* Your assessment is very important for improving the work of artificial intelligence, which forms the content of this project
Download Human immunodeficiency virus type 1 Vprmediated G2 cell cycle
Tissue engineering wikipedia , lookup
Extracellular matrix wikipedia , lookup
Signal transduction wikipedia , lookup
Cell nucleus wikipedia , lookup
Cell growth wikipedia , lookup
Cell encapsulation wikipedia , lookup
Cell culture wikipedia , lookup
Cytokinesis wikipedia , lookup
Cellular differentiation wikipedia , lookup
Organ-on-a-chip wikipedia , lookup
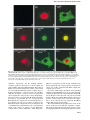
The EMBO Journal Vol. 19 No. 15 pp. 3956±3967, 2000 - Retracted - Human immunode®ciency virus type 1 Vpr-mediated G2 cell cycle arrest: Vpr interferes with cell cycle signaling cascades by interacting with the B subunit of serine/threonine protein phosphatase 2A Mohammed Hrimech, Xiao-Jian Yao, Philip E.Branton1 and EÂric A.Cohen2 Laboratoire de ReÂtrovirologie Humaine, DeÂpartement de microbiologie et immunologie, Faculte de meÂdecine, Universite de MontreÂal, Montreal, Quebec, Canada H3C 3J7 and 1Department of Biochemistry, McGill University, Montreal, Quebec, Canada H3G 1Y6 2 Corresponding author e-mail: [email protected] The Vpr protein of primate lentiviruses arrests cell cycling at the G2/M phase through an inactivation of cyclin B±p34cdc2 and its upstream regulator cdc25. We provide here biochemical and functional evidence demonstrating that human immunode®ciency virus type 1 (HIV-1) Vpr mediates G2 arrest by forming a complex with protein phosphatase 2A (PP2A), an upstream regulator of cdc25. Vpr associates with PP2A through a speci®c interaction with the B55 regulatory subunit. This interaction is necessary but not suf®cient for G2 arrest. Interestingly, we found that Vpr association with B55-containing PP2A targets the enzymatic complex to the nucleus and, importantly, enhances the recruitment and dephosphorylation of the cdc25 substrate. Our data suggest that Vpr mediates G2 arrest by enhancing the nuclear import of PP2A and by positively modulating its catalytic activity towards active phosphorylated nuclear cdc25. Keywords: cell cycle/human immunode®ciency virus/ nuclear import/protein phosphatase 2A/Vpr Introduction Human immunode®ciency virus type 1 (HIV-1) Vpr is a small protein (96 amino acids, Mr 14 kDa) that is incorporated into virions through a speci®c interaction with the p6 domain of the p55gag precursor protein and was thus proposed to have a function early in virus replication (Cohen et al., 1990; Paxton et al., 1993). Two distinct biological functions are associated with Vpr. First, Vpr has been found to enhance the nuclear migration of the preintegration complex (PIC) in newly infected non-dividing cells (Heinzinger et al., 1994). Both the presence of Vpr in the virion and its function in nuclear import account for the requirement for Vpr for ef®cient replication of HIV-1 in non-dividing cell types such as macrophages (Heinzinger et al., 1994; Subbramanian et al., 1998a). The second biological activity of Vpr prevents the passage of cells through mitosis at the G2 stage of the cell cycle (Jowett et al., 1995; Rogel et al., 1995). Cells expressing Vpr, from the complete HIV-1 genome or from an expression plasmid encoding Vpr, do not proliferate but accumulate at the G2 phase of the cell cycle (He et al., 1995; Re et al., 3956 1995). Vpr prevents mitosis in human and primate cells from a variety of tissues, as well as in yeast (Macreadie et al., 1995; Planelles et al., 1996), suggesting that Vpr targets a general cellular pathway that controls progression from G2 to mitosis. The functional role of the G2 cell cycle arrest mediated by Vpr in HIV-1 pathogenesis is as yet unclear. Experimental evidence in in vitro systems indicates that the establishment of a cell cycle arrest enables HIV-1 to optimize virus production and cripple the immune response, thus facilitating the persistence of the virus within the infected individual (Stewart et al., 1997; Goh et al., 1998). In mammalian cells, the transition from G2 into mitosis is controlled by activation of the complex between the cyclin-dependent kinase p34cdc2 and its regulatory partner, cyclin B. This is achieved by a series of coordinated phosphorylation and dephosphorylation events (Jackman and Pines, 1997). Phosphorylation of p34cdc2 on Thr161 by cyclin-associated kinase (CAK) is one of the ®rst events in this process. Activation of p34cdc2 is prevented, however, by additional phosphorylation on Thr14 and Tyr15 by the protein kinases Wee1 and Myt1. Dephosphorylation of Thr14/Tyr15 by the protein phosphatase cdc25 eventually activates the p34cdc2±cyclin B complex, allowing progression to mitosis. Both Wee1/Myt1 and cdc25 activities are regulated by upstream kinase/phosphatase networks, and protein phosphatase 2A (PP2A)-like phosphatase activities have been implicated in keeping Wee1 active and cdc25 inactive during S and G2 phases (Clarke et al., 1993; Tang et al., 1993). Cells expressing Vpr have very low or undetectable levels of cyclin B±p34cdc2 kinase activity (He et al., 1995; Re et al., 1995). It is unlikely that Vpr blocks the activity of the cyclin B±p34cdc2 kinase complex directly since cdc25 phosphatase is found in its inactive, hypophosphorylated state in Vpr-expressing cells (Re et al., 1995), and forced activation of cyclin B±p34cdc2 by treatment of cells with the phosphatase (PP1A and PP2A) inhibitor okadaic acid overcomes Vpr-mediated G2 cell arrest. It is, therefore, more likely that Vpr acts upstream of cyclin B± p34cdc2 and cdc25 to affect their coordinate regulation. Interestingly, recent genetic studies with the ®ssion yeast Schizosaccharomyces pombe suggest the involvement of Wee1, PP2A and Rad24 in induction of cell cycle arrest by HIV-1 Vpr (Masuda et al., 2000). However, the identity of the cellular pathway(s) or factor(s) targeted by Vpr to mediate G2 cell cycle arrest is still not clear and remains a matter of intense research (Withers-Ward et al., 1997; Mahalingam et al., 1998). The reversible phosphorylation of proteins, catalyzed by protein kinases and protein phosphatases (PP), is the key mechanism for the regulation of diverse cellular functions. PP2A is one of the four major classes of protein serine/ threonine phosphatase (Hunter, 1995). PP2A is involved in ã European Molecular Biology Organization HIV-1 Vpr interacts with the B subunit of PP2A a broad range of cellular processes, including signal transduction, transcriptional regulation and control of DNA replication and cell cycle progression (Lee, 1995; SchoÈnthal, 1995). This diversity of PP2A functions is conferred by a diversity of targeting/regulatory subunits and several levels of post-translational modi®cations. The diverse heterotrimeric forms of PP2A in vivo are generated by the association of a ubiquitous core heterodimer, consisting of a 36 kDa catalytic C subunit and a 68 kDa structural/regulatory A subunit, with a variable regulatory B subunit, which binds to the core enzyme yielding the holoenzyme (Mumby and Walter, 1993). The A and C subunits are each encoded by two highly related (85 and 97% identity, respectively) and widely expressed genes that are named a and b. Over 15 different variable B subunits are expressed in a tissue- and developmentalspeci®c manner. These proteins are generated as isoforms and splice variants from three unrelated gene families designated B, B¢ (also called B56) and B¢¢ (Mumby and Walter, 1993). The B family has three members, Ba, Bb and Bg, each with a molecular mass of ~55 kDa (Pallas et al., 1992; Zolnierowicz et al., 1994). The B¢ family consists of numerous recently identi®ed isoforms and splice variants whose molecular masses range from 54 to ~70 kDa (McCright and Virshup, 1995). The B¢¢ family has two members, which have molecular masses of 72 and 130 kDa and are splice variants of the same gene (Hendrix et al., 1993). The different B subunits interact via the same or overlapping sites within the A subunit of the AC dimer so that the binding of different B subunits to AC is mutually exclusive (Ruediger et al., 1992). B subunits have important functions in regulating the substrate speci®city (Kamibayashi et al., 1994) and the subcellular localization of PP2A (McCright et al., 1996; Zhao et al., 1997). To gain insight into the mechanism of Vpr-mediated G2 cell cycle arrest, we examined whether HIV-1 Vpr interacts with PP2A. Here, we show that HIV-1 Vpr associates with PP2A through an interaction with the B55 regulatory subunit. This association enhances the nuclear import of B55-containing PP2A and positively modulates the enzyme activity. Importantly, Vpr association with PP2A increases the recruitment and dephosphorylation of the cdc25 substrate. Results Vpr associates with the PP2A holoenzyme complex through a speci®c interaction with the Ba subunit Ruediger et al. (1997) have recently reported that the balance between PP2A holoenzyme and core enzyme is important for HIV-1 gene expression and virus production. This study found that increasing the ratio of PP2A core enzyme to holoenzyme by overexpression of an N-terminal mutant of the A subunit (deletion of repeat 5), AD5, which binds the C but not the B subunit, inhibited transcription from the HIV-1 long terminal repeat (LTR) and decreased virus production. To investigate the role of PP2A in the pathway leading to Vpr-mediated G2 cell cycle arrest, we initially examined whether changes in the ratio of PP2A core enzyme and holoenzyme interfered with Vpr-mediated G2 arrest. The results of these experi- ments indicated that an increase in PP2A core enzyme levels in cells by overexpression of PP2A Aa subunit mutant AD5 alleviated the effect of Vpr on cell cycle progression and consequently provided ®rst evidence that PP2A holoenzyme was involved in the pathway targeted by Vpr to induce cell cycle arrest (data not shown). We next investigated whether Vpr interacted with the PP2A complex. To test this possibility, we initially developed a co-precipitation assay in 293T cells that overexpress tagged versions of Vpr (T7-Vpr) and PP2A B55a subunit (Flag-B55a) as well as native PP2A Aa or mutant AD5 subunits. The tagged version of B55a allowed us to distinguish the exogenous B55a protein from endogenous B subunits belonging to the three families. In addition, using a different set of monoclonal antibodies directed against the native A subunit or mutant AD5 subunit (Figure 1A), we were able to precipitate PP2A core enzyme or holoenzyme differentially as previously described (Kremmer et al., 1997). 293T cells were co-transfected with expression plasmids encoding T7-Vpr, Flag-B55a and Aa or AD5 as depicted in Figure 1A. At 48 h post-transfection, cells were counted and divided into three equal aliquots, lysed and incubated with antibodies directed against T7-Vpr (antiT7), Aa (6F9) or AD5 (5H4) subunits, or Flag-B55a (anti-Flag), respectively (Figure 1A). For aliquots that were intended to be immunoprecipitated with antibodies against Aa (6F9) or AD5 (5H4), cells were treated prior to lysis with the cell membrane-permeable cross-linking agent dimethyl 3,3¢-dithiobispropionimidate´2 HCl (DTBP) as indicated in Materials and methods. The resulting immunocomplexes were then analyzed further for the presence of the different components of the putative Vpr±PP2A complex, including Aa, B55a, C subunits and Vpr, by western blotting using the appropriate antibodies (Figure 1A). Antibodies directed against T7-Vpr, Aa or Flag-B55a all co-precipitated a complex comprising Vpr (14 kDa), exogenous Flag-B55a (55 kDa), Aa (68 kDa) and endogenous C subunit (36 kDa) (Figure 1B, lanes 2, 5 and 8). Precipitation of the Vpr±PP2A complex was observed in conditions where the DTBP cross-linker was present (Figure 1B, lanes 4±6) or absent (lanes 1±3 and 7± 9). Interestingly, although the 6F9 Aa monoclonal antibody precipitated the endogenous C subunit in mocktransfected 293T cells (Figure 1B, lane 4), the anti-FlagB55a antibody did not precipitate detectable amounts of endogenous A or C subunits in the presence of AD5 (lane 9). The inability of anti-Flag-B55a antibodies to precipitate endogenous core enzyme (A and C subunits) when AD5 is expressed probably results from the limiting amount of endogenous native core enzyme available for interaction with Flag-B55a. The expression of the AD5 mutant was previously shown to replace the wild-type A subunit in pre-existing core and holoenzyme and compete with newly synthesized wild-type A subunit for newly synthesized C subunit, thereby causing an increase in the level of AD5±C core enzyme and a parallel decrease in the level of holoenzyme (Ruediger et al., 1997). Indeed, as shown later in Figures 1D and 7A, anti-T7 or anti-Flag antibodies were able to precipitate endogenous PP2A Aa or C subunits in 293T cells expressing only T7-Vpr or Flag-B55a (lanes 2 and 3). 3957 M.Hrimech et al. Fig. 1. Vpr associates with the PP2A holoenzyme complex through a speci®c interaction with the B55a subunit. (A) Schematics of PP2A Aa, AD5, Flag-B55a and T7-Vpr expression vectors. The CMV promoter is represented by a dark circle. Antibodies used against each protein in immunoprecipitation (IP) and western blotting (WB) are indicated. (B) 293T cells (106) were mock transfected (lanes 1, 4 and 7) or co-transfected with different combinations of expression plasmids encoding T7-Vpr (2.5 mg), Flag-B55a (2.5 mg) and wild-type PP2A Aa (10 mg) or AD5 mutant (10 mg), as indicated at the bottom of each panel. At 48 h post-transfection, cells were treated (lanes 4±6) or not (lanes 1±3 and 7±9) with the membrane-permeable DTBP cross-linker, lysed and immunoprecipitated with anti-T7 (lanes 1±3), 6F9 or 5H4 (lanes 4±6) or anti-Flag (lanes 7±9) antibodies, as described in Materials and methods. Immunocomplexes were then separated by SDS±PAGE and analyzed further by immunoblotting using anti-T7, anti-Flag, 6G3 or anti-C subunit antibodies to detect T7-Vpr, FlagB55a, PP2A Aa and AD5, or PP2A C subunits, respectively, as indicated on the right side of each blot. (C) 293T (106) cells were co-transfected with pcDNA3-Flag-B55a (2.5 mg) and SVCMVT7-VPRmac239 (2.5 mg) or SVCMV-T7-VPXmac239 (2.5 mg), as indicated at the bottom of the ®gure. Cells were lysed, immunoprecipitated with anti-Flag (upper panel) or anti-T7 (lower panel) antibodies and the resulting immunocomplexes analyzed by western blotting using anti-T7 antibodies. (D) 293T cells (106) were mock transfected (lane 1) or transfected with 2.5 mg of SVCMV-T7-VPR. Cells were lysed, immunoprecipitated with anti-T7 antibodies and analyzed further by western blotting using anti-PP2A Aa (6G3) or anti-C subunit antibodies. The endogenous PP2A Aa and C subunits are indicated on the right side of the blots. The data of Figure 1B also indicate that the association of Vpr with PP2A occurs through an interaction with the B55a subunit. Immunoprecipitation of protein lysates from 293T cells overexpressing the mutant AD5, which binds C but not B subunits, with anti-T7-Vpr or anti-FlagB55a antibodies, respectively, clearly reveals the presence of Vpr±B55a complexes (Figure 1B, lanes 3 and 9). The A and C subunits of PP2A do not appear to interact with Vpr, since immunoprecipitation with the 5H4 monoclonal antibody, which recognizes both AD5 and endogenous 3958 native A subunit, precipitated the endogenous C subunit but did not co-precipitate Vpr (Figure 1B, lane 6). To verify the speci®city of the Vpr±PP2A interaction, we took advantage of the fact that the cell cycle arrest and PIC nuclear import functions of HIV-1 Vpr are segregated in two distinct viral proteins, Vpr and Vpx, respectively, in simian immunode®ciency virus isolated from macaques (SIVmac) (Fletcher et al., 1996; Planelles et al., 1996). Using the transient expression system described above, we tested whether SIVmac T7-Vpx or T7-Vpr was able to interact with PP2A B55a subunit. The results in Figure 1C clearly indicate that while SIVmac T7-Vpr and T7-Vpx were expressed at similar levels in co-transfected cells (lanes 2 and 3, lower panel), only SIVmac T7-Vpr coprecipitated with the PP2A B55a subunit (compare lanes 2 and 3, upper panel). All together, these results provide strong evidence that Vpr associates speci®cally with the PP2A holoenzyme through a direct or indirect interaction with the B55a subunit. The interaction of SIVmac Vpr but not Vpx with B55a strongly suggests that the formation of a Vpr±PP2A complex plays a critical role in Vpr's ability to mediate a G2 cell cycle arrest. To examine whether Vpr can interact with endogenous PP2A holoenzyme, 293T cells were transfected with SVCMV-T7-VPR only, and the presence of a Vpr±PP2A complex was examined by immunoprecipitation using anti-T7 antibodies and analysis by western blotting as described above. As shown in Figure 1D, both the structural/regulatory Aa (68 kDa) and the catalytic C (36 kDa) subunits were co-immunoprecipitated by the anti-T7-Vpr antibodies, thus demonstrating that Vpr forms a complex with endogenous PP2A (lane 2). Vpr interacts speci®cally with B subunits belonging to the B family To test whether the association of Vpr with PP2A occurs through a speci®c interaction with a particular family of B subunits, we evaluated the binding of Vpr to different subunits belonging to B (55a, 55b and 55g), B¢ (56e and 56g) and B¢¢ (PR72). Cells were co-transfected with plasmids expressing T7-Vpr and Flag-tagged B subunits belonging to the three gene families. At 48 h posttransfection, cell lysates were immunoprecipitated using anti-Flag antibodies and the resulting immunocomplexes were probed by immunoblotting for the presence of T7tagged Vpr. The results shown in Figure 2 reveal that Vpr interacts speci®cally with the PP2A B subunit belonging to the B family. Interestingly, the interaction of Vpr with B55a was found to be more ef®cient than the interaction with B55b or g (Figure 2, compare lane 1 with lanes 2 and 3) even though the three B subunits were expressed at similar levels (lanes 1±3). In contrast, no interaction was detected between Vpr and B subunits belonging to the B¢ (B56g and B56e) or B¢¢(PR72) families (Figure 2, lanes 4±6). Mutations in the C-terminal domain of Vpr abolish the interaction with PP2A B55a subunit Secondary structure predictions of Vpr as well as structural studies of Vpr polypeptides by NMR have indicated the presence of two amphipathic a-helical domains located between residues 17 and 46 and between residues 53 and 74 (Mahalingam et al., 1995; Yao et al., 1995; HIV-1 Vpr interacts with the B subunit of PP2A Fig. 2. Vpr associates speci®cally with B subunits belonging to the B family. 293T cells (106) were mock transfected (lane 7) or cotransfected with 2.5 mg of SVCMV-T7-VPR and 2.5 mg of pcDNA3Flag-B55a (lane 1), pcDNA3-Flag-B55b (lane 2), pcDNA3-Flag-B55g (lane 3), pcDNA3-Flag-B56e (lane 4), pcDNA3-Flag-B56g (lane 5) or pcDNA3-Flag-B¢¢ (lane 6). Cells were lysed, immunoprecipitated with anti-Flag (upper panel) or anti-T7 (lower panel) antibodies and analyzed by western blotting for the presence of T7-Vpr or Flag-B subunits, using anti-T7 or anti-Flag antibodies, respectively, as indicated. Subbramanian et al., 1998b; Schuler et al., 1999; Wecker and Roques, 1999). The protein also contains, between residues 60 and 81, a hydrophobic leucine/isoleucine-rich (LR) domain and a partially overlapping C-terminal arginine-rich region (Di Marzio et al., 1995; Wang et al., 1996) (Figure 3A). A consensus emerging from structure± activity studies indicates that three main activities of Vpr (i.e. virion incorporation, nuclear import and cell cycle arrest) are encoded by distinct domains of the protein (Di Marzio et al., 1995; Yao et al., 1995; Mahalingam et al., 1997; Subbramanian et al., 1998b). Indeed, several studies have shown that point mutations disrupting the N-terminal a-helical region preferentially reduced the effect of Vpr on cell cycle progression and nuclear localization, while mutations in the second amphipathic helix of Vpr speci®cally affected PIC nuclear import. Mutations in the C-terminal basic domain affected both nuclear localization and cell cycle functions, while sparing incorporation of mutant proteins into HIV-1 virions (Di Marzio et al., 1995; Mahalingam et al., 1997). To examine the functional relevance of the Vpr±PP2A interaction, we tested the ability of the B55a subunit to bind a panel of Vpr mutants, including mutants that are not able to arrest cells in G2 (Figure 3A). Each mutant Vpr expression vector was co-transfected in 293T cells with pcDNA3-Flag-B55a. Cell lysates were ®rst immunoprecipitated with anti-Flag antibodies and the presence of each Vpr mutant in immunocomplexes was analyzed by western blotting using anti-T7 antibodies. The results in Figure 3B (lower panel) reveal that each T7-Vpr mutant was expressed at comparable levels in co-transfected cells. Interestingly, two Vpr mutants, VprSR79/80 and VprR80A, which are defective for cell cycle G2 arrest (Di Marzio et al., 1995; Forget et al., 1998), were unable to interact with the B55a subunit (Figure 3B, lanes 7 and 8). In contrast, mutants such as VprR62P or VprQ65E, which induce G2 arrest as wild-type Vpr (Forget et al., 1998), interacted with B55a (Figure 3B, lanes 5 and 6). Surprisingly, VprE25K and VprA30F mutants that were previously shown to induce G2 cell cycle arrest, albeit with reduced ef®ciency (30% of wild-type Vpr; Figure 3A), interacted with the B55a subunit with ef®ciency comparable to wild-type Vpr (Figure 3B, compare lanes 3 and 4 with lane 2). The same analysis was also carried out by precipitating the lysates with anti-T7 antibodies and probing the immunocomplexes with anti-Flag or anti-Aa subunit (6G3). All mutants that interacted with B55a were shown to be part of the PP2A holoenzyme complex (data not shown). These results indicate that the Vpr C-terminal domain plays a critical role in the interaction of the protein with PP2A B55a subunit. Furthermore, the data indicate that the interaction of Vpr with PP2A B55a subunit is necessary but not suf®cient to induce G2 cell cycle arrest. Vpr targets PP2A B55a subunit to the nucleus Vpr has been localized primarily in the nuclear compartment using cellular fractionation approaches as well as conventional optical immuno¯uorescence and laser confocal microscopy (Subbramanian et al., 1998b; Vodicka et al., 1998). To assess whether the binding of Vpr to PP2A Ba subunits modi®es the cellular localization of PP2A, we co-expressed native Vpr with Flag-tagged B55a in Cos-7 cells and characterized the localization of wild-type Vpr and B55a by double staining immuno¯uorescence techniques and laser confocal microscopy. As shown in Figure 4, cells expressing native Vpr alone display a pronounced nuclear staining (Figure 4B). In contrast, cells expressing Flag-tagged B55a exhibit primarily a cytoplasmic staining with a clear exclusion from the nucleus (Figure 4C). When Vpr and B55a were co-expressed in Cos-7 cells, the B55a cellular localization shifted from the cytoplasm to the nucleus (Figure 4E) while Vpr localization remained nuclear (Figure 4D). Highly sensitive confocal laser sectioning and subsequent computer-assisted image merging clearly revealed that Vpr co-localized with the B55a protein (Figure 4F). Not surprisingly, when B55a and the Vpr R80A mutant that is defective for G2 arrest and PP2A binding were co-expressed in the same cell, they failed to co-localize to any appreciable level (Figure 4G± I). Together, these results strongly suggest that upon binding to Vpr, the B55a-containing PP2A holoenzyme complex is targeted from a cytoplasmic to a nuclear compartment. Vpr stimulates PP2A activity To determine whether the association of HIV-1 Vpr with PP2A in¯uenced the enzyme activity, lysates from 293T cells transiently expressing Vpr and/or Flag-B55a were immunoprecipitated with anti-Flag antibodies and the PP2A-containing immunocomplexes were assayed for PP2A activity in the presence of increasing concentrations of a PP2A-speci®c phosphopeptide substrate as described in Materials and methods. The results depicted in Figure 5A (left panel) clearly show that expression of Vpr increased the catalytic activity of PP2A by ~5-fold. To assess whether Vpr stimulation of PP2A activity was dependent on the interaction of the protein with PP2A, we performed similar experiments with Vpr mutant R80A, 3959 M.Hrimech et al. Fig. 3. The C-terminal domain of HIV-1 Vpr is critical for the interaction of the protein with PP2A B55a subunit. (A) The primary amino acid sequence of Vpr is shown. The substitution mutations introduced in each Vpr mutant are indicated below. In addition, the relative G2 arrest ability of each Vpr mutant is also indicated. Wild-type Vpr was set arbitrarily at 100%. (B) 293T cells (106) were mock transfected (lane 1) or co-transfected with 2.5 mg of Flag-B55a expression vector (pcDNA3-Flag-B55a) and 2.5 mg of plasmid encoding T7-tagged wild-type Vpr (lane 2) or different Vpr mutants including VprE25K (lane 3), VprA30F (lane 4), VprR62P (lane 5), VprQ65E (lane 6), VprSR79/80ID (lane 7) or VprR80A (lane 8), as indicated. Cells were lysed, immunoprecipitated with anti-Flag antibodies and analyzed by western blotting with anti-T7 antibodies to reveal co-precipitated T7-Vpr (upper panel). To verify the expression of each Vpr mutant in transfected cells, each cell lysate was subsequently immunoprecipitated with anti-T7 antibodies and analyzed by western blotting using anti-T7 antibody (lower panel). which fails to interact with PP2A and is defective for G2 cell cycle arrest. As shown in Figure 5A, measurement of PP2A activity in lysates from cells expressing B55a and R80A reveals that the enzyme catalytic activity remained unchanged as compared with the control (cells expressing B55a and Vpr± negative control). In contrast, Vpr mutant A30F, which retains the ability to bind PP2A B55a but displays a reduced capacity to mediate G2 arrest, stimulates PP2A activity by ~2-fold (Figure 5A). As shown in Figure 5B, comparable amounts of B55a, Vpr or Vpr mutant proteins were detected in each immunocomplex used to assay phosphatase activity. To investigate whether recombinant Vpr could increase PP2A activity in vitro, immunoprecipitated Flag-B55a± PP2A complexes were incubated with a highly puri®ed preparation of recombinant Vpr produced from baculovirus-infected insect cells and PP2A activity was determined as described above. The results in Figure 5A (right panel) reveal that recombinant Vpr is also able to enhance PP2A catalytic activity towards a synthetic phosphopeptide substrate. This stimulatory effect of Vpr on PP2A did 3960 not result from a contaminating phosphatase activity carried over by Vpr since the recombinant Vpr preparation did not display any phosphatase activity. Moreover, when Vpr was depleted using rabbit polyclonal anti-Vpr antibodies prior to its addition to immunoprecipitated PP2A, no increase in phosphatase activity was detected (data not shown). Overall, these results strongly suggest that the interaction between Vpr and PP2A B55a stimulates the catalytic activity of the PP2A holoenzyme towards its substrate. cdc25c is present in the PP2A±Vpr complex We next tested whether cdc25, a PP2A cellular substrate whose phosphorylation pro®le is affected by Vpr (Re et al., 1995), was part of the Vpr±PP2A complex. 293T cells were transfected with SVCMV-T7-VPR or pcDNA3-FlagB55a or co-transfected with Vpr and B55a expression plasmids. At 48 h post-transfection, cells were incubated with the cross-linking reagent DTBP as described in Materials and methods. Treated cells were lysed and immunoprecipitated with anti-cdc25c, anti-T7 or anti-Flag HIV-1 Vpr interacts with the B subunit of PP2A Fig. 4. Vpr targets B55a subunit-containing PP2A complex to the nucleus. Cos-7 cells were mock transfected (A) or transfected with 5 mg of SVCMV-VPR (B) or B55a expressor, pcDNA3-Flag-B55a (C), or co-transfected with 5 mg of Vpr and B55a expressor plasmids (D±F) or Vpr mutant (R80A) and B55a expressor plasmids (G±I). Following ®xation, cells were incubated ®rst with rabbit anti-Vpr antibodies or goat anti-Flag antibodies, as indicated, and then labeled with lisamine/rhodamine-conjugated goat anti-rabbit (for Vpr) (B, D and G) or FITC-conjugated anti-goat/ sheep (for the B55a subunit) (C, E and H) antibodies. Labeled cells were then analyzed by confocal laser microscopy. A laser section through a representative cell is shown for each transfection. The images on the far right column labeled as F and I depict superimposed images of the Vpr-speci®c (red) and Flag-B55a (green) signals in the same cells. antibodies, respectively, and the resulting immunocomplexes probed for the presence of cdc25c, PP2A Aa and C subunits, and Vpr by immunoblotting. The results in Figure 6A reveal that endogenous cdc25c can be found associated with the PP2A holoenzyme complex (lane 3). Indeed, when B55a is expressed alone, the anti-Flag antibodies precipitate a fraction of the total endogenous cdc25c (Figure 6A, compare lanes 3 and 1). Surprisingly, the level of cdc25c associated with the PP2A holoenzyme increased by ~6-fold when Vpr was co-expressed with B55a (Figure 6A, compare lanes 4 and 3). The presence of Vpr changed only the level of cdc25c recruited to the PP2A holoenzyme complex. The levels of endogenous Aa or C subunits in the complex did not vary to any signi®cant extent in the presence or absence of Vpr (Figure 6A, compare lanes 3 and 4). Furthermore, when endogenous PP2A was precipitated by anti-T7-Vpr antibodies from T7-Vpr-expressing cells, a large proportion of endogenous cdc25c was found complexed with PP2A (Figure 6A, compare lanes 2 and 1). In contrast, when similar experiments were performed with the Vpr mutant R80A, no cdc25c was detected in the complex precipitated by the anti-T7 antibodies (Figure 6B, lane 4). However, when the same cells were reacted with anti-Flag B55a, the resulting PP2A immunocomplex was shown to contain cdc25c, but at levels that were comparable to those found in cells only expressing Flag-B55a (Figure 6B, compare lanes 6 and 2). We next determined the phosphorylation status of the cdc25c found associated with the Vpr±PP2A complex. The results in Figure 6C show that in the absence of Vpr, both hyperphosphorylated and hypophosphorylated forms 3961 M.Hrimech et al. Fig. 5. Vpr increases the catalytic activity as well as the af®nity of PP2A holoenzyme towards a speci®c phosphopeptide substrate. (A) Left panel: 293T cells (106) were co-transfected with 5 mg of pcDNA3-Flag-B55a and SVCMV-T7-VPR, T7-VPR±, T7-VPRA30F or T7-VPRR80A, as indicated. Cells were lysed and immunoprecipitated with anti-Flag antibodies. An increasing concentration of synthetic phosphopeptide substrate was added to equivalent amounts of each Flag-B55a-containing PP2A immunocomplex and incubated at 30°C for 30 min to assay PP2A activity. Right panel: 293T cells (106) were transfected with 5 mg of pcDNA3-Flag-B55a. Cells were lysed and immunoprecipitated as described above. Immunocomplexes were incubated with recombinant Vpr for 2 h at 4°C and phosphatase activity was monitored. (B) Expression of Flag-B55a and Vpr. Each transfected cell sample was divided into two aliquots. One was used to monitor phosphatase activity and the second was treated with DTBP and immunoprecipitated with 6F9 antibodies. After protein separation by SDS±PAGE and western blotting, the presence of Flag-B-55a and Vpr was detected using anti-Flag (upper panel) and anti-T7 (lower panel) antibodies, respectively. of cdc25c can be detected in cells (lane 1). Only hypophosphorylated forms of cdc25c were found complexed to PP2A in B55a-expressing cells (Figure 6C, lane 2). Interestingly, the pool of hypophosphorylated cdc25 found associated with PP2A increased substantially in the presence of Vpr (Figure 6C, lane 3). In addition, a small fraction of hyperphosphorylated cdc25c, which probably represents newly recruited cdc25c, was detected in the Vpr±PP2A complex (Figure 6C, lane 3). As expected, treatment of cells with aphidicolin, an agent that causes a block in G1/S phase, led to an inactivation of cdc25c, which accumulated in a hypophosphorylated form (Figure 6C, lane 4). 3962 Fig. 6. Association of cdc25c with the Vpr±PP2A complex. (A) 293T cells (106) were mock transfected (lane 1), transfected with 2.5 mg of SVCMV-T7-VPR (lane 2) or pcDNA3-Flag-B55a (lane 3), or cotransfected with 2.5 mg of T7-Vpr and Flag-B55a expression plasmids (lane 4). Cells were treated with the plasma membrane-permeable DTBP cross-linker, lysed and immunoprecipitated with anti-cdc25c, anti-T7 or anti-Flag antibodies, respectively, and analyzed by SDS± PAGE and western blotting using the appropriate antibodies to detect PP2A Aa subunit (6G3), endogenous cdc25c (anti-cdc25c), PP2A C subunit (anti-C subunit) and T7-Vpr (anti-T7), as indicated on the right side of the blots. (B) 293T cells (106) were mock transfected (lane1), transfected with 2 mg of pcDNA3-Flag-B55a (lane 2), or co-transfected with 2.5 mg of pcDNA3-Flag-B55a and 2.5 mg of SVCMV-T7-Vpr (lane 3) or SVCMV-T7-VprR80A (lane 4). Cells were treated with DTBP, lysed and immunoprecipitated with anti-cdc25c, anti-Flag or anti-T7 antibodies as indicated. Resulting immunoprecipitates were then separated by SDS±PAGE and analyzed by western blotting using anti-cdc25c. Cell lysates similar to those from lanes 3 and 4 were immunoprecipitated with anti-Flag antibodies and the resulting immunocomplexes were analyzed for the presence of cdc25c by immunoblotting using anti-cdc25c antibodies (lanes 5 and 6). (C) 293T cells (106) were mock transfected (lane 1), transfected with 2.5 mg of pcDNA3-Flag-B55a (lane 2) or co-transfected with 2.5 mg of pcDNA3Flag-B55a and 2.5 mg of SVCMV-T7-Vpr (lane 3) or treated with aphidicolin (lane 4). Cells were treated with DTBP, lysed and immunoprecipitated with anti-cdc25c or anti-Flag antibodies as indicated. Resulting immunoprecipitates were then separated by 8% SDS±PAGE and analyzed by western blotting using anti-cdc25c. Overall, these results indicate that a fraction of cdc25c present within cells can be found associated with the PP2A holoenzyme. Moreover, association of Vpr with PP2A through its interaction with the B55a subunit increases the recruitment and dephosphorylation of the cdc25c substrate. Discussion Our analyses of physical interactions and cell cycle modulation lead to the conclusion that PP2A plays a central role in the G2 cell cycle arrest mediated by the Vpr HIV-1 Vpr interacts with the B subunit of PP2A Fig. 7. Model for Vpr-mediated G2 cell cycle arrest. (A) Normally, the active form of cdc25 accumulates in the nucleus and activates cdc2±cyclin B to trigger mitosis. PP2A holoenzyme containing B55 subunits interacts with cdc25 and regulates its activity. (B) In HIV-1-infected cells, Vpr associates with PP2A via an interaction with B regulatory subunits. The Vpr±PP2A interaction enhances PP2A catalytic activity and targets the hyperactive holoenzyme to the nucleus where active cdc25 substrate is located. Inactivation of cdc25, by ef®cient dephosphorylation, keeps cdc2±cyclin B in its hyperphosphorylated form, thereby stopping cells from entering mitosis. protein encoded by primate lentiviruses. Speci®cally, our ®ndings provide evidence that HIV-1 Vpr and PP2A interact in cells and that this interaction has biological consequences with regard to Vpr-mediated G2 cell cycle arrest. First, immunoprecipitation experiments with antibodies directed to either T7-Vpr, PP2A Aa or epitopetagged B55a subunits reveal that Vpr exists in a complex that includes all three subunits of the phosphatase within Vpr-expressing cells (Figure 1B±D), and that this complex contains phosphatase activity that is speci®c towards a PP2A-speci®c phosphopeptide substrate (Figure 5) and is sensitive to low levels of okadaic acid, a characteristic of PP2A (data not shown). Furthermore, similar co-precipitation experiments in cells overexpressing a deletion mutant of the Aa subunit, AD5, which binds C but not B subunits, reveal that the association of Vpr with PP2A occurs through a speci®c interaction with the B regulatory subunit (Figure 1B). Only B subunits belonging to the B family were found to interact with Vpr (Figure 2). Interestingly, although the precise roles of regulatory B subunits are still not clearly understood, a functional role has been assigned to the B55a subunit in the regulation of mitosis, as suggested by the ®nding that deletion of this gene is associated with cytokinesis defects in both yeast and Drosophila (Healy et al., 1991; Mayer-Jaekel et al., 1993). It therefore appears that Vpr, via its ability to bind B55 subunits, forms a complex with a subspecies of PP2A holoenzyme involved in the regulation of mitosis. Secondly, considerable functional evidence correlating this interaction with Vpr-mediated G2 arrest was obtained. (i) Overexpression of the PP2A Aa subunit mutant, AD5, in Vpr-expressing cells, reduces the levels of PP2A complexed with Vpr and signi®cantly diminishes Vprmediated cell cycle arrest (Figure 1 and data not shown). Since overexpression of this mutant was shown to reduce the levels of holoenzyme relative to core enzyme within transfected cells by at least 2-fold, we believe that this mutant alleviates Vpr-mediated G2 cell cycle arrest by reducing the levels of B55 subunit-containing PP2A holoenzyme available for interaction with Vpr. (ii) Our data demonstrate that PP2A holoenzyme containing the B55a subunit interacts with HIV-1 and SIVmac Vpr, which are both capable of inducing a G2 cell cycle arrest. In contrast, the holoenzyme was unable to form a complex with SIVmac Vpx, which mediates the other Vpr function, i.e. nuclear import of the PIC (Fletcher et al., 1996) (Figure 1C). (iii) Mutation analysis of Vpr indicates that a correlation exists between Vpr's ability to bind PP2A and its capacity to mediate G2 cell cycle arrest. Vpr mutants containing substitution mutations at amino acid R80 or S79 and R80 at the C-terminus of the protein, and that are known to be defective for G2 arrest (Di Marzio et al., 1995; Mahalingam et al., 1997; Yao et al., 1998), lost their 3963 M.Hrimech et al. ability to interact with PP2A B55a subunit (Figure 3). In addition, mutations in the N-terminal ®rst a-helix (E25K and A30F) affected cell cycle arrest (30% of wild-type levels) more than they in¯uenced the physical interaction, thus indicating that the interaction of Vpr with PP2A, although necessary, was not suf®cient to mediate G2 cell cycle arrest. These results further suggest the possibility that other functional domains of Vpr or additional Vprassociated factors may cooperate in facilitating Vprmediated G2 cell cycle arrest. Interestingly, using indirect immuno¯uorescence and confocal microscopy, we demonstrate that upon Vpr co-expression, B55a subunits that have primarily a cytoplasmic localization are targeted to the nucleus where they co-localize with Vpr (Figure 4). As expected, co-expression of PP2A B55a subunit with Vpr mutant R80A, which is unable to form a complex with PP2A, did not change the cytoplasmic localization of the B55a subunit (Figure 4). These observations indicate that Vpr, by binding to the B55a subunit, provides PP2A with a nuclear targeting component and strongly suggest that Vpr nuclear localization function is required for G2 cell cycle arrest. The third signi®cant ®nding described in this report is that Vpr stimulates PP2A catalytic activity towards a PP2A-speci®c phosphopeptide substrate (Figure 5). Measurement of phosphatase activity in epitope-taggedB55a-containing immunocomplexes isolated from epitope-tagged-B55a- or Vpr/epitope-tagged-B55a-expressing cells reveals that the presence of Vpr stimulates phosphatase activity by ~5-fold. Furthermore, similar effects on phosphatase catalytic activity were obtained when recombinant Vpr was added directly to epitopetagged-B55a immunocomplexes, thus demonstrating that Vpr can positively modulate PP2A in vitro. Stimulation of phosphatase activity by Vpr correlated with the protein's ability to mediate a cell cycle arrest since G2 arrestdefective mutant VprR80A was unable to stimulate phosphatase activity in this assay. Furthermore, the A30F mutant, which displayed an impaired G2 cell cycle arrest (30% of wild type) and a reduced nuclear localization (Yao et al., 1995) but interacted with PP2A as well as wild-type Vpr, slightly stimulated phosphatase activity. Thus, these results suggest that conformational changes in this region (N-terminal a-helix) of the protein may affect phosphatase activity once PP2A is bound to Vpr. The availability of an in vitro assay system should now permit the isolation of Vpr mutants that bind and stimulate PP2A in vitro but do not mediate G2 cell cycle arrest in transfected cells. Such Vpr mutants may correspond to proteins that lost the ability to target B55±PP2A complex to the nucleus and consequently may help identify nuclear localization domain(s) relevant to Vpr-mediated G2 cell cycle arrest. The fourth major ®nding of this study is that the presence of Vpr in the PP2A complex increases the recruitment and dephosphorylation of the cdc25c phosphatase substrate in transfected cells (Figure 6). Enhanced recruitment of cdc25c to the Vpr±PP2A complex is speci®c since it is not observed with another known substrate of PP2A, the MAP kinase Raf1, which is known to interact with PP2A (Dent et al., 1995) (data not shown). Moreover, it requires the association of Vpr with the PP2A complex since we did not detect such enhancement of cdc25c recruitment in the presence of the VprR80A 3964 mutant. These results provide the ®rst evidence that cdc25c phosphatase is a substrate of PP2A holoenzyme containing the B55a subunit. Moreover, these ®ndings suggest that Vpr, by binding to B55a, increases the speci®city and activity of PP2A towards the cdc25 substrate. Virus and PP2A Viruses have evolved a number of mechanisms that allow them to interact with the normal host cell cycle. Hence, the ability to negatively modulate the activity of PP2A via the association of viral protein subunits with the AC core heterodimer is exploited by the DNA tumor viruses SV40 and polyoma virus, as a mechanism to interfere with intracellular signaling cascades and promote cell proliferation (Mumby, 1995). In contrast, the Vpr protein encoded by primate lentiviruses negatively modulates cell growth, interacts with a complex that includes all of the PP2A subunits and interferes with signal transduction cascades regulating G2/M cell cycle progression by conferring the PP2A enzymatic complex speci®city for the cdc25 regulator. On the basis of its interaction with PP2A, Vpr resembles a viral protein encoded by the open reading frame 4 of the adenovirus E4 gene (E4orf4). Like Vpr, the adenovirus E4orf4 protein forms a complex with the heterotrimeric form of PP2A in adenovirus-infected cells (Kleinberger and Shenk, 1993). The interaction between E4orf4 and PP2A has recently been shown to be important for the induction of apoptosis by E4orf4 and for downregulation of virally induced signal transduction (Kleinberger and Shenk, 1993; Shtrichman et al., 1999). In contrast to SV40 and polyoma small and middle T antigens, which inhibit PP2A activity, both Vpr and E4orf4 act as positive regulators of the enzyme activity. Model of HIV-1 Vpr-mediated G2 arrest There is increasing evidence that the cell cycle is controlled in part by localizing speci®c regulators to the right place at the right time. For instance, Lopez-Girona et al. (1999) have shown that, in the ®ssion yeast S.pombe, the cdc25 phosphatase is exported from the nucleus in response to DNA damage. Indeed, activation of the DNA damage checkpoint causes the net nuclear export of cdc25 by a process that requires phosphorylation by chk1 kinase and association with Rad24, a protein that belongs to the 14-3-3 protein family, which acts as an attachable nuclear export sequence. Export separates cdc25 from its substrate, the cyclin B±cdc2 kinase, thereby stopping cells from entering mitosis. By analogy, Vpr may mediate a G2 cell cycle arrest by acting at least in part as a PP2A/B55attachable nuclear import signal. Normally, cdc25 continually shuttles between the cytoplasm and the nucleus where its active phosphorylated form activates cdc2± cyclin B1 complexes by dephosphorylating cdc2 (Figure 7A). Recent evidence indeed suggests that cdc25 accumulates in the cytoplasm during interphase and progressively localizes in the nucleus as cells move towards the G2/M phase of the cell cycle (Dalal et al., 1999) (data not shown). The cellular location where PP2A interacts with cdc25 to regulate the levels of active phosphorylated cdc25 is still not de®ned precisely. However, preliminary evidence from our laboratory indicates that in Vpr G2-arrested cells, cdc25 accumulates in the nucleus and HIV-1 Vpr interacts with the B subunit of PP2A totally co-localizes with Vpr (data not shown), thus suggesting that the Vpr±PP2A complex interacts with cdc25 in the nucleus. Detailed subcellular localization studies are currently under way to address this important question. Overall, our data support a model in which Vpr mediates G2 cell cycle arrest by ef®ciently targeting PP2A/B55 to the nucleus and positively modulating the holoenzyme catalytic activity towards the active phosphorylated nuclear cdc25 substrate (Figure 7B). Ef®cient recruitment and dephosphorylation of active nuclear cdc25 would in turn lead to a situation where Wee1 and Myt1 kinase activities are greater than cdc25 phosphatase activity, thus resulting in cdc2±cyclin B inactivation and G2 cell cycle arrest. The identi®cation of PP2A as the cellular protein complex targeted by Vpr to mediate G2 cell cycle arrest should provide important information on how these proteins may regulate and perturb cell growth, and should present opportunities for the development of both HIV therapeutics and anticancer strategies. Materials and methods Antibodies, recombinant proteins and chemicals Mouse monoclonal anti-T7-Tag antibodies were obtained from Novagen. The rabbit anti-Vpr serum was raised against bacterially expressed recombinant Vpr as described previously (Subbramanian et al., 1998b). Goat anti-Flag antibodies, rabbit anti-cdc25c or anti-Raf1 sera and goat anti-human PP2A C subunit were obtained from Santa Cruz. Rat monoclonal antibodies 6F9, 5H4 and 6G3 were raised against puri®ed native PP2A Aa subunit (6F9, 5H4) or an 11 amino acid peptide (6G3) corresponding to the N-terminus of the Aa subunit as described previously (Kremmer et al., 1997). His-tagged Vpr was expressed in Sf9 insect cells following infection with a recombinant baculovirus and puri®cation on an NTA matrix as described previously (Popov et al., 1998). The cross-linking agent DTBP was from Pierce, the protease inhibitor cocktail from Boehringer Mannheim, and the propidium iodide and aphidicolin from Sigma. Plasmids and cloning strategies The PP2A Aa subunit expression plasmids pcDNA3-PP2A Aa and pcDNA3-PP2A AD5 used in this study were described previously (Ruediger et al., 1997). Plasmid expressor encoding Flag-tagged PP2A human B (B55a, B55b, B55g) (Mayer et al., 1991), B¢ (B56e, B56g) (McCright and Virshup, 1995) or B¢¢ (PR72) (Hendrix et al., 1993) subunits were constructed by fusing a Flag sequence at the 5¢ end of KpnI±EcoRI-digested cDNA fragments encompassing each of the B subunit genes. The resulting DNA fragments encoding Flag-tagged B subunits were inserted in pcDNA-3 to generate pcDNA3-FlagB vectors. The HIV-1 Vpr expressor plasmid, SVCMV-VPR, and the negative control plasmid, SVCMV-VPR±, encoding a Vpr gene harboring a point mutation in the Vpr translation initiation codon, were described previously (Yao et al., 1995). Plasmids encoding mutant Vpr (SVCMV-VPRE25K, SVCMV-VPRA30F, VPRR62P, VPRQ65E, VPRSR79/80ID and VPRR80A) were described elsewhere (Yao et al., 1995; Subbramanian et al., 1998b). To construct SVCMV-T7-VPR, a PCR-generated Vpr cDNA derived from the proviral plasmid HxBRU (Yao et al., 1995) was fused to the C-terminus of the T7-Tag sequence and inserted into SVCMV-Vpr to replace Vpr. SVCMV-GFP-VPR or SVCMV-GFP-VPR± were constructed by inserting a cDNA fragment containing the cytomegalovirus (CMV) immediate early promoter, the green ¯uorescent protein (GFP) gene and the glutathione terminator sequence (derived from the pQBI 25 plasmid; Quantum Biotechnologies Inc.) into the unique BamHI site of SVCMV-VPR or SVCMV-VPR±, respectively. SVCMV-T7-VPXmac239 and SVCMV-T7-VPRmac239 encoding the Vpx and the Vpr genes, respectively, from SIVmac239 (p239SpSp5¢) (Kestler et al., 1990) were constructed using similar strategies to those described for the construction of SVCMV-T7-VPR. Cell lines and transfections Cos-7 African green monkey kidney cells and human embryonic kidney 293T cells were maintained in Dulbecco's modi®ed Eagle's medium (DMEM) supplemented with 10% fetal calf serum and 1% penicillin and streptomycin. All cells were maintained at 37°C and 5% CO2. For all experiments, we used the standard calcium phosphate co-precipitation technique to transfect cells, except where indicated. Cell cycle analysis Vpr-mediated cell cycle arrest was evaluated by propidium iodide staining and ¯ow cytometry analysis as described previously (Yao et al., 1998). Immunoprecipitations and immunoblotting analyses 293T cells (106) were ®rst transfected or co-transfected with different plasmids, as indicated in each experiment. At 48 h post-transfection, cells were washed twice with cold phosphate-buffered saline (PBS) and subsequently lysed at 4°C for 1 h with NP-40 lysis buffer (50 mM Tris± HCl pH 7.4, 400 mM NaCl, 0.2% NP-40 and a protease inhibitor cocktail). Cell lysates were then microcentrifuged at 14 000 r.p.m. for 30 min to remove cell debris. Supernatants were immunoprecipitated with either mouse anti-T7, goat anti-Flag, rat anti-PP2A Aa (6F9), rat antiPP2A AD5 (5H4) or rabbit anti-cdc25c antibodies as described previously (Yao et al., 1998). When 6F9 or 5H4 antibodies were used for immunoprecipitation, cells were treated with the cell membranepermeable cross-linking reagent DTBP prior to lysis. DTBP was resuspended in PBS buffer pH 7.5 and added to each sample to a ®nal concentration of 0.25 mM in a 5 ml volume. After a DTBP treatment of 1 h at 4°C, cells were treated with 1 M Tris buffer pH 7.5 to stop the reaction, washed three times with PBS to remove excess DTBP, lysed with NP-40 lysis buffer and incubated with the appropriate antibodies overnight at 4°C. Immunocomplexes were recovered by addition of protein A± Sepharose beads (Pharmacia) for 2 h at 4°C and microcentrifugation. Beads were washed extensively using lysis buffer and boiled in Laemmli buffer. After protein separation on SDS±polyacrylamide gels, proteins were transferred to a nitrocellulose membrane (0.45 mM pore size; BioRad) by electroblotting for 3 h at 30 V in a Bio-Rad Trans Blot cell. The membrane was then incubated for 1 h in blocking Tris-buffered saline (TBS) solution containing 1% Tween-20 and 3% non-fat dry milk (Carnation; NestleÂ) and incubated for an additional 3 h at room temperature with the appropriate antibody diluted in TBS as follows: mouse anti-T7-tagged Vpr (1:5000), rat anti-PP2A Aa wild type and AD5 subunit (6G3) (1:10 000), goat anti-PP2A C subunit (1:1000), goat antiFlag (1:1000), anti-cdc25c (1:1000) or anti-Raf1 (1:1000). Bound antibodies were then probed with horseradish peroxidase-conjugated anti-mouse (1:7500), anti-rat (1:5000), anti-rabbit (1:7500) or anti-goat (1:8000) antibodies, respectively, washed extensively and revealed using a sensitive enhanced chemiluminescence detection system (ECL detection kit; Amersham). Phosphatase activity assay 293T cells (106) were transfected with pcDNA3-Flag-B55a (5 mg) or cotransfected with pcDNA3-Flag-B55a (5 mg) and SVCMV-VPR or Vpr mutant expressors (5 mg) using Lipofectamine (Life Technologies) according to the manufacturer's instructions. A total of 2 3 106 cells were collected 48 h post-transfection and lysed with NP-40 buffer. Lysates were microcentrifuged at 14 000 r.p.m. for 30 min and immediately used for PP2A holoenzyme puri®cation by immunoprecipitation using goat anti-Flag covalently coupled to protein G±Sepharose beads (Pharmacia). Similar amounts of puri®ed PP2A immunocomplex, as adjusted by measurement of total protein concentration using the BioRad Protein assay (Bio-Rad), were used for in vitro phosphatase activity assay. The catalytic activity of PP2A holoenzyme was measured using the UBI phosphatase assay kit according to the manufacturer's instructions. This assay used a phosphopeptide (KRpTIRR) as substrate for PP2A enzyme (Harder et al., 1994). Free phosphate generated by PP2A catalytic activity was revealed by addition of Malachite green solution, which turns green in the presence of free phosphate, and quanti®ed using a spectrophotometer at a wavelength of 650 nm. Immuno¯uorescence and imaging of cells Cos-7 cells were co-transfected with 5 mg of SVCMV-VPR or SVCMVVPRR80A and pcDNA3-Flag-B55a (5 mg). Cells were washed with cold PBS, trypsinized and replated onto glass coverslips 24 h post-transfection. After a 24 h incubation at 37°C, cells were washed twice with PBS, ®xed in PBS±4% paraformaldehyde for 5 min, permeabilized in PBS±0.2% Triton X-100 for 5 min and processed for immunolabeling. For single or 3965 M.Hrimech et al. double labeling protocols, cells were incubated with the ®rst corresponding antibody diluted in PBS containing 0.03% sodium azide and 2% nonfat dry milk (Carnation; NestleÂ) for 12 h. First antibodies were used at the following dilutions: rabbit anti-Vpr (1:500) and goat anti-Flag (1:200). Following several washes, cells were incubated for 3 h with lisamine/ rhodamine-labeled goat anti-rabbit antibodies (1:100) for Vpr detection and ¯uorescein isothiocyanate (FITC)-labeled anti-goat/sheep antibodies (1:100) for B55a subunit detection. Confocal laser microscopy was performed on a Zeiss LSM 410 (Carl Zeiss, Germany) equipped with a Plan-Apochromat 633 oil immersion objective and an Ar/Kr laser. The FITC images were obtained by scanning the cells with the 488 nm laser and ®ltering the emission with a 515±540 nm band-pass. For the lisamine/ rhodamine images, the 568 nm laser was used in combination with a 575± 640 nm band-pass ®lter. For each cell studied and each image, the additive signal through the whole cell thickness was ®rst digitized. Then the confocal serial sections were scanned. Acknowledgements We thank N.Rougeau for excellent technical assistance. We also thank G.Walter and R.Rudieger for generously supplying the 6F9, 5H4 and 6G3 monoclonal antibodies as well as the PP2A Aa and mutant AD5 expression vectors, D.M.Virshup and B.A.Hemmings for the B subunit expression vectors, M.Bukrinsky for the puri®ed baculovirus preparation of recombinant Vpr, and R.Subbramanian for Vpr mutants. We also thank R.C.Desrosiers for providing SIVmac proviral clone p293SpS5¢ that was obtained through the AIDS Research and Reference Reagent Program, Division of AIDS, NIAID, NIH. We thank G.Lemay and R.Sekaly for helpful discussion and comments on the manuscript, D.Boivin and R.Marcellus for advice and important reagents, S.Senechal for FACS analysis, and H.Dilhuydy for laser confocal microscopy. M.H. is the recipient of a studentship from the Fonds pour la Formation de Chercheurs et l'Aide aÁ la Recherche (FCAR). EÂ.A.C. is a Medical Research Council (MRC) of Canada Scientist. This work was supported by grants from the MRC and FCAR to EÂ.A.C. References Clarke,P.R., Hoffmann,I., Draetta,G. and Karsenti,E. (1993) Dephosphorylation of cdc25-c by a type-2A protein phosphatase: speci®c regulation during the cell cycle in Xenopus egg extracts. Mol. Cell. Biol., 4, 397±411. Cohen,EÂ.A., Terwilliger,E.F., Jalinoos,Y., Proulx,J., Sodroski,J.G. and Haseltine,W.A. (1990) Identi®cation of HIV-1 vpr product and function. J. Acquir. Immune De®c. Syndr., 3, 11±18. Dalal,S.N., Schweitzer,C.M., Gan,J. and DeCaprio,J.A. (1999) Cytoplasmic localization of human cdc25C during interphase requires an intact 14-3-3 binding site. Mol. Cell. Biol., 19, 4465±4479. Dent,P., Jelinek,T., Morrisson,D.K., Weber,M.J. and Sturgill,T.W. (1995) Reversal of Raf-1 activation by puri®ed and membraneassociated protein phosphatases. Science, 268, 1902±1906. Di Marzio,P., Choe,S., Ebright,M., Knoblauch,R. and Landau,N.R. (1995) Mutational analysis of cell cycle arrest, nuclear localization and virion packaging of human immunode®ciency virus type 1 Vpr. J. Virol., 69, 7909±7916. Fletcher,T.M., Brichacek,B.I., Sharova,N., Newman,M.A., Stivahtis,G., Sharp,P.M., Emerman,M., Hahn,B. and Stevenson,M. (1996) Nuclear import and cell cycle arrest functions of the HIV-1 Vpr are encoded by two separate genes in HIV-2/SIV-SM. EMBO J., 15, 6155±6165. Forget,J., Yao,X.J., Mercier,J. and Cohen,EÂ.A. (1998) Human immunode®ciency virus type 1 vpr protein transactivation function: mechanism and identi®cation of domains involved. J. Mol. Biol., 284, 915±923. Goh,W.C., Rogel,M.E., Kinsey,C.M., Michael,S.F., Fultz,P.N., Nowak, M.A., Hahn,B.H. and Emerman,M. (1998) HIV-1 Vpr increases viral expression by manipulation of the cell cycle: a mechanism for selection of Vpr in vivo. Nature Med., 4, 65±71. Harder,K.W., Owen,P., Wong,L.K., Aebersold,R., Clark-Lewis,I. and Jirik,F.R. (1994) Characterization and kinetic analysis of the intracellular domain of human protein tyrosine phosphatase b (HPTP b) using synthetic phosphopeptides. Biochem. J., 298, 395± 401. He,J., Choe,S., Walker,R., Di Marzio,P., Morgan,D.O. and Landau,N.R. (1995) Human immunode®ciency virus type 1 viral protein R (Vpr) 3966 arrests cells in the G2 phase of the cell cycle by inhibiting p34cdc2 activity. J. Virol., 69, 6705±6711. Healy,A.M., Zolnierowicz,S., Stapleton,A.E., Goebl,M., DePaoliRoach,A.A. and Pringle,J.R. (1991) CDC55, a Saccharomyces cerevisiae gene involved in cellular morphogenesis: identi®cation, characterization and homology to the B subunit of mammalian type 2A protein phosphatase. Mol. Cell. Biol., 11, 5767±5780. Heinzinger,N.K. et al. (1994) The Vpr protein of human immunode®ciency virus type 1 in¯uences nuclear localization of viral nucleic acids in nondividing host cells. Proc. Natl Acad. Sci. USA, 91, 7311± 7315. Hendrix,P., Mayer-Jackel,R.E., Cron,P., Goris,J., Hofsteenge,J., Merlevede,W. and Hemmings,B.A. (1993) Structure and expression of a 72-kDa regulatory subunit of protein phosphatase 2A. Evidence for different size forms produced by alternative splicing. J. Biol. Chem., 268, 15267±15276. Hunter,T. (1995) Protein kinases and phosphatases: the yin and yang of protein phosphorylation and signaling. Cell, 80, 225±236. Jackman,M.R. and Pines,J.N. (1997) Cyclins and the G2/M transition. In Kastan,M.B. (ed.), Checkpoint Controls and Cancer. Vol. 29. Cold Spring Harbor Laboratory Press, Cold Spring Harbor, NY, pp. 47±73. Jowett,J.B., Planelles,V., Poon,B., Shah,N.P., Chen,M.L. and Chen,I.S. (1995) The human immunode®ciency virus type 1 vpr gene arrests infected T cells in the G2 + M phase of the cell cycle. J. Virol., 69, 6304±6313. Kamibayashi,C., Estes,R., Lickteig,R.L., Yang,S.I., Craft,C. and Mumby,M.C. (1994) Comparison of heterotrimeric protein phosphatase 2A containing different B subunits. J. Biol. Chem., 269, 20139±20148. Kestler,H. et al. (1990) Induction of AIDS in rhesus monkeys by molecularly cloned simian immunode®ciency virus. Science, 248, 1109±1112. Kleinberger,T. and Shenk,T. (1993) Adenovirus E4orf4 protein binds to protein phosphatase 2A and the complex down regulates E1Aenhanced junB transcription. J. Virol., 67, 7556±7560. Kremmer,E., Ohst,K., Kiefer,J., Brewis,N. and Walter,G. (1997) Separation of PP2A core enzyme and holoenzyme with monoclonal antibodies against the regulatory A subunit: abundant expression of both forms in cells. Mol. Cell. Biol., 17, 1692±1701. Lee,T.H. (1995) The role of protein phosphatase 2A in the Xenopus cell cycle: initiation of the G2/M transition. Semin. Cancer Biol., 6, 203± 209. Lopez-Girona,A., Furnari,B., Mondesert,O. and Russell,P. (1999) Nuclear localization of cdc25 is regulated by DNA damage and a 14-3-3 protein. Nature, 397, 172±175. Macreadie,I.G., Castelli,L.A., Hewish,D.R., Kirkpatrick,A., Ward,A.C. and Azad,A.A. (1995) A domain of human immunode®ciency virus type 1 Vpr containing repeated H(S/F)RIG amino acid motifs causes cell growth arrest and structural defects. Proc. Natl Acad. Sci. USA, 92, 2770±2774. Mahalingam,S., Khan,S.A., Murali,R., Jabbar,M.A., Monken,C.E., Collman,R.G. and Srinivasan,A. (1995) Mutagenesis of the putative a-helical domain of the Vpr protein of human immunode®ciency virus type 1: effect on stability and virion incorporation. Proc. Natl Acad. Sci. USA, 92, 3794±3798. Mahalingam,S., Ayyavoo,V., Patel,M., Kieber-Emmons,T. and Weiner,D.B. (1997) Nuclear import, virion incorporation and cell cycle arrest/differentiation are mediated by distinct functional domains of human immunode®ciency virus type 1 Vpr. J. Virol., 71, 6339±6347. Mahalingam,S., Ayyavoo,V., Patel,M., Kieber-Emmons,T., Kao,G.D., Muschel,R.J. and Weiner,D.B. (1998) HIV-1 Vpr interacts with a human 34-kDa mov34 homologue, a cellular factor linked to the G2/M phase transition of the mammalian cell cycle. Proc. Natl Acad. Sci. USA, 95, 3419±3424. Masuda,M., Nagai,Y., Oshima,N., Tanaka,K., Murakami,H., Igarashi,H. and Okayama,H. (2000) Genetic studies with the ®ssion yeast Schizosaccharomyces pombe suggest involvement of wee1, ppa2 and rad24 in induction of cell cycle arrest by human immunode®ciency virus type 1 Vpr. J. Virol., 74, 2636±2646. Mayer,R.E., Hendrix,P., Cron,P., Matthies,R., Stone,S.R., Goris,J., Merlevede,W., Hofsteenge,J. and Hemmings,B.A. (1991) Structure of the 55-kDa regulatory subunit of protein phosphatase 2A: evidence for a neuronal-speci®c isoform. Biochemistry, 30, 3589±3597. Mayer-Jaekel,R.E., Ohkura,H., Gomes,R., Sunkel,C.E., Baumgartner,S., Hemmings,B.A. and Glover,D.M. (1993) The 55 kd regulatory subunit HIV-1 Vpr interacts with the B subunit of PP2A of Drosophila protein phosphatase 2A is required for anaphase. Cell, 72, 621±633. McCright,B. and Virshup,D.M. (1995) Identi®cation of a new family of protein phosphatase 2A regulatory subunits. J. Biol. Chem., 270, 26123±26128. McCright,B., Rivers,A.M., Audlin,S. and Virshup,D.M. (1996) The B56 family of protein phosphatase 2A (PP2A) regulatory subunits encodes differentiation-induced phosphoproteins that target PP2A to both nucleus and cytoplasm. J. Biol. Chem., 271, 22081±22089. Mumby,M. (1995) Regulation by tumor antigens de®nes a role for PP2A in signal transduction. Semin. Cancer Biol., 6, 229±237. Mumby,M.C. and Walter,G. (1993) Protein serine/threonine phosphatases: structure, regulation and functions in cell growth. Physiol. Rev., 73, 673±699. Pallas,D.C., Weller,W., Jaspers,S., Miller,T.B., Lane,W.S. and Roberts,T.M. (1992) The third subunit of protein phosphatase 2A (PP2A), a 55-kilodalton protein which is apparently substituted for by T antigens in complexes with the 36- and 63-kilodalton PP2A subunits, bears little resemblance to T antigens. J. Virol., 66, 886±893. Paxton,W., Connor,R.I. and Landau,N.R. (1993) Incorporation of Vpr into human immunode®ciency virus type 1 virions: requirement for the p6 region of gag and mutational analysis. J. Virol., 67, 7229±7237. Planelles,V., Jowett,J.B.M., Li,Q.-X., Xie,Y., Hahn,B. and Chen,I.S.Y. (1996) Vpr-induced cell cycle arrest is conserved among primate lentiviruses. J. Virol., 70, 2516±2524. Popov,S. et al. (1998) Viral protein R regulates nuclear import of the HIV-1 pre-integration complex. EMBO J., 17, 909±917. Re,F., Braaten,D., Franke,E.K. and Luban,J. (1995) Human immunode®ciency virus type 1 Vpr arrests the cell cycle in G2 by inhibiting the activation of p34cdc2±cyclin B. J. Virol., 69, 6859± 6864. Rogel,M.E., Wu,L.I. and Emerman,M. (1995) The human immunode®ciency virus type 1 vpr gene prevents cell proliferation during chronic infection. J. Virol., 69, 882±888. Ruediger,R., Roeckel,D., Fait,J., Bergqvist,A., Magnusson,G. and Walter,G. (1992) Identi®cation of binding sites on the regulatory A subunit of protein phosphatase 2A for the catalytic C subunit and for tumor antigens of simian virus 40 and polyomavirus. Mol. Cell. Biol., 12, 4872±4882. Ruediger,R., Brewis,N., Ohst,K. and Walter,G. (1997) Increasing the ratio of PP2A core enzyme to holoenzyme inhibits Tat-stimulated HIV-1 transcription and virus production. Virology, 238, 432±443. SchoÈnthal,A.H. (1995) Regulation of gene expression by serine/ threonine protein phosphatases. Semin. Cancer Biol., 6, 239±248. Schuler,W., Wecker,K., de Rocquigny,H., Baudat,Y., Sire,J. and Roques,B.P. (1999) NMR structure of the (52±96) C-terminal domain of the HIV-1 regulatory protein Vpr: molecular insights into its biological functions. J. Mol. Biol., 285, 2105±2117. Shtrichman,R., Sharf,R., Barr,H., Dobner,T. and Kleinberger,T. (1999) Induction of apoptosis by adenovirus E4orf4 protein is speci®c to transformed cells and requires an interaction with protein phosphatase 2A. Proc. Natl Acad. Sci. USA, 96, 10080±10085. Stewart,S., Poon,B., Jowett,J.B.M. and Chen,I.S.Y. (1997) Human immunode®ciency virus type 1 Vpr induces apoptosis following cell-cycle arrest. J. Virol., 71, 5579±5592. Subbramanian,R.A., Kessous-Elbaz,A., Lodge,R., Forget,J., Yao,X.J., Bergeron,D. and Cohen,EÂ.A. (1998a) Human immunode®ciency virus type 1 Vpr is a positive regulator of viral transcription and infectivity in primary human macrophages. J. Exp. Med., 187, 1103±1111. Subbramanian,R.A., Yao,X.J., Dilhuydy,H., Rougeau,N., Bergeron,D., Robitaille,Y. and Cohen,EÂ.A. (1998b) Human immunode®ciency virus type 1 Vpr localization: nuclear transport of a viral protein modulated by a putative amphipathic helical structure and its relevance to biological activity. J. Mol. Biol., 278, 13±30. Tang,Z., Coleman,T.R. and Dunphy,W.G. (1993) Two distinct mechanisms for negative regulation of the Wee1 protein kinase. EMBO J., 12, 3427±3436. Vodicka,M.A., Koepp,D.M., Silver,P.A. and Emerman,M. (1998) HIV-1 Vpr interacts with the nuclear transport pathway to promote macrophage infection. Genes Dev., 12, 175±185. Wang,L., Mukherjee,S., Narayan,O. and Zhao,L.J. (1996) Characterization of a leucine-zipper-like domain in Vpr protein of human immunode®ciency virus type 1. Gene, 178, 7±13. Wecker,K. and Roques,B.P. (1999) NMR structure of the (1±51) N-terminal domain of the HIV-1 regulatory protein Vpr. Eur. J. Biochem., 266, 359±369. Withers-Ward,E.S. et al. (1997) Human immunode®ciency virus type Vpr interacts with HHR23A, a cellular protein implicated in nucleotide excision DNA repair. J. Virol., 71, 9732±9742. Yao,X.J., Subbramanian,R.A., Rougeau,N., Boisvert,F., Bergeron,D. and Cohen,EÂ.A. (1995) Mutagenic analysis of human immunode®ciency virus type 1 Vpr: role of a predicted N-terminal a-helical structure in Vpr nuclear localization and virion incorporation. J. Virol., 69, 7032± 7044. Yao,X.J., Mouland,A.J., Subbramanian,R.A., Forget,J., Rougeau,N., Bergeron,D. and Cohen,EÂ.A. (1998) Vpr stimulates viral expression and induces cell killing in human immunode®ciency virus type 1infected dividing Jurkat T cells. J. Virol., 72, 4686±4693. Zhao,Y., Boguslawski,G., Zitomer,R.S. and DePaoli-Roach,A.A. (1997) Saccharomyces cerevisiae homologs of mammalian B and B¢ subunits of protein phosphatase 2A direct the enzyme to distinct cellular functions. J. Biol. Chem., 272, 8256±8262. Zolnierowicz,S., Csortos,C., Bondor,J., Verin,A., Mumby,M.C. and DePaoli-Roach,A.A. (1994) Diversity in the regulatory B-subunits of protein phosphatase 2A: identi®cation of a novel isoform highly expressed in brain. Biochemistry, 33, 11858±11867. Received March 20, 2000; revised and accepted June 14, 2000 3967 The EMBO Journal Vol. 21 No. 14 p. 3918, 2002 Retraction Human immunode®ciency virus type 1 Vpr-mediated G2 cell cycle arrest: Vpr interferes with cell cycle signaling cascades by interacting with the B subunit of serine/threonine protein phosphatase 2A Mohammed Hrimech, Xiao-Jian Yao, Philip E.Branton and EÂric A.Cohen The EMBO Journal, 19, 3956±3967, 2000 In the course of carrying out experiments that were a direct extension of the above paper, we (the authors) discovered differences from those presented in the original article such that the primary conclusions of the paper are in question. Because of this, we are retracting the entire paper on the interaction of HIV-1 Vpr and the B55 subunit of protein phosphatase 2A (PP2A) and its implications on Vpr-mediated G2 cell cycle arrest. We are deeply regretful for any scienti®c misconceptions that have resulted from this study and apologize for any delay that readers may have incurred in their research. 3918 ã European Molecular Biology Organization