* Your assessment is very important for improving the work of artificial intelligence, which forms the content of this project
Download Slide 1
Aromaticity wikipedia , lookup
Elias James Corey wikipedia , lookup
Physical organic chemistry wikipedia , lookup
George S. Hammond wikipedia , lookup
Enantioselective synthesis wikipedia , lookup
Diels–Alder reaction wikipedia , lookup
Hydrogenation wikipedia , lookup
Asymmetric induction wikipedia , lookup
Vinylcyclopropane rearrangement wikipedia , lookup
Ring-closing metathesis wikipedia , lookup
Baylis–Hillman reaction wikipedia , lookup
Ene reaction wikipedia , lookup
Aromatization wikipedia , lookup
Stille reaction wikipedia , lookup
Discodermolide wikipedia , lookup
Wolff rearrangement wikipedia , lookup
Hydroformylation wikipedia , lookup
Aza-Cope rearrangement wikipedia , lookup
Tiffeneau–Demjanov rearrangement wikipedia , lookup
Wolff–Kishner reduction wikipedia , lookup
Nucleophilic acyl substitution wikipedia , lookup
Hofmann–Löffler reaction wikipedia , lookup
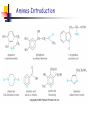
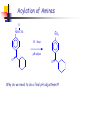
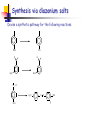
Unit 4 Amines Structure and properties Nomenclature Reactions (some review) Synthesis (some review) Spectroscopy – mass spec, IR, NMR Amines-Introduction Amines and amine derivatives are found throughout biological systems. Amino acids (proteins) Bioregulators Neurotransmission Vitamins drugs Amines-Introduction Amines-Introduction O H 3C O CH3 N N N N CH3 caffeine Biologically active basic amines obtained from plants are called alkaloids. Amines-Introduction There are many man-made drug that are amines. H H N N CH3 CH3 methamphetamine levomethamphetamine vasoconstrictor H N OH H H N pseudoephedrine amphetamine Nasal decongestant CH3 Amine- Structures Amines are derivatives of ammonia NH3 Amines are classified based on the number of alkyl groups attached to nitrogen. R-NH2 primary amine (1°) R2-NH secondary amine (2°) R3-N tertiary amine (3°) R4-N+, X- quaternary ammonium salt(4°) Amine- Nomenclature Common amines are named based on the alkyl groups attached to the nitrogen. Alkylamine dialkylamine trialkylamine tetralkylamine ethylamine dimethylamine butyldiethylamine tetraethylammonium chloride Amine- Nomenclature Name and classify the following amines. (CH3CH2)2NCH3 NH CH3 H CH2NH2 N NH2 Amine- Nomenclature The amine is named as a substituent group when there is a higher priority group. OH NHCH3 NH2CH2CH2CH2CO2H 4-aminobutanoic acid 2-methylaminophenol NH2 O N CH2CH3 4-(ethylmethylamino)cyclohexanone N(CH3)2 OH 3-aminocyclohexene CH3 Amine- Nomenclature (IUPAC) IUPAC names are based on the longest carbon chain. Drop the –e of the alkane name and add-amine. Substituents on nitrogen have N- prefix. NH2 NH2 CH3CH2CHCH3 CH3CHCHCH3 CH3 N(CH3)2 CH3CH2CHCHCHCH3 CH3 CH3 NHCH3 CH3CH2CHCH3 Aromatic Amines When the amino group is bonded to an aromatic ring the parent compound is called an aniline. NH2 NH2 N(CH3)2 CH3 aniline N,N-dimethylaniline 4-methylaniline or P-toluidine Heterocyclic Amines H N H N H N N pyrrole pyrrolidine pyridine aziridine H N N N N H H piperidine piperazine N pyrimidine N N N N purine H Structure of Amines Nitrogen atoms with 3 or 4 single bonds are sp3 hybridized. H H N H H N CH3 CH3 H-N-H bond angle is about 107° The steric interactions of larger substituents increases the bond angle C-N-C is about 108 ° Structure of Amines The nitrogen atom is potentially a stereocenter. However nitrogen inversion occurs rapidly at room temperature. Chiral Amines: Amines with a chiral carbon. Quaternary ammonium cpds with four different groups bonded to nitrogen. Amines that are unable to achieve the planar transition state (ie small rings). pp. 875 Structure of Amines Quaternary ammonium salt with four different groups bonded to nitrogen. The counter ion can be Cl-, Br-, OH- etc. (R) + N (CH3)2CH CH2CH3 H3C (S) N CH3CH2 + CH(CH3)2 CH3 Physical properties of Amines Amines (1 °,2 ° and 3 °) are highly polar materials because of the lone pair of electrons on nitrogen. The N-H bond is less polar than the O-H bond. Weaker hydrogen bonding. Tertiary amines cannot hydrogen bond but are hydrogen bond acceptors. Physical properties of Amines The strength and number of hydrogen bonds found in an amine influence melting points, boiling points, and water solubility. Compound bp (C) Type MW (CH3)3N: 3 3 amine 59 CH3OCH2CH3 8 ether 60 CH3NHCH2CH3 37 2 amine 59 CH3CH2CH2NH2 48 1 amine 59 CH3CH2CHO 49 aldehyde 58 CH3COCH3 56 ketone 58 CH3CH2CH2OH 97 alcohol 60 Physical properties of Amines Methylamine ethylamine n-propylamine type 1° 1° 1° MW 31 45 59 mp -93 -81 -83 bp -7 17 48 dimethylamine diethylamine 2° 2° 45 73 -96 -42 7 56 trimethylamine triethylamine 3° 3° 59 101 -117 -115 3.5 90 Physical properties of Amines Amines up to about 6 carbons are water soluble. Amines accept hydrogen bonds from water and alcohols. Branching increases solubility. Amines have very strong odors (rotting fish). 1,5-pentanediamine (cadaverine). 1,4-butanediamine (putrescine). Pure amine are clear (liq. or solids) but oxidation from atmospheric oxygen often results in the materials being dark colored. Physical properties of Amines Amines are bases, in the presence of acids the lone pair of electrons act as a proton acceptor (Bronsted-Lowry bases). R NH2 + HCl R NH3 + Cl- The lone pair of electrons on nitrogen can also act as a nucleophile (Lewis base). H H R NH2 + H H C Cl H + R N H H H Cl- Physical properties of Amines H R N Kb + H O H R N H H H H ammonium ion [RNH3+] [OH - ] ______________ Kb = [RNH2 ] Amines are weak bases: Kb = 10-3 - 10-10 Larger Kb is stronger base. pKb = 3 - 10 Smaller pKb is stronger base. + OH - hydroxide ion Physical properties of Amines type ammonia methylamine ethylamine n-propylamine 1° 1° 1° pKb 4.74 3.36 3.36 3.32 dimethylamine diethylamine 2° 2° 3.28 3.01 trimethylamine triethylamine 3° 3° 4.26 3.24 aniline pyridine 1° heterocyclic 9.4 8.75 see page 878 Physical properties of Amines Amines are converted to the water soluble ammonium salt by treatment with an acid. Examples: HCl, HBr, H2SO4 and organic acids. The salt can be converted back to the free amine by treatment with a strong base. Examples: NaOH, KOH, Na2CO3 and bicarbonate. Amines are often isolated and purified using acid/base extraction. page 881 Amine salts are more stable to air oxidation than the free amine and have little or no odor. Physical properties of Amines Spectroscopy of Amines Spectroscopy of Amines propylamine Spectroscopy of Amines piperidine Spectroscopy of Amines Benzylamine Spectroscopy of Amines N-methylaniline Spectroscopy of Amines Reactions of Amines (review) Primary amines react with ketones and aldehydes to give imines. Analogous products are obtained for hydroxylamine, hydrazine, semicarbazide and carbazide. Write the mechanism for this reaction. (Note: The acid conditions.) Formation of Imines The formation of an imine involves an initial nucleophilic attack by ammonia or a primary amine on the carbonyl carbon. Followed by subsequent loss of a water molecule. The C=O becomes a C=N-R group where R= H, alkyl or aryl H+ H3C H3C H2N H H CH3 R R N C OH C O C O Ph Ph CH3 R N C OH H Ph CH3 H2O H CH3 H+ R N C O H Ph H H R N C OH Ph H + H3O+ Ph CH3 CH3 R N C R N C H H Ph CH3 R N C Ph H2O Ph Reactions of Amines (review) The aromatic ring of aniline derivatives are highly activated for electrophilic aromatic substitution reactions. As shown above the sigma complex is stabilized at the ortho and para positions by the non-bonding electron on the nitrogen. Is the ring activated in the presents of an acid? Reactions of Amines (review) NH2 NH2 NO2 xs Cl2 Cl NO2 + 2 HCl NaHCO3 Cl What function does the sodium bicarbonate serve in the above reaction? Give two reasons why anilines can not be nitrated directly using HNO3/H2SO4? Reactions of Pyridine The pyridine ring is deactivated towards electrophilic aromatic substitution because of the electronegative nitrogen in the ring. Additionally the non-bonding electron on the nitrogen would react with the electrophile. Reaction only occurs under extreme conditions. Note: That substitution is in the 3-position. Reactions of Pyridine The pyridine ring is activated towards nucleophilic aromatic substitution. With substitution occurring at either the 2- or 4position. The sigma complex is stabilized by the negative charge being on nitrogen. Alkylation of Amines Amines are good nucleophiles that react with alkyl halides via a Sn2 mechanism. Reaction with primary halides give alkylated ammonium halides. Secondary halides are less reactive than primary halides and often give poor yields or elimination products. Tertiary halides do not react because of steric hindrance. Note: The that °3 halides can still under go elimination. Alkylation of Amines The above reaction gives an incomplete picture of the chemistry involved in the alkylation of an amine with an alkyl halide. Alkylation of Amines The reaction of one mole of primary amine with one mole of alkyl halide will give a mixture of starting primary amine, secondary amine, tertiary amine and quaternary ammonium salts. There two methods that can be used to avoid obtaining mixtures of products when alkylating amines with alkyl halides. Alkylation of Amines Exhaustive alkylation (methylation) involves reacting the amine with an excess of alkyl halide in the presence of an acid scavenger. R NH2 + 3 CH3 Cl NaHCO3 R N (CH3)3 Cl - (> 90%) Alkylation of Amines The use of a large excess of ammonia results in monoalkylation. The excess ammonia is simply allowed to boil of at the end of the reaction. This synthetic approach is useful in many situations but is often limited by the cost of the alkyl halide. NH3 10 moles + R' CH2 X 1 mole R' + CH2 N H3 X- Acylation of Amines Amides are produced by the treatment of primary and secondary amines with acid halides in the presents of a non-nucleophilic acid scavenger. The reaction involves the nucleophilic attack of the amine on the carbonyl carbon (the electrophilic center) of the acid halide followed by loss HX. Amides are far less basic and nucleophilic than amines. As a result mono-acylation product is normally produced. Why is the amide less basic and nucleophilic than the amine? Acylation of Amines O- O R' C Cl + R' R NH2 Cl NH2 R O O R' R' Cl C NHR + Cl - H NH2 R O R' C NHR + Cl - O Base R' C NHR + H Base + H The base is typically pyridine, a tertiary amine or bicarbonate. The yields are generally very good ( > 90 %). Cl - Acylation of Amines The acyl group in an amide can be easily removed by hydrolysis with aqueous acid. As a result acylation can be used as a means of temporary protecting the amine group as the amide while conducting other reactions that would produce undesired change in the amine. The acyl group acts as a Masking agent. Many reactions can not be done directly on the aromatic ring of aniline either because the amine will react with the reagents or multiple substitution occur. Acylation of Amines H O NH2 Cl + N AlCl3 + aluminum complexs O The Friedel-Crafts acylation of the ring fails in the above reaction. However, if the amine of the aniline is acylation first then the ring can be acylated. O O O NH2 O + CH3CCl Base NHCCH3 Cl NHCCH3 AlCl3 O Acylation of Amines O NHCCH3 NH2 H+ / heat pH adjust O O Why do we need to do a final pH adjustment? Sulfonylation of Amines The reaction of amines with sulfonyl chlorides is analogous to that of acyl halides. O R' S O O NaOH Cl + R NH2 R' S NHR O The sulfa drugs are antibacterial agents that contain the sulfonamide group. Elimination Reactions involving Amines Amines can under go two different types of elimination reactions that give alkenes. Hofmann elimination: An amine is exhaustively methylated to the quaternary ammonium salt. The halide salt is converted to the hydroxide salt by treatment with silver oxide. The quaternary ammonium hydroxide is then thermally decomposed via a conserted E2 mechanism to give the alkene. Cope Elimination: A tertiary amine is converted to the amine oxide by treatment with hydrogen peroxide or a peroxyacid. The amine oxide is then thermally decomposed to give an alkene. Both reactions generally give the least-substituted alkene. Elimination Reactions involving Amines Hofmann elimination: acid scavenger R NH2 + R N(CH3)3 I 3 CH3 I - + 2 HI trimethyl amine a good leaving group R N(CH3)3 I CH3CH2CH N(CH3)3 OH + - 1/2 Ag2O R N(CH3)3 OH heat CH3CH2CH CH2 + H2O + N(CH3)3 CH3 For R = 2-butyl the product is 95 % 1-butene, the least substituted alkene. Onle 5% of the product is the Saytzeff product. Hofmann elimination: Requirements an anti coplanar stereochemistry. Hofmann elimination: Predict the products of the following reactions. CH3 1) CH3I N CH3 2) Ag2O 3) heat CH3 NH2 1) CH3I 2) Ag2O 3) heat 1) CH3I NHCH2CH3 2) Ag2O 3) heat Cope elimination: The oxidation of amines can lead to a number of different products depending on the type of starting amine. Oxidation of °1 amines results in the formation of hydroxyl amines which are oxidized to nitroso cpds which are then oxidized to nitro cpds. °2 amines are oxidized to hydroxyl amines. °3 amines are oxidized to amine oxides. The Cope elimination involves the thermal decomposition of these amine oxides. Cope elimination: The oxidation of the °3 amine is easily done using either 30% or 50% hydrogen peroxide. The reaction is very exothermic and the addition of the peroxide must be carefully regulated to avoid over heating the reaction. R R N R °3 amine R + H2O2 R N O R °3 amine oxide + H2O Cope elimination: Heating the amine oxide results in the elimination of a dialkyl hydroxylamine. The reaction occurs via a concerted E2 mechanism that requires syn stereochemistry to occur. The reaction occurs under milder conditions than the Hofmann elimination. CH3 CH3CH2CH N O CH3 CH3 heat CH3CH2CH CH2 + HO N(CH3)2 Cope elimination: Predict the products of the following reactions. 1) H2O2 N 2) heat 1) H2O2 N 2) heat (CH3)2N 1) H2O2 2) heat Reactions with Nitrous Acid Treatment of primary aromatic amines with sodium nitrite under acidic conditions arenediazonium salt. This process is called diazotization of an amine. Once the diazonium salt is made the diazonium group can be replace by many different groups. NH2 NaNO2 HCl What about diazonium salts made from alkylamines? N2 Cl - Reactions with Nitrous Acid Reactions with Nitrous Acid H3O + R N N OH H R N N O H R N N diazonium ion + H2O Reactions with Nitrous Acid Reactions of diazonium salts. Synthesis via diazonium salts Devise a synthetic pathway for the following reactions. NO2 CN O O H2N HO N2 Cl - O2N NO2 N N OH Synthesis of Amines via reduction Reductive amination is a two step process that adds an alkyl group to ammonia, a primary or secondary amine. 1. The first step is the formation of the imine or oxime derivative of an aldehyde or ketone. 2. The imine/oxime is then reduced to give the amine. The reaction is done on an industrial scale using H2 over a Ni catalyst. Laboratory scale process generally rely on hydride reducing agents such as LAH. Synthesis of Amines via reduction Primary amines via reductive amination: O R N H2NOH R' H+ R OH NH2 reduction R' R R' R=R'= alkyl, aryl and hydrogen R=R' The hydrogenation can be done with 15-60 psig of H2 at 140-60°C over 0.2% supported Ni catalyst. Synthesis of Amines via reduction Secondary amines via reductive amination: a primary amine O R R" R' N NH2 H+ R=R'= alkyl, aryl and hydrogen R=R' R H R" N reduction R' N-substituted imine R R" R' Synthesis of Amines via reduction Tertiary amines via reductive amination: a secondary amine R" O R R" R' R" N NH + R R" R" N reduction R' R R" R' H R=R'= alkyl, aryl and hydrogen R=R' iminium salt Since many iminium salts are unstable they are generally not isolate. As a result the reducing agent is added to the reaction mixture so that the iminium salt can be reduced as it is formed. Only very weak reducing agents can be used in this reaction to avoid reduction of the starting aldehyde or ketone. Sodium triacetoxyborohydride and sodium cyanoborohydride will reduce the iminium salt without reducing the carbonyl compound. Industrially, the reaction is done using hydrogen and a Ni catalyst. Synthesis of Amines via reduction Propose a synthetic route for the following. NH C18H37NH2 N C18H37N(CH3)2 H C18H37NH2 O C18H37NCH2CH(CH2CH3)CH2CH2CH2CH3 NH2 cpd with no more than 5 carbons Br NH2 N Synthesis of Amines via acylation reduction Amines are mono acylated by treatment with with an acid chloride. Since the resulting amide is a poor nucleophile multiply acylations are not likely. The amide is then converted to the amine by treatment with LAH. The type of amine produced depends on the type of starting amine used. Starting amine Ammonia 1° amines 2° amines product 1° amines 2° amines 3° amines Synthesis of Amines via acylation reduction H CH2CH2CH2CH3 N from aniline Synthesis of of primary Amines Direct alkylation: Primary amines can be prepared from alkyl halide by using a large excess of ammonia to avoid / minimize multiply alkylations on nitrogen. Gabriel synthesis: This synthetic method allows for the selective preparation of primary amines with out the use of a large excess of amine. The synthesis involved the alkylation of the phthalimide anion, which is simply a protected ammonia that can only be alkylated once. Synthesis of primary Amines (Gabriel Synthesis) excess NH3 R CH2 X X= Cl, Br and I R CH2 NH2 Synthesis from azides and nitriles The azide and nitrile groups can be reduced to primary amines using either LAH or catalytic hydrogenation. The azide ion N3- and cyanide ion CN- are both very good nucleophiles that will react readily with primary and secondary halides. Synthesis from azides and nitriles Reaction of an alkyl halide with azide ion produces an alkyl azide. Reduction of the azide gives corresponding the 1° amine. The main drawback to the synthetic method is that alkyl azides are explosive. The net reaction is the replacement of the halide with NH2. Synthesis from azides and nitriles Reaction of an alkyl halide with cyanide ion produces an a nitrile. Nitriles are very stable and are easily handled. Reduction of the nitrile gives a primary amine that is one carbon (CH2) longer then the initial alkyl halide. CN CH2Br - 1) LAH CH2C N CH2CH2NH2 2) H2O Synthesis from nitro compounds The nitro group can be reduced to the amine by: 1) catalytic hydrogenation 2) active metal and H+ NH2 NO2 H2 CH3 CH3 Ni 90% Hofmann rearrangement The Hofmann rearrangement allows for the preparation of primary amines that have 1°, 2° and 3° alkyl groups, or aryl amines. The reaction involves the treatment of an amide with a halogen under strongly basic conditions. This results in a rearrangement of the amide to an amine in which the carbonyl of the starting amide has been lost. CH3 O C CH3 Cl2 / OH C NH2 H2O - CH3 C NH2 CH3 Hofmann rearrangement Hofmann rearrangement