* Your assessment is very important for improving the work of artificial intelligence, which forms the content of this project
Download ppt - Avraham Samson`s Lab
Bottromycin wikipedia , lookup
Artificial gene synthesis wikipedia , lookup
Ribosomally synthesized and post-translationally modified peptides wikipedia , lookup
Multi-state modeling of biomolecules wikipedia , lookup
Magnesium transporter wikipedia , lookup
Gene expression wikipedia , lookup
List of types of proteins wikipedia , lookup
Biochemistry wikipedia , lookup
G protein–coupled receptor wikipedia , lookup
Protein (nutrient) wikipedia , lookup
Interactome wikipedia , lookup
Ancestral sequence reconstruction wikipedia , lookup
Metalloprotein wikipedia , lookup
Western blot wikipedia , lookup
Protein moonlighting wikipedia , lookup
Protein design wikipedia , lookup
Circular dichroism wikipedia , lookup
Implicit solvation wikipedia , lookup
Protein adsorption wikipedia , lookup
Protein–protein interaction wikipedia , lookup
Two-hybrid screening wikipedia , lookup
Intrinsically disordered proteins wikipedia , lookup
Structural alignment wikipedia , lookup
Protein folding wikipedia , lookup
Nuclear magnetic resonance spectroscopy of proteins wikipedia , lookup
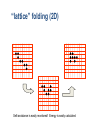
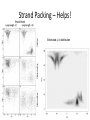
Structure prediction: Ab-initio Lecture 9 Structural Bioinformatics Dr. Avraham Samson 81-871 Let’s think! Levinthal's paradox In 1969, Cyrus Levinthal noted that, because of the very large number of degrees of freedom in a polypeptide chain, the molecule has an astronomical number of possible conformations. For example, a polypeptide of 100 residues will have 99 peptide bonds, and therefore 198 different phi and psi bond angles. If each of these bond angles can be in one of three stable conformations, the protein may misfold into a maximum of 3198 (~10100) different conformations. Therefore, a polypeptide would require a time longer than the age of the universe to arrive at its correct native conformation. This is true even if conformations are sampled at rapid (picosecond) rates. The "paradox" is that most small proteins fold spontaneously on a millisecond or even microsecond time scale. Protein Structure Prediction • Two main categories of protein structure prediction methods: – Homology modeling (class of last week!) – Ab-initio methods (class of today!) • Methods can also be characterized: – Based on physical principles (simulations) – Based on statistics derived from known structures (knowledge-based) 3 Secondary Structure Prediction • Methods attempt to decide which type of secondary structure (helix, strand or coil) each amino acid in a protein sequence is likely to adopt. • The based methods are currently able to achieve success rates of over 75% based on sequence profiles. 4 Folding Simulations • Accurate folding simulations will allow us to predict the structure of any protein. • However, this approach is impractical due to limitations of computing power. • Our understanding of the principles of protein folding are far short of the level needed to achieve this. 5 Homology Modeling • Similar sequences Almost identical structures • Sometimes referred to as “Comparative modeling” • The most reliable technique for predicting protein structure • Comparing the sequence of the new protein with the sequences of proteins of known structure – Strong similarity (% identity, % similarity, alignment) – No strong similarities comparative modeling cannot be used. 6 Predicting Small Conformational Changes • Native fold of a protein can be found by finding the conformation of the protein which has the lowest energy as defined by a suitable potential energy function. • Even between very similar proteins, there are differences. • Some of these differences might be functionally important (different binding loop conformations) • Predicting what the effects of these small structural changes is the real challenge in modeling 7 Ab initio Prediction • Ab initio (i.e. ‘from scratch’) • Use only the information in the target sequence itself • Two branches – Knowledge-based methods • Predict structure by applying statistical rules • Rules: observations made on known protein structures – Simulation methods • Predict structures by applying physical parameters (Van-derWaals, dipole-dipole, etc) 8 Simulation Methods • Most ambitious approach • Simulate the protein-folding process using basic physics • Only useful for short peptides and small molecules • Very useful for predicting unknown loop conformations as part of homology modeling 9 Energy Function • Find a potential function • Construct an algorithm capable of finding the global minimum of this function • The exact form of this energy function is as yet unknown • It is reasonable to assume that it would incorporate terms pertaining to the types of interactions observed in protein structures – Hydrogen bonding – Van der Waals effects 10 Searching Conformational Space • Consider a protein chain of N residues • The size of its conformational space is roughly 10N states. • 10 main chain torsion angle triples for each residue • Not consider the additional conformational space provided by the side chain torsion yet. 11 How to Find Global Energy Minimum Efficiently • Clearly proteins do not fold by searching their entire conformational space (Levinthal’s paradox) • Proteins fold by means of a folding pathway encoded in the protein sequence ? • Short-chain segments (5-7 residues) could quite easily locate their global minimum. • Location of the native fold is driven by the folding of such short fragments ? 12 One Subtle Point • The native conformation need not necessarily correspond to the global minimum of free energy. 13 Secondary Structure Prediction • Although predicting just the secondary structure of a protein is a long way from predicting its tertiary structure, information on the locations of helices and strands in a protein can provide useful insights as to its possible overall fold. • It is also worth noting that the origins of the protein structure prediction field lie in this area 14 Intrinsic Propensities for Secondary Structure Formation • Are some residues more likely to form -helices or -strands than others? • Yes – Ex. proline residues are not often found in -helices • 1974, statistical analysis of 15 proteins with known 3-D structures • For each of the 20 amino acids, calculate the probability of finding any residue in -helices and in -strands • Also calculate the probability of finding any residue in helices and in -strands 15 Example (Chou and Fasman, 1974) • Suppose there was a total of 2000 residues in their 15 protein data set Total number of residues Number of alanines 100 Number of helical residues 500 Number of alanines in helices 2000 50 We would calculate the propensity of alanine for helix formation as follows: P(Ala in Helix) = 50/500 = 0.1 P(Ala) = 100/2000 = 0.05 Helix propensity (PA) of Ala = P(Ala in Helix)/P(Ala) = 0.1/0.05 = 216 ab-initio prediction • Prediction from sequence using first principles AVVTW...GTTWVR Ab-initio prediction • “In theory”, we should be able to build native structures from first principles using sequence information and molecular dynamics simulations: “Ab-initio prediction of structure” – Simulation of the villin head piece (36-residues). (Pande et al.) http://www.youtube.com/watch?v=1eSwDK ZQpok&feature=related http://www.youtube.com/watch?NR=1&v= meNEUTn9Atg&feature=endscreen ... the bad news ... • It is not possible to span simulations to the “seconds” range • Simulations are limited to small systems and fast folding/unfolding events in known structures – steered dynamics – biased molecular dynamics • Simplified systems typical shortcuts • Reduce conformational space – 1,2 atoms per residue – fixed lattices • Statistic force-fields obtained from known structures – Average distances between residues – Interactions • Use building blocks: 3-9 residues from PDB structures “lattice” folding (2D) Self-avoidance is easily monitored! Energy is easily calculated Example PROSA potential Very stable Low stability Hydrophobic C-C Total http://lore.came.sbg.ac.at:8080/CAME/CAME_EXTERN/ProsaII/index_html Results from ab-initio • Average error 5 Å - 10 Å • Long simulations Some protein from E.coli predicted at 7.6 Å (CASP3, H.Scheraga) “loops” in homology modeling Ab initio PDB Final test • The model must justify experimental data (i.e. differences between unknown sequence and templates) and be useful to understand function. Rosetta energy function • • • • • Residue environment (solvation) Residue pair interaction (electrostatic, disulfides) Steric repulsion Radius of gyration (vdw attraction, solvation) Cb density (solvation, correction for excluded volume) • SS pairing (hydrogen bonding) • Strand arrangement into sheet • Helix-strand packing Protein Structure Prediction using ROSETTA Worldwide distributed computing Ab Initio Methods • Ab initio: “From the beginning”. • Assumption 1: All the information about the structure of a protein is contained in its sequence of amino acids. • Assumption 2: The structure that a (globular) protein folds into is the structure with the lowest free energy. • Finding native-like conformations require: - A scoring function (potential). - A search strategy. Rosetta • The scoring function is a model generated using various contributions. It has a sequence dependent part (including for example a term for hydrophobic burial), and a sequence independent part (including for example a term for strandstrand packing). • The search is carried out using simulated annealing. The move set is defined by a fragment library for each three and nine residue segment of the chain. The fragments are extracted from observed structures in the PDB. The Rosetta Scoring Function Hydrophobic Burial Residue Pair Interaction The Sequence Independent Term vector representation Strand Packing – Helps! Estimated f-q distribution Sheer Angles – Help not! Parameter Estimation Parameter Estimation Parameter Estimation Parameter Estimation Fragment Selection Validation Data Set CASP3 Protocol Construct a multiple sequence alignment from f-blast. Edit the multiple sequence alignment. Identify the ab initio targets from the sequence. Search the literature for biological and functional information. • Generate 1200 structures, each the result of 100,000 cycles. • Analyze the top 50 or so structures by an all-atom scoring function (also using clustering data). • Rank the top 5 structures according to protein-like appearance and/or expectations from the literature. • • • • CASP3 Predictions Why is Rosetta so fast? Monte Carlo (Random Sampling) http://www.chemistryexplained.com/images/c hfa_03_img0571.jpg • Randomly (or pseudorandomly) pick a configuration and evaluate its energy. • If acceptably low, store result. • If not, move a distance away from that point as a function of the energy (Metropolis criterion, a.k.a. simulated annealing) and evaluate again • When some convergence threshold or time limit is met, stop and return stored results. What have we learned? • Can tackle sampling today • Forcefields sufficient? Folding to the native state folding rate prediction • Role of water – Explicit solvent not crucial to rate determination? – Compare to explicit solvent simulation • Universal mechanism of folding? – Maybe no universal mechanism: all proteins could be different?