. Identification of a putative PP2A binding site in IKK γ Role of PP2A in IKK signaling IKKβ T loop phosphorylation Rescue studies of IKK γ-deficient cells Conclusion: These data demonstrate that the wild type and PP2A-binding defective IKKγ constructs were efficiently integrated into endogenous IKK complexes containing the IKKα and IKKβ catalytic subunits. Conclusion: These data with reconstituted MEFs provide strong evidence that PP2A plays a positive regulatory role in IKK signaling following cellular stimulation with the pro inflammatory cytokine TNF. Conclusion These functional results, together with data shown, further reinforce the model that PP2A binds to IKK, facilitating the induction of IκB kinase activity, targeted degradation of IκB, and release of NF-κB to its nuclear site of action. Instead, our studies support the idea that this region of IKKγ is a novel protein-protein interaction domain, which facilitates PP2A’s interaction with the IKK complex, allowing this phosphatase to function as a positive regulator of IKK signaling. Thank you