* Your assessment is very important for improving the workof artificial intelligence, which forms the content of this project
Download Camp 1 - University of California, Santa Cruz
Butyric acid wikipedia , lookup
Metalloprotein wikipedia , lookup
Multi-state modeling of biomolecules wikipedia , lookup
Biosynthesis wikipedia , lookup
Mitochondrion wikipedia , lookup
Fatty acid synthesis wikipedia , lookup
Amino acid synthesis wikipedia , lookup
Basal metabolic rate wikipedia , lookup
Photosynthesis wikipedia , lookup
Electron transport chain wikipedia , lookup
Light-dependent reactions wikipedia , lookup
Microbial metabolism wikipedia , lookup
Photosynthetic reaction centre wikipedia , lookup
Nicotinamide adenine dinucleotide wikipedia , lookup
Fatty acid metabolism wikipedia , lookup
Phosphorylation wikipedia , lookup
Evolution of metal ions in biological systems wikipedia , lookup
Oxidative phosphorylation wikipedia , lookup
Glyceroneogenesis wikipedia , lookup
Lactate dehydrogenase wikipedia , lookup
Adenosine triphosphate wikipedia , lookup
Biochemistry wikipedia , lookup
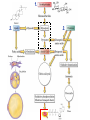
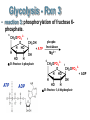
Chapter 27 Specific Catabolic Pathways: Carbohydrate, Lipid & Protein Metabolism 1. 2. 3. Glycolysis: Follow Along: Figure 27.3 Glycolysis • 10 enzyme-catalyzed reactions by which glucose is oxidized to two molecules of pyruvate. glycolys is C6 H1 2 O6 Glucos e HO HO O 2 CH3 CCOO - + 2 H+ Pyruvate CH2 OH O + OH -D -Glucose OH - O O O-P-O-P-O-AMP - O - O ATP hexokinas e 2+ Mg 2• During glycolysis, there is net conversion of 2ADP to 2ATP. CH OPO HO HO 2 O 3 + OH C6 H1 2 O6 + 2 ADP + 2 OH Pi -D -Glucose 6-p hosphate Glucose - O O-P-O-AMP O O 2 CH3 CCOO - + 2 ATP ADP Pyruvate NOTE: ATP = Energy molecule of choice Glycolysis - Rxn 1 • reaction 1: phosphorylation of -D-glucose. HO HO CH2 OH O O O + -O-P-O-P-O-AMP OH -D -Glucose OH HO HO - O - O ATP CH 2 OPO3 O hexokinas e 2+ Mg 2- OH OH -D -Glucose 6-p hosphate O + - O-P-O-AMP O ADP Glycolysis - Rxn 2 • reaction 2: isomerization of glucose 6-phosphate to fructose 6-phosphate. 6 HO HO 6 2- CH2 OPO3 O 2 OH ph os phoglu coisomeras e 1 OH -D-Glu cose 6-phosp hate 2- CH2 OPO3 1 CH2 OH O H HO 2 H OH HO H -D -Fru ctos e 6-phosp hate Glycolysis - Rxn 2 • This isomerization is most easily seen by considering the open-chain forms of each monosaccharide; it is one keto-enol tautomerism followed by another. 1 CHO H 2 OH HO H H OH H OH CH2 OPO3 2 Glucose 6-p hos phate H C OH C OH HO H H OH H OH CH2 OPO3 2(A n ened iol) 1 CH2 OH 2C O HO H H OH H OH CH2 OPO3 2 Fru ctose 6-phosp hate Glycolysis - Rxn 3 • reaction 3: phosphorylation of fructose 6- phosphate. 6 CH2 OPO 3 2 - 1 CH2 OH O H HO + A TP H OH HO H -D-Fructose 6-phosphate ATP ADP phosphofructokinase Mg 2 + 6 CH2 OPO 3 2 - 1 CH2 OPO 3 2 O H HO + A DP H OH HO H -D-Fructose 1,6-bisphosphate Glycolysis - Rxn 4 • reaction 4: cleavage of fructose 1,6-bisphosphate to two triose phosphates. CH2 OPO3 2- C=O HO H H al dol ase H OH OH CH2 OPO3 2 - Fructose 1,6-b isph osp hate CH2 OPO3 2 C=O CH2 OH CHO H C OH CH2 OPO3 2 - D ih yd roxyacetone p hos phate D -Glyceraldehyde 3-p hosph ate Glycolysis - Rxn 5 • reaction 5: isomerization of triose phosphates. • Catalyzed by phosphotriose isomerase. • Reaction involves two successive keto-enol tautomerizations. • Only the D enantiomer of glyceraldehyde 3-phosphate is formed. CH2 OH C= O CHOH C-OH CH2 OPO 3 2 - CH2 OPO 3 2 - Dihydroxyacetone phosphate An enediol intermediate CHO H C OH CH2 OPO 3 2 D-Glyceraldehyde 3-phosphate Glycolysis - Rxn 6 • Reaction 6: oxidation of the -CHO group of D-glyceraldehyde 3-phosphate. • The product contains a phosphate ester and a highenergy mixed carboxylic-phosphoric anhydride. CHO H C OH 2CH2 OPO3 D -Glyceraldehyde 3-ph os phate + + NAD + Pi glyceraldeh yd e 3-p hosphate d ehydrogenase O 2C-OPO3 H C OH + NADH 2CH2 OPO3 1,3-Bis phosp hoglycerate Glycolysis - Rxn 7 O phosp ho2C-OPO3 O glycerate kinas e • + O-P-O-AMP H C OH 2+ 2Mg O CH2 OPO3 1,3-Bisp hos phoAD P glycerate O phosp hoCOOO O 2C-OPO3 O glycerate kinas e + O-P-O-P-O-AMP H C OH + O-P-O-AMP H C OH 22+ O O 2CH OPO Mg 2 3 O CH2 OPO3 3-Ph os phoglycerate ATP 1,3-Bisp hos phoAD P glycerate COOO O + -O-P-O-P-O-AMP H C OH O O CH2 OPO3 2 3-Ph os phoglycerate ATP Reaction 7: transfer of a phosphate group from 1,3-bisphosphoglycerate to ADP. Glycolysis - Rxn 8 & 9 • Reaction 8: isomerization of 3-phosphoglycerate to 2-phosphoglycerate. phosph oglycerate COOCOOmutas e H C OH H C OPO3 2 CH2 OH CH2 OPO3 22-Ph os phoglycerate 3-Phos phoglycerate • Reaction 9: dehydration of 2-phosphoglycerate. COOCOOen olase 22+ H2 O H C OPO3 C OPO 2+ 3 Mg CH2 OH CH2 2-Phosph oglycerate Phosph oen olp yruvate COO O 2C OPO3 + O-P-O-AMP CH2 O- kinas e Mg2 + Glycolysis - Rxn 10 AD P os phoenol• Reaction Ph 10: phosphate transfer to ADP - COO 2C OPO3 CH2 Ph os phoenolpyruvate pyruvate O + O-P-O-AMP OAD P pyru vate kinas e Mg2 + O O COOC=O + O-P-O-P-O-AMP O- OCH3 A TP Pyruvate O O COOC=O + O-P-O-P-O-AMP O- OCH3 A TP Pyruvate Glycolysis: SUMMARY: 1 ATP used 1 ATP used Figure 27.3 2 ATP produced Note: Glycer-3 phosphate X 2 2 ATP produced Glycolysis: Summary • The net equation for glycolysis: C6 H1 2 O6 + 2 N A D+ + 2 HPO 4 2 - + 2 A DP Glucos e O 2 CH3 CCOO - + 2 NADH + Pyruvate glycolys is 2 ATP + 2 H 2 O + 2 H + Confirming your knowledge • Of the 36 molecules of ATP produced by the complete metabolism of glucose, how many are produced in glycolysis alone? ____ • What is the net total ATP production? ___ ATP Total: 36 ATP Reactions of Pyruvate: Fig 27.4 • Pyruvate metabolized three ways: • depends on organism & presence/absence of O2 12 aerobic conditions p lants and animals Acetyl CoA 13 Citric acid cycle OH O - 11 anaerob ic conditions CH3 CHCOOCH3 CCOO contracting mu scle Lactate Pyruvate 10 anaerob ic conditions CH3 CH2 OH + CO2 fermentation in yeast Ethanol NOTE: Anaerobic = w/o Oxygen Aerobic = w/ Oxygen Reactions of Pyruvate • A key to understanding the biochemical logic behind two of these reactions of pyruvate is to recognize that glycolysis needs a continuing supply of NAD+. • If no oxygen is present to reoxidize NADH to NAD+, then another way must be found to reoxidize it. Pyruvate to Lactate • Under anaerobic (NO Oxygen) conditions, the most important pathway for the regeneration of NAD+ is reduction of pyruvate to lactate. Pyruvate, the oxidizing agent, is reduced to lactate. lactate O dehydrogenase + CH3 CCOO + NA DH + H Pyruvate OH CH3 CHCOO- + NA D+ Lactate Pyruvate to Lactate • While reduction to lactate allows glycolysis to continue, it increases the concentration of lactate and also of H+ in muscle tissue lactate OH C6 H1 2 O6 fermentation 2 CH3 CHCOO- + 2 H+ Glucos e Lactate ((ACID!)) • When blood lactate reaches about 0.4 mg/100 mL, muscle tissue becomes almost completely exhausted, esp. b/c H+ ions. Pyruvate to Ethanol • Yeasts and several other organisms regenerate NAD+ by this two-step pathway: • decarboxylation of pyruvate to acetaldehyde. pyruvate O decarboxylase + CH3 CCOO + H Pyruvate O CH3 CH + CO 2 Acetaldehyde • Acetaldehyde is reduced to ethanol. alcohol O dehydrogenase + CH3 CH + N AD H + H Acetaldehyde CH3 CH2 OH + NA D + Ethanol Pyruvate to Acetyl-CoA • Under aerobic conditions, pyruvate undergoes oxidative decarboxylation. • The carboxylate group is converted to CO2. • The remaining two carbons are converted to the acetyl group of acetyl CoA. oxidative O decarboxylation CH3 CCOO - + NAD+ + CoASH Pyruvate O CH3 CSCoA + CO2 + N ADH Acetyl-CoA Confirming your knowledge • The end product of glycolysis (pyruvate) cannot enter as such into the citric acid cycle. • What is the name of the process that converts this C3 compound to a C2 compound? Catabolism of Glycerol • Glycerol enters glycolysis via dihydroxyacetone phosphate. CH2 OH ATP ADP CHOH CH2 OH 7.3 kcal/mole Glycerol + NAD CH2 OH CHOH CH2 OPO3 2Glycerol 1-phosph ate 3.4 kcal/mole. NADH CH2 OH C=O CH2 OPO3 2 D ihydroxyacetone phosp hate Challenge Question • Which yields more Energy upon hydrolysis: ATP or glycerol 1- phosphate and Why? CH2 OH ATP CHOH CH2 OH Glycerol ADP + NAD CH2 OH CHOH CH2 OPO3 2Glycerol 1-phosph ate NADH CH2 OH C=O CH2 OPO3 2 D ihydroxyacetone phosp hate Summary • Glycolysis yields 2 net ATP • ATP is the molecule of choice for cell(s) Energy • ATP is a High E yielding molecule (7.3 kcal/mole) • Pyruvate is the END product of Glycolysis oxidative O » under aerobic conditions Acetyl CoA further ATP!!! decarboxylation + CH3 CCOO »+under NADanaerobic + CoASH conditions Lactate (muscle fatigue) Pyruvate O CH3 CSCoA + CO2 + N ADH Acetyl-CoA