* Your assessment is very important for improving the workof artificial intelligence, which forms the content of this project
Download cardiovascular drugs and autacoids
Discovery and development of direct thrombin inhibitors wikipedia , lookup
Drug interaction wikipedia , lookup
Discovery and development of integrase inhibitors wikipedia , lookup
Discovery and development of cyclooxygenase 2 inhibitors wikipedia , lookup
Nicotinic agonist wikipedia , lookup
Toxicodynamics wikipedia , lookup
Discovery and development of antiandrogens wikipedia , lookup
Metalloprotease inhibitor wikipedia , lookup
Discovery and development of beta-blockers wikipedia , lookup
Discovery and development of proton pump inhibitors wikipedia , lookup
5-HT3 antagonist wikipedia , lookup
Cannabinoid receptor antagonist wikipedia , lookup
NK1 receptor antagonist wikipedia , lookup
Neuropharmacology wikipedia , lookup
Psychopharmacology wikipedia , lookup
Discovery and development of ACE inhibitors wikipedia , lookup
Neuropsychopharmacology wikipedia , lookup
Discovery and development of angiotensin receptor blockers wikipedia , lookup
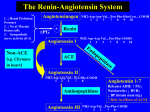
PHL 313 & 315 VASODILATORS Autacoids ALHARBI, Ph.D. Autacoids TEXTBOOK Basic and Clinical Pharmacology 12th edition Author: Katzung, B. Publisher: Lang Medical Books. GENERAL PROPERTIES OF VASODILATORS 1- Vasodilator drugs can be classified based on their site of action (arterial versus venous) 3- Some drugs primarily dilate resistance vessels (arterial dilators; e.g., hydralazine) 4- while others primarily affect venous capacitance vessels (venous dilators; e.g., nitroglycerine). 5- Most vasodilator drugs have mixed arterial and venous dilator properties (mixed dilators; e.g., alphaadrenoceptor antagonists, angiotensin converting enzyme inhibitors). Therapeutic Uses of VASODILATORS 1- Hypertension 2- Congestive heart failure 3- Coronary artery insufficiency 4- Hemostasis Slow bleeding into surgical field 5- Impotence Increased erectile function 6- Peripheral vascular disease Common Side-Effects of Vasodilators 1- Systemic vasodilation and arterial pressure reduction can lead to a baroreceptormediated reflex stimulation of the heart LEADING TO 2- TACHYCARDIA This increases oxygen demand, which is undesirable if the patient also has coronary artery disease. Common Side-Effects of Vasodilators 3- OROTHSTATIC HYPOTENSION Vasodilators can impair normal baroreceptor-mediated reflex vasoconstriction when a person stands up, which can lead to orthostatic hypotension and syncope upon standing. 4- RENAL RETENTION Of SODIUM AND WATER Vasodilators can lead to renal retention of sodium and water, which increases blood volume and cardiac output and thereby compensates for the reduced systemic vascular resistance. Common Side-Effects of Vasodilators 5- HEADACHE Due to Vasodilation of cerebral blood vessel 6- Cutaneous Flushing Due to vasodilation of skin blood vessels. MAJOR DRUG CLASSES OF VASODILATORS 1- Nitrodilators 2- potassium-channel openers 3- direct acting vasodilators (multiple actions) only Hydralazine 4- calcium channel blockers (CCBs) 5- Endothelin receptor antagonist 6- Renin inhibitors 7- Angiotensin converting enzyme inhibitors (ACE-I) 8- Angiotensin-ii receptor blockers (ARBs) 9- Phosphodiestrase-type 5 inhibitors 10- Activation of dopamine receptors (D1-receptors) - Fenoldopam 1- Nitrodilators There are two basic types of nitrodilators: 1- Release NO spontaneously (e.g., sodium nitroprusside) 2- organic nitrates that require an enzymatic process to form NO. - Organic nitrates do not directly release NO - nitrate groups interact with enzymes and intracellular sulfhydryl groups that reduce the nitrate groups to NO or to S-nitrosothiol, - which then is reduced to NO. - Nitric oxide activates smooth muscle soluble guanylyl cyclase (GC) to form cGMP. - Increased intracellular cGMP inhibits calcium entry into the cell, thereby decreasing intracellular calcium concentrations and causing smooth muscle relaxation 1- Examples of Nitrodilators Nitroglycerin Isosorbide dinitrate (Isordil, Sorbitrate, Dilatrate) Isosorbide Mononitrate Sodium nitroprusside (Nitropress) Hydralazine (Apresoline) (actually its action is not through nitric oxide) ORGANIC NITRATES 1- These agents are prodrugs that are sources of nitric oxide (NO). 2- NO activates the soluble isoform of guanylyl cyclase, thereby increasing intracellular levels of cyclic GMP. 3- In turn, cyclic GMP promotes the dephosphorylation of the myosin light chain and the reduction of cystolic Ca2+ and leads to the relaxation of smooth muscle cells in a broad range of tissues. NONPROPRIETARY NAMES AND TRADE NAMES Nitroglycerin (glyceryl trinitrate; NITRO-BID, NITROSTAT, NITROL, NITRO-DUR, others) CHEMICAL STRUCTURE PREPARATIONS, USUAL DOSES, AND ROUTES OF ADMINISTRATIONa T: 0.3-0.6 mg as needed S: 0.4 mg per spray as needed C: 2.5-9 mg 2-4 times daily B: 1 mg every 3-5 h O: 2.5-5 cm, topically to skin every 4-8 h D: 1 disc (2.5-15 mg) for 12-16 h per day IV: 10-20 g/min; increments of 10 g/min to a maximum of 400 Isosorbide dinitrate (ISORDIL, SORBITRATE, DILATRATESR, others) Isosorbide-5-mononitrate (IMDUR, ISMO, others) g/min T: 2.5-10 mg every 2-3 h T(C): 5-10 mg every 2-3 h T(O): 5-40 mg every 8 h C: 40-80 mg every 12 h T: 10-40 mg twice daily C: 60-120 mg daily Cardiovascular Effects 1- Low concentrations of nitroglycerin preferentially dilate veins more than arterioles. 2- This venodilation decreases venous return, leading to a fall in left and right ventricular chamber size and enddiastolic pressures 3- Systemic arterial pressure may fall slightly, and heart rate is unchanged or may increase slightly in response to a decrease in blood pressure. 4- Doses of nitroglycerin that do not alter systemic arterial pressure may still produce arteriolar dilation in the face and neck, resulting in a facial flush, or dilation of meningeal arterial vessels, causing headache. Cardiovascular Effects 1- Higher doses of organic nitrates cause further venous pooling and may decrease arteriolar resistance as well 2- thereby decreasing systolic and diastolic blood pressure and cardiac output and causing pallor, weakness, dizziness, and activation of compensatory sympathetic reflexes. 3- The reflex tachycardia and peripheral arteriolar vasoconstriction tend to restore systemic vascular resistance; this is superimposed on sustained venous pooling. 4- Coronary blood flow may increase transiently as a result of coronary vasodilation but may decrease subsequently if cardiac output and blood pressure decrease sufficiently. 5- In diabetic patient with neuropathy, nitrates may reduce arterial pressure and coronary perfusion pressure significantly, producing potentially lifethreatening hypotension and even aggravating angina. Cardiovascular Effects organic nitrates cause dilation and prevent vasoconstriction of large epicardial vessels without impairing autoregulation in the small vessels, which are responsible for ~90% of the overall coronary vascular resistance. sublingual nitroglycerin can dilate epicardial stenoses and reduce the resistance to flow In patients with angina owing to coronary artery spasm, the ability of organic nitrates to dilate epicardial coronary arteries, and particularly regions affected by spasm, may be the primary mechanism by which they are of benefit. Cardiovascular Effects Mechanism of Relief of Symptoms of Angina Pectoris The ability of nitrates to dilate epicardial coronary arteries, even in areas of atherosclerotic stenosis, is modest The main action is reduction in myocardial work leading to decrease in myocardial O2 demand, as their primary effect in chronic stable angina. This accomplish by decrease preload Tolerance 1- repeated or continuous exposure to high doses of organic nitrates leads to a marked attenuation in the magnitude of most of their pharmacological effects. 2- The magnitude of tolerance is a function of dosage and frequency of use. 3- Tolerance may result from a reduced capacity of the vascular smooth muscle to convert nitroglycerin to NO, 4- Multiple mechanisms have been proposed to account for nitrate tolerance, including volume expansion, neurohumoral activation, cellular depletion of sulfhydryl groups, and the generation of free radicals Tolerance 5- associated with oxidative stress that is why hydralazine (anti-oxidant) decreases organic nitrate tolerance 6- A more effective approach to restoring responsiveness is to interrupt therapy for 8-12 hours each day, which allows the return of efficacy. It is usually most convenient to omit dosing at night in patients with exertional angina either by adjusting dosing intervals of oral or buccal preparations or by removing cutaneous nitroglycerin 7- Coronary and digital arteriospasm during withdrawal and its relaxation by nitroglycerin also have been demonstrated radiographically. Because of the potential problem of nitrate dependence, it seems prudent not to withdraw nitrates abruptly from a patient who has received such therapy chronically. Interaction of Nitrates with PDE5 Inhibitors 1- Erectile dysfunction is a frequently encountered problem whose risk factors parallel those of coronary artery disease. 2- Thus many men desiring therapy for erectile dysfunction already may be receiving (or may require, especially if they increase physical activity) anti-anginal therapy. 3- The combination of sildenafil and other phosphodiesterase 5 (PDE5) inhibitors with organic nitrate vasodilators can cause extreme hypotension. Therapeutic Uses 1- ANGINA 2- CONGESTIVE HEART FAILURE 3- UNSTABLE ANGINA PECTORIS AND NON-ST-SEGMENT– ELEVATION MYOCARDIAL INFARCTION Should include antiplatelets 4- ACUTE MYOCARDIAL INFARCTION 5- VARIANT (PRINZMETAL) ANGINA 6- Diffuse Esophageal Spasm In a limited number of patients with diffuse esophageal spasm without gastroesophageal reflux†, isosorbide dinitrate has been used effectively to relieve pain, dysphagia, and spasm. Drug Uses Nitroglyce Angina pectoris rin and (coronary artery nitrates disease), CHF, Vessels Affected Mechanism Venous Direct effect, conversion to NO, increase cGMP Sodium nitroprusside Arteriolar Direct effect, conversion to Hypertensive and emergencies, acute NO,* increase cGMP venous CHF Hydralazine Hypertension, CHF Arteriolar Direct effect, partially EDRF-dependent formation of NO,* increase cGMP; possible K (with nitrate) channel agonist; inhibition of inositol triphosphate-induced Ca++ release + Hydralazine Hydralazine dilates arterioles but not veins. Hydralazine may be used more effectively, particularly in severe hypertension. The combination of heart failure Hydralazine with nitrates is effective in and should be considered in patients with both hypertension and heart failure, especially in African-American patients Hydralazine Hydralazine has been shown to prevent tolerance toward organic nitrate This is due to antioxidant properties hydralazine is a highly potent radical scavenger. Thus, the combination with isosorbide dinitrate (ISDN) will favorably influence the nitroso-redox balance in the cardiovascular system in patients with congestive heart failure Thus explain at least in part the improvement of prognosis in patients with chronic congestive heart failure. Adverse Effects Hydralazine -The most common adverse effects of hydralazine are: -In patients with ischemic heart disease, reflex tachycardia and sympathetic stimulation may provoke angina or ischemic arrhythmias. dizziness, drowsiness, headache. CV: tachycardia, angina, arrhythmias, edema, orthostatic hypotension. sodium retention. drug-induced lupus syndrome. -. Minoxidil 1- Minoxidil is a very efficacious orally active vasodilator. 2- The effect results from the opening of potassium channels in smooth muscle membranes by minoxidil sulfate, the active metabolite. 3- Increased potassium permeability stabilizes the membrane at its resting potential and makes contraction less likely. 4- Like hydralazine, minoxidil dilates arterioles but not veins. Because of its greater potential antihypertensive effect, minoxidil should replace hydralazine when maximal doses of the latter are not effective or in patients with renal failure and severe hypertension, USE IN PATIENTS who do not respond well to hydralazine . Minoxidil ADVESRSE EFFECTS: 1- Tachycardia, palpitations, angina, and edema are observed when doses of CARDIAC DEPRESSANTS blockers and diuretics are inadequate. 2- Headache, sweating WHY ? 3- Hypertrichosis (GROWTH OF HAIR), which is particularly bothersome in women, are relatively common. 4- Minoxidil illustrates how one person's toxicity may become another person's therapy. Topical minoxidil (as Rogaine) is used as a stimulant to hair growth for correction of baldness. Sodium Nitroprusside 1- Sodium nitroprusside is a powerful parenterally administered vasodilator 2- It is used in treating hypertensive emergencies as well as severe heart failure. 3- Nitroprusside dilates both arterial and venous vessels, resulting in reduced peripheral vascular resistance and venous return. 4- The action occurs as a result of activation of guanylyl cyclase, either via release of nitric oxide or by direct stimulation of the enzyme. 5- The result is increased intracellular cGMP, which relaxes vascular smooth muscle. Sodium Nitroprusside 1- Nitroprusside is a complex of iron, cyanide groups, and a nitroso moiety. 2- It is rapidly metabolized by uptake into red blood cells with liberation of cyanide. 3- Cyanide in turn is metabolized by the mitochondrial enzyme rhodanase, in the presence of a sulfur donor, to the less toxic thiocyanate. 4- Thiocyanate is distributed in extracellular fluid and slowly eliminated by the kidney. Sodium Nitroprusside ADVERSE EFFECTS: 1- the most serious toxicity is related to accumulation of cyanide 2- metabolic acidosis 3- arrhythmias 4- excessive hypotension 2-. Methemoglobinemia during infusion of nitroprusside has also been reported. Diazoxide 1- Diazoxide is an effective and relatively long-acting parenterally administered arteriolar dilator 2- Diminishing usage suggests that it may be withdrawn. 3- Injection of diazoxide results in a rapid fall in systemic vascular resistance and mean arterial blood pressure. 4- its mechanism suggest that it prevents vascular smooth muscle contraction by opening potassium channels and stabilizing the membrane potential at the resting level. Use Hypoglycemia related to islet cell adenoma, carcinoma, hyperplasia, or adenomatosis; nesidioblastosis; leucine sensitivity; extrapancreatic malignancy Diazoxide ADVERSE EFFECTS: 1- hypotension 2- The reflex sympathetic response can provoke angina 2- in patients with ischemic heart disease, and diazoxide should be avoided in this situation. 3- Diazoxide inhibits insulin release from the pancreas (probably by opening potassium channels in the beta cell membrane) and is used to treat hypoglycemia secondary to insulinoma Fenoldopam 1- Fenoldopam is a peripheral arteriolar dilator used for hypertensive emergencies And postoperative hypertension with renal compromised function. 2- Mechanism of Action A selective postsynaptic dopamine agonist (D1-receptors) which exerts hypotensive effects by decreasing peripheral vasculature resistance with increased renal blood flow leading to diuresis, and natriuresis 3- It is 6 times as potent as dopamine in producing renal vasodilatation and has minimal adrenergic effects 4- Fenoldopam is administered by continuous intravenous infusion. 5-As with other direct vasodilators, the major toxicities are reflex tachycardia, headache, and flushing. 5- Fenoldopam also increases intraocular pressure and should be avoided in patients with glaucoma. Inhibitors of Angiotensin Mechanism & Sites of Action 1- Renin release from the kidney cortex is stimulated by: a- reduced renal arterial pressure b- sympathetic neural stimulation c- reduced sodium delivery d- increased sodium concentration at the distal renal tubule * Renin acts upon angiotensinogen to split off the inactive precursor decapeptide angiotensin I. * Angiotensin I is then converted, primarily by endothelial ACE, to the arterial vasoconstrictor octapeptide angiotensin II Inhibitors of Angiotensin Mechanism & Sites of Action * Angiotensin-ii is in turn converted in the adrenal gland to angiotensin III. * Angiotensin II has vasoconstrictor and sodium-retaining activity. * Angiotensin II and III both stimulate aldosterone release * Angiotensin may contribute to maintaining high vascular resistance in hypertensive states associated with high plasma renin activity, * renal arterial stenosis has high renin level - Angiotensin system also induce cardiac hypertrophy Inhibitors of Angiotensin Action Mechanism & Sites of Inhibitors of Angiotensin Action Mechanism & Sites of . The converting enzyme involved in tissue angiotensin II synthesis is also inhibited by ACE inhibitors. Three classes of drugs act specifically on the reninangiotensin system: 1- ACE inhibitors 2- the competitive inhibitors of angiotensin at its receptors (antagonist), including losartan 3- aliskiren, an orally active renin antagonist 4- aldosterone receptor inhibitors (eg, spironolactone, eplerenone) Angiotensin-Converting Enzyme (ACE) Inhibitors 1- Captopril - drugs in this class inhibit the converting enzyme peptidyl dipeptidase that hydrolyzes angiotensin I to angiotensin II - (under the name plasma kininase) inactivates bradykinin, a potent vasodilator, which works by stimulating release of nitric oxide and prostacyclin The hypotensive activity of captopril results both from an inhibitory action on the reninangiotensin system and a stimulating action on the kallikrein-kinin system The latter mechanism has been demonstrated by showing that a bradykinin receptor antagonist blunts the blood pressure-lowering effect of captopril 2- Enalapril is an oral prodrug that is converted by hydrolysis to a converting enzyme inhibitor, enalaprilat, with effects similar to those of captopril. Enalaprilat itself is available only for intravenous use, primarily for hypertensive emergencies. 4- Benazepril, fosinopril, moexipril, perindopril, quinapril, ramipril, and trandolapril are other long-acting members of the class. All are prodrugs, like enalapril, and are converted to the active agents by hydrolysis, primarily in the liver. 5- Angiotensin II inhibitors lower blood pressure principally by decreasing peripheral vascular resistance. Cardiac output and heart rate are not significantly changed. 6- Unlike direct vasodilators, these agents do not result in reflex sympathetic activation and can be used safely in persons with ischemic heart disease. 7- The absence of reflex tachycardia may be due to downward resetting of the baroreceptors or to enhanced parasympathetic activity. Angiotensin-Converting Enzyme (ACE) Inhibitors 8- ACE inhibitors have a particularly useful role in treating patients with chronic kidney disease because they diminish proteinuria and stabilize renal function (even in the absence of lowering of blood pressure). 9- This effect is particularly valuable in diabetes, and these drugs are now recommended in diabetes even in the absence of hypertension. 10- These benefits probably result from improved intrarenal hemodynamics, with decreased glomerular efferent arteriolar resistance and a resulting reduction of intraglomerular capillary pressure. 10 - ACE inhibitors have also proved to be extremely useful in the treatment of heart failure, and after myocardial infarction, and there is recent evidence that ACE inhibitors reduce the incidence of diabetes in patients with high cardiovascular risk Adverse effects of angiotensin-Converting Enzyme (ACE) Inhibitors 1- Severe hypotension can occur after initial doses of any ACE inhibitor in patients who are hypovolemic as a result of diuretics, salt restriction, or gastrointestinal fluid loss. 2- Other adverse effects common to all ACE inhibitors include acute renal failure (particularly in patients with bilateral renal artery stenosis or stenosis of the renal artery of a solitary kidney) 3- hyperkalemia Hyperkalemia is more likely to occur in patients with renal insufficiency or diabetes 4- dry cough sometimes accompanied by wheezing 5- Angioedema.. Bradykinin and substance P seem to be responsible for the cough and angioedema seen with ACE inhibition. 6- ACE inhibitors are contraindicated during the second and third trimesters of pregnancy because of the risk of fetal hypotension, anuria, and renal failure, sometimes associated with fetal malformations or death. Adverse effects of angiotensin-Converting Enzyme (ACE) Inhibitors 7- recent evidence also implicates first-trimester exposure to ACE inhibitors in increased teratogenic risk. 8- Captopril, particularly when given in high doses to patients with renal insufficiency, may cause neutropenia or proteinuria. 9- Minor toxic effects seen more typically include altered sense of taste 10- allergic skin rashes, and drug fever, which may occur in up to 10% of patients. 11- Important drug interactions include those with potassium supplements or potassium-sparing diuretics, which can result in hyperkalemia. 12- Nonsteroidal anti-inflammatory drugs may impair the hypotensive effects of ACE inhibitors by blocking bradykinin-mediated vasodilation, which is at least in part, prostaglandin mediated. ANGIOTENSIN II RECEPTOR ANTAGONISTS ANGIOTENSIN II RECEPTOR ANTAGONISTS 1- Candesartan 2- olmesartan 3- irbesartan 4- eprosartan 5- telmisartan 6- valsartan (the active metabolite of losartan) 7- losartan. ANGIOTENSIN II RECEPTOR ANTAGONISTS The AngII receptor blockers bind to the AT1 receptor with • high affinity and are more than 10,000-fold selective for the AT1 receptor over the AT2 receptor. The rank-order affinity of the AT1 receptor for ARBs is: * candesartan = olmesartan > irbesartan = eprosartan > telmisartan = valsartan = • (the active metabolite of losartan) > losartan. • ANGIOTENSIN II RECEPTOR ANTAGONISTS * Do ARBs have therapeutic efficacy equivalent to that of ACE inhibitors? Although both classes of drugs block the RAS they differ in several important aspects: •ARBs reduce activation of AT1 receptors more effectively than do ACE inhibitors. • ACE inhibitors reduce the biosynthesis of AngII by the action of ACE, but do not inhibit alternative non-ACE AngII-generating pathways. •ARBs block the actions of AngII via the AT1 receptor regardless of the biochemical pathway leading to AngII formation. ANGIOTENSIN II RECEPTOR ANTAGONISTS •In contrast to ACE inhibitors, ARBs permit activation of AT2 receptors. • Because ARBs block AT1 receptors, this increased level of AngII is available to activate AT2 receptors. •ACE inhibitors increase the levels of a number of ACE substrates, including bradykinin and Ac-SDKP. Whether the pharmacological differences between ARBs and ACE inhibitors result in significant differences in therapeutic outcomes is an open question. ANGIOTENSIN II RECEPTOR ANTAGONISTS Candesartan Cilexetil (Atacand) Candesartan cilexetil is an inactive ester prodrug that is completely hydrolyzed to the active form, candesartan, The plasma clearance of candesartan is affected by renal insufficiency but not by mild-to-moderate hepatic insufficiency. Candesartan cilexetil should be administered orally once or twice daily for a total daily dose of 432 mg. Eprosartan (Teveten) . Clearance is by renal elimination and biliary excretion. The plasma clearance of eprosartan is affected by both renal insufficiency and hepatic insufficiency. Irbesartan (Avapro) . cleared by renal elimination (20%) and biliary excretion (80%). The plasma clearance of irbesartan is unaffected by either renal or mild-to-moderate hepatic insufficiency. The oral dosage of irbesartan is 150-300 mg once daily. . Losartan (Cozaar) * Losartan is converted to the 5-carboxylic acid metabolite EXP 3174, which is more potent than losartan as an AT1 receptor antagonist. The plasma clearances of losartan and EXP 3174 are due to renal clearance and hepatic clearance (metabolism and biliary excretion). The plasma clearance of losartan and EXP 3174 is affected by hepatic but not renal insufficiency Losartan should be administered orally once or twice daily for a total daily dose of 25-100 mg. In addition to being an ARB, losartan is a competitive antagonist of the thromboxane A2 receptor and attenuates platelet aggregation . Olmesartan Medoxomil (Benicar) Olmesartan medoxomil is an inactive ester prodrug that is • completely hydrolyzed to the active form, olmesartan * Plasma clearance of olmesartan is due to both renal • elimination and biliary excretion. Although renal impairment and hepatic disease decrease the • plasma clearance of olmesartan * no dose adjustment is required in patients with mild-to-• moderate renal or hepatic impairment. The oral dosage of olmesartan medoxomil is 20-40 mg once daily.• Telmisartan (Micardis) Telmisartan is cleared from the circulation mainly • by biliary secretion of intact drug. • The plasma clearance of telmisartan is affected by hepatic but not renal insufficiency. The recommended oral dosage of telmisartan is 4080 mg once daily. Valsartan (Diovan) Valsartan is cleared from the circulation by the liver (~70% of total clearance). The plasma clearance of valsartan is affected by hepatic but not renal insufficiency. The oral dosage of valsartan is 80-320 mg once daily. . Therapeutic Uses of AngII Receptor Antagonists 1- All ARBs are approved for the treatment of hypertension. 2- In addition, irbesartan and losartan are approved for diabetic nephropathy 3- losartan is approved for stroke prophylaxis 4- and valsartan is approved for heart failure and to reduce cardiovascular mortality in clinically stable patients with left ventricular failure or left ventricular dysfunction following myocardial infarction. 5- The efficacy of ARBs in lowering blood pressure is comparable with that of ACE inhibitors and other established antihypertensive drugs, with a favorable adverse-effect profile.. Current recommendations are to use ACE inhibitors as first-line agents for the treatment of heart failure and to reserve ARBs for treatment of heart failure in patients who cannot tolerate or have an unsatisfactory response to ACE inhibitors. ARBs are renoprotective in type 2 diabetes mellitus, in part via blood pressure– independent mechanisms Based on these results, many experts now consider them the drugs of choice for renoprotection in diabetic patients. Losartan is superiority of an ARB compared with alpha 1 adrenergic receptor antagonist with regard to reducing stroke in hypertensive patients with left ventricular hypertrophy). irebesartan appears to maintain sinus rhythm in patients with persistent, longstanding atrial fibrillation. Losartan is reported to be safe and highly effective in the treatment of portal hypertension in patients withcirrhosis and portal hypertension without compromising renal function... Adverse Effects of ARBs 1- The incidence of angioedema and cough with ARBs is less than that with ACE inhibitors. 2- ARBs have teratogenic potential and should be discontinued in pregnancy. 3- In patients whose arterial blood pressure or renal function is highly dependent on the RAS (e.g., renal artery stenosis), ARBs can cause hypotension, oliguria, progressive azotemia, or acute renal failure. 4- ARBs may cause hyperkalemia in patients with renal disease or in patients taking K+ supplements or K+-sparing diuretics. 5- There are rare postmarketing reports of anaphylaxis, abnormal hepatic function, hepatitis, neutropenia, leukopenia, agranulocytosis, pruritus, urticaria, hyponatremia, alopecia, and vasculitis, including Henoch-Schönlein purpura. DIRECT RENIN INHIBITORS DRIs are a novel class of antihypertensive drugs that inhibit the RAS at its origin. Angiotensinogen is the only specific substrate for renin, and its conversion to AngI presents a rate-limiting step for the generation of downstream components of the RAS. Aliskiren (TEKTURNA) is the only DRI approved for clinical use. Aliskiren, a second-generation renin inhibitor that was approved by the U.S. FDA in 2007 for the treatment of hypertension. Aliskiren has blood pressure–lowering effects similar to those of ACE inhibitors and ARBs. DIRECT RENIN INHIBITORS . Aliskiren Aliskiren is a potent competitive inhibitor of renin. It binds the active site of renin to block conversion of angiotensinogen to AngI, thus reducing the consequent production of AngII. In healthy volunteers, aliskiren (40-640 mg/day) induces a dosedependent decrease in blood pressure, aliskiren is similar to the high dose of valsartan in lowering blood pressure, reducing albuminuria, normalizing serum creatinine, and protecting against end-organ damage Therapeutic Uses of Aliskiren 1- Aliskiren is an effective antihypertensive agent that is well tolerated in monotherapy and combination therapy. 2- It has cardioprotective and renoprotective effects in combination therapy 3- Aliskiren is recommended in patients who are intolerant to other antihypertensive therapies or for use in combination with other drugs for further blood pressure control. Adverse Aliskiren 1- hyperkalemia in diabetics on combination therapy, 2- Angioedem. 3- Like other RAS inhibitors, aliskiren is not recommended in pregnancy. CA2+ CHANNEL ANTAGONISTS DRUGS VASODILATION (CORONARY FLOW) SUPPRESSION OF CARDIAC CONTRACTILITY SUPPRESSION OF AUTOMATICITY (SA NODE) SUPPRESSION OF CONDUCTION (AV NODE) Amlodipine 5 1 1 0 felodipine 5 1 1 0 Isradipine NR NR NR NR Nicardipine 5 0 1 0 Nifedipine 5 1 1 0 Diltiazem 3 2 5 4 Verapamil 4 4 5 5 Clevidipine (I.V. Only) DRUGS USED IN DIASTOLIC HEART FAILURE 1- BETA BLOCKER 2- CALCIUM CHANNEL BLOCKER 3- ACE INHIBITORS OR ARB 4- Ranolazine a novel anti anginal drug known to inhibit late sodium current (INaL) Ranolazine Prevents Heart Failure with Preserved Ejection Fractionuggested that Ranolazine (RAN), a novel anti anginal drug known to inhibit late sodium current (INaL) may improve diastolic dysfunction (DD) caused by HTN induced oxidative stress. By improving diastolic dysfunction RAN can improve Heart Failure with Preserved Ejection Fraction (HFPEF). Methods: A mouse model DRUGS USED IN HEART FAILURE Commonly Used Agent(s) Group Class HCTZ Thiazide diuretics Diuretics Furosemide Loop diuretics Spironolactone Potassium sparing diuretics Digoxin Digitalis preparations Dobutamine Hydralazine Inotropic drugs Vasodilators Isosorbide Enalapril Cardiac glycosides ACE inhibitors Captopril Angiotensin II receptor blockers Losartan Angiotensin II receptor blockers Vasoactive drugs DRUGS USED IN HEART FAILURE Commonly Used Agent(s) Metoprolol CR/XL Carvedilol Amiodarone Dofetilide Spironolactone Eplerenone Nesiritide Tolvaptan Group Class -Blockers Antiarrhythmic drugs Aldosterone receptor antagonists Natriuretic peptides Vasopressin receptor antagonists Autacoids TOPICS WILL BE COVERED 1- Pharmacology of Histamine and its analogues 2- Pharmacology of Antihistamines(H1 &H2) & mast cell sabilizers 3- Pharmacology of The Eicosanoids:Products of Cyclooxygenases - Prostaglandins, Thromboxanes, & Related Compounds - Analogues of Cyclooxygenases Products and their inhibitors Second Assessment Test 4- Autacoids: Pharmacology of The Eicosanoids:Lipoxygenase products and their blockers 5- Pharmacology of Serotonin, agonists and antagonists & the Ergot AlkaloidsAutacoids 6- Pharmacology of Nitric oxide and Vasoactive Peptides and their blockers such as endothelins, angiotensin, neurotensin, substance P. AUTACOIDS Introduction • Means self remedy • Naturally occurring substances • Localized in tissues • Do not normally circulate • Diverse physiological and pharmacological activities • Differ from hormones and neurotransmitters • Short duration of action • Usually involved in a response to injury • Sites of action restricted to the synthesis area CLASSIFICATION 1. Biogenic amines: Histamine, 5-hydroxytryptamine 2. Biogenic Peptides: Angiotensin and kinins 3. Small Proteins: cytokineins 4. Membrane derived lipids: LTs, PGs, TxA2 & PAF 5. Endothelium-derived agents:NO (gas); ET (peptide) Histamine 1- Synthesis 2- Histamine Storage - mast cell 3- DISTRIBUTION OF HISTAMINE 4- Metabolism Histamine • The more important of these involves ring methylation to form Nmethylhistamine It increases mastocytosis Release Histamine unexpected anaphylactoid reactions As a result of the interaction of antigen with immunoglobulin E (IgE) antibodies on the mast cell surface • Histamine Release by Drugs, Peptides, Venoms, and Other Agents. • These drugs stimulate the release of histamine from mast cells directly and without prior sensitization. Examples of Agents Release Histamine 1-amides, amidines, quaternary ammonium compounds, pyridinium compounds, piperidines, and alkaloids (Tubocurarine, succinylcholine, morphine 2- radiocontrast media 3- certain carbohydrate elicit the response. plasma expanders also may Release Histamine 4- some antibiotics, Vancomycin-induced "red-man syndrome" involving upper body and facial flushing and hypotension may be mediated through histamine release. Beta-lactam antibiotics such amoxil as well as cephalosporin Polymyxin B is also very active. 5- food PHARMACOLOGY OF HISTAMINE Histamine receptors 1- H1 receptor - Smooth muscle - brain 2- H2 receptor - Gastric mucosa 3- H3 receptor - Presynaptic: brain, myenteric plexus 4- H4 receptor - Eosinophils, neutrophils, CD4 T cells 1- Central Nervous System • Histamine increases wakefulness via H1 receptors • explaining the potential for sedation by classical antihistamines • Histamine is a powerful stimulant of sensory nerve endings, especially those mediating pain and itching via H1 Gastric Acid Secretion • Acting at H2 receptors, histamine is a powerful gastric secretagogue • and evokes a copious secretion of acid from parietal CELL • it also increases the output of pepsin and intrinsic factor. IMMUNE SYSTEM H4 receptors are on immune active cells such as eosinophils and neutrophils Activation of H4 receptors on eosinophils induces: - H4 antagonists may be useful inhibitors of allergic and inflammatory responses IMMUNE SYSTEM - suggesting that the histamine released from mast cells acts at H4 receptors to recruit eosinophils. - H4 antagonists may be useful inhibitors of allergic and inflammatory responses Histamine-induces Vasodilation Vasodilation involves both H1 and H2 receptors distributed throughout the resistance vessels in most vascular beds 1- Activation H1 receptors Medaites its action via endothelium-NO-dependent dilation that is relatively rapid in onset and short-lived. Increase calcium which lead to release Nitric oxide . Histamine-induces Vasodilation 2- activation of H2 receptors H2 receptors couple via GS to the activation of adenylyl cyclase - (stimulating the cyclic AMP-PKA pathway in smooth muscle) - causes dilation that develops more slowly and is more sustained Histamine-induces Vasodilation Increased "Capillary" Permeability • Causes edema formation. • H1 receptors on endothelial cells are the major mediators of this response • Role of H2 receptors is uncertain. Triple Response of Lewis it elicits a characteristic phenomenon known as the triple response (Lewis, 1927). (1) a localized red spot - The initial red spot results from the direct vasodilating effect of histamine (H1-receptor-mediated NO production) extending for a few millimeters around the site of injection that appears within a few seconds and reaches a maximum in about a minute Triple Response of Lewis 2) a brighter red flush, or "flare," - the flare is due to histamine-induced stimulation of axon reflexes - that cause vasodilation indirectly - extending about 1 cm or so beyond the original red spot - and developing more slowly Triple Response of Lewis (3) a wheal that is discernible in 1 to 2 minutes - and occupies the same area as the original small red spot at the injection site. - the wheal reflects histamine's capacity to increase capillary permeability (edema formation). CLINICAL USES OF HISTAMINE HISTAMINE ANALOQUE • BetaSERC® 16 tablets COMPOSITION: • Each tablet contains betahistine dihydrochloride 16 mg. CLINICAL USES OF HISTAMINE • SERC® 16 tablets PHARMACOLOGICAL ACTION: Betahistine The mechanism of action: • • Pharmacological testing in animals has shown that: - the blood circulation in the striae vascularis of the inner ear improves • - probably by means of a relaxation of the precapillary sphincters of the microcirculation of the inner ear. CLINICAL USES OF HISTAMINE • SERC® 16 tablets In pharmacological studies, betahistine was found to have weak H1 receptor agonistic • - and considerable H3 antagonistic properties in the CNS and autonomic nervous system. • - Betahistine was also found to have a dose dependent inhibiting effect on spike generation of neurons in lateral and medial vestibular nuclei. • The importance of this observation in the action against Ménière’s syndrome or vestibular vertigo, however, remains unclear. CLINICAL USES OF HISTAMINE • SERC® 16 tablets INDICATIONS: Symptomatic treatment of the vertigo associated with Ménière’s Syndrome. CONTRA-INDICATIONS: - Patients with active peptic ulcer. - Patients with phaeochromocytoma. DOSAGE AND DIRECTIONS FOR USE: Adult dosage The usual initial dose is 8 to 16 mg three times daily to be taken preferably with meals. Maintenance doses of up to 48 mg daily have been recommended. CLINICAL USES OF HISTAMINE • SERC® 16 tablets SIDE-EFFECTS AND SPECIAL PRECAUTIONS: Gastro-intestinal disturbances, headache and skin rashes have been reported. • Special precautions Caution should be exercised when betahistine dihydrochloride is given to patients with a history of peptic ulcer or asthmatic patients. Concomitant use with antihistamines should be avoided. ANTIHISTAMINES H1-RECEPTOR BLOCKERS FIRST GENERATION ANTIHISTAMINES (H1 BLOCKERS)SEDATING 1- Ethanolamines - Carbinoxamine maleate - Clemastine fumarate - Diphenhydramine HCl - Dimenhydrinate (DIZINIL)-ANTIMOTION SICKNESS) 2- Ethylenediamines - Pyrilamine maleate - Tripelennamine HCl - Tripelennamine citrate 3- Alkylamines - Chlorpheniramine maleate - Brompheniramine maleate 4- Piperazines - Hydroxyzine HCl - Hydroxyzine pamoate - Cyclizine HCl - Cyclizine lactate - Meclizine HCl 5- Phenothiazines - Promethazine HCl 6- Piperidines - Cyproheptadine HCl(periactin) - Phenindamine tartrate 7-OTHERS - ketotifen and olopatadine - effective in allergic conjunctivitis and rhinitis. Nasal sprays or topical ophthalmic preparations Second-Generation H1 blockers (non-sedating H1 blockers) 1- Alkylamines - Acrivastine 2- Piperazines - Cetirizine hydrochloride(ZYRTEC) 3- Phthalazinones - Azelastine hydrochloride 4- Piperidines - Levocabastine hydrochloride - Loratadine - Desloratadine - Ebastine - Mizolastine - Fexofenadine PHARMACOLOGY OF ANT-IHISTAMINES(H1) 1- Competitive antagonism for the same receptor 2- do not prevent histamine release 3- do not cause physiological antagonism (smooth muscle dilatation) - NOT SUITABLE FOR ACUTE BRONCHIAL ASTHMA Do not block gastric acid production (blocked by H2) PHARMACOLOGY OF ANTIHISTAMINES(H1) 4- Protect against shock and allergic reactions 5- CNS depression, a side effect first generation WHILE SECOND GENERATION DO NOT) 6- ANTICHOLINERGIC ACTIVITY (mainly sedating H1-blockers) the principle of antimotion sickness - dimenhydrinate PHARMACOLOGY OF ANTI-HISTAMINES(H1) 6- Local anesthetic effect suh as promethazine 7- Adrenoceptor-blocking actions such as promethazine 8- Serotonin-blocking action such as cyproheptadine Therapeutic uses of H1 BLOCKERS 1-allergic reactions such as hay fever 2- motion sickness, verigo 3- NAUSEA AND VOMITING OF PREGNANCY 4- sedation 5-common cold Common Adverse Effects of H1 blockers 1- The most frequent side effect in the first-generation H1 antagonists is sedation. - Concurrent ingestion of alcohol or other CNS depressants produces an additive effect that impairs motor skills. 2- dizziness, tinnitus, lassitude, incoordination, fatigue 3- blurred vision, diplopia (due to atropine like action) 4- Tachycardia Common Adverse Effects 5- some H1 antagonists increase appetite such as cyproheptadine due to serotonin blocking activity 6- antimuscarinic actions of some of the first-generation H1receptor antagonists include: - dryness of the mouth and respiratory passages (sometimes inducing cough) - urinary retention or frequency, and dysuria. These effects are not observed with second-generation H1 antagonists Common Adverse Effects OF SECOND GENERATION H1-BLOCKERS 1- Lethal ventricular arrhythmias in early second-generation agents 1- terfenadine 2- or astemizole Common Adverse Effects OF SECOND GENERATION H1-BLOCKERS 3- The mechanism of this toxicity involves blockade of potassium channels in the heart 4- The result is prolongation of the action potential 5- excessive prolongation leads to arrhythmias. 6- Both terfenadine and astemizole were withdrawn from the United States market in recognition of these problems. H1 blockers-Drug Interactions - Drugs inhibit CYP3A4 such as: - ketoconazole - itraconazole - macrolide antibiotics such as erythromycin OR CLARITHROMYCIN. These antimicrobial drugs cause significant increases in blood concentrations of the antihistamines(SECOND GENERATION). H1 blockers-Drug Interactions 7- Terfenadine and astemizole should be considered to be contraindicated in patients taking: - ketoconazole - itraconazole - macrolides - and in patients with liver disease. 8- Grapefruit juice also inhibits CYP3A4 and has been shown to increase terfenadine's blood levels significantly as well as other drugs. MAST CELL STABILIZERS Mechanism of Action inhibits mast cell degranulation CROMOLYN & NEDOCROMIL Cromolyn sodium (disodium cromoglycate) and nedocromil sodium) they effectively inhibit both antigen- and exercise-induced asthma, CROMOLYN & NEDOCROMIL have no effect on 1- airway smooth muscle tone 2- and are ineffective in reversing asthmatic bronchospasm 3-they are only of value when taken prophylactically CLINICAL USE OF CROMOLYN & NEDOCROMIL 1-allergen inhalation, by sulfur dioxide and by a variety of causes of occupational asthma. 2- Cromolyn solution is also useful in reducing symptoms of allergic rhinoconjunctivitis.(EYE DROP) ADVERSE EFFECTS Because the drugs are so poorly absorbed, adverse effects of cromolyn and nedocromil are minor and are localized to the sites of deposition. These include such minor symptoms as: throat irritation, cough, mouth dryness, H2 Antagonists Effect: Block all phases of gastric acid secretion due to histamine. proton pump inhibitors (NEXIUM OR LOSEC, OMEPRAZOLE) are steadily replacing H2 antagonists for most clinical indications. H2 Antagonists 1- Cimetidine 2- Ranitidine 3- Nizatidine 4- Famotidine CLINICAL USES OF H2 ANATGONIST 1- GASTROESOPHAGEAL REFLUX DISEASE (GERD) 2- PEPTIC ULCER DISEASE ranitidine, 150 mg; famotidine, 20 mg 3- PREVENTION OF BLEEDING FROM STRESS-RELATED GASTRITIS ADVERSE EFFECTS OF H2 ANATGONIST 1- CENTRAL NERVOUS SYSTEM - confusion, hallucinations, agitation) given I.V. These events may be more common with cimetidine. - rarely occur in ambulatory patients. If ADVERSE EFFECTS OF H2 ANATGONIST 2- ENDOCRINE EFFECTS - Cimetidine inhibits binding of dihydrotestosterone to androgen receptors - inhibits metabolism of estradiol - and increases serum prolactin levels. When used long-term or in high doses - it may cause gynecomastia - impotence in men and galactorrhea in women. These effects are specific to cimetidine and do not occur with the other H2 antagonists. ADVERSE EFFECTS OF H2 ANATGONIST 3- Increases liver enzymes BUT reversible H2 blockers-Drug Interactions 1- Cimetidine interferes with several important hepatic cytochrome P450 drug metabolism pathways, - including those catalyzed by CYP1A2, CYP2C9, CYP2D6, and CYP3A4 - Half-lives of drugs metabolized by these pathways may be prolonged. SEROTONIN (5-HT) 1- It formed from L-tryptophan 2- In the pineal gland, serotonin serves as a precursor of melatonin, a melanocyte-stimulating hormone. 3- over 90% of the serotonin in the body is found in enterochromaffin cells in the gastrointestinal tract. Physiological role of SEROTONIN (5-HT) 1- Brain serotonergic neurons are involved in numerous diffuse functions such as - mood - sleep - appetite - temperature regulation Physiological role of SEROTONIN (5-HT) 2- the perception of pain 3- the regulation of blood pressure, and vomiting 4- Serotonin also appears to be involved in clinical conditions such as depression, anxiety, and migraine(blood vessels). 5- Serotonergic neurons are also found in the enteric nervous system of the gastrointestinal tract and around carcinoid tumor. PHARMACOLOGY OF SEROTONIN (5-HT) 1- Cardiovascular system * 5-HT2 - Serotonin directly causes the contraction of vascular smooth muscle, mainly through 5-HT2 receptors. - In humans, serotonin is a powerful vasoconstrictor (AGONIST MAY PRECIPITATE ANGINA AND HEART ATTACK ) - Serotonin can also elicit reflex bradycardia by activation of 5-HT3 receptors on chemoreceptor nerve endings. Cardiovascular system - Serotonin also constricts veins venoconstriction with a resulting increased capillary filling appears to be responsible for the flush that is observed following release from a carcinoid tumor. 2- Gastrointestinal tract 5-HT2 Serotonin is a powerful stimulant of gastrointestinal smooth muscle increasing tone and facilitating peristalsis. This action is caused by the direct action of serotonin on 5-HT2 smooth muscle receptors 5-HT4 Activation of 5-HT4 receptors in the enteric nervous system causes increased acetylcholine release increase motility or "prokinetic" effect of partial serotonin agonists such as Tegaserod Overproduction of serotonin in carcinoid tumor is associated with severe diarrhea. 2- Gastrointestinal tract Zelnorm® (tegaserod maleate) INDICATIONS AND USAGE IBS with Constipation Zelnorm® (tegaserod maleate) is indicated for the short-term treatment of women with irritable bowel syndrome (IBS) whose primary bowel symptom is constipation. lished. Chronic Idiopathic Constipation Zelnorm® (tegaserod maleate) is indicated for the treatment of patients less than 65 years of age with chronic idiopathic constipation. 2- Gastrointestinal tract Zelnorm® (tegaserod maleate) Post Marketing Experience Voluntary reports of adverse events occurring with the use of Zelnorm include the following: ischemic Colitis 1- mesenteric ischemia, gangrenous bowel, 2- electrolyte disorders 3- suspected sphincter of Oddi spasm, bile duct stone, cholecystitis with elevated transaminases, 5-HT1D/1B Agonists & Migraine Headache The 5-HT1D/1B agonists (triptans) are used almost exclusively in migraine headache. Migraine in its "classic" form is characterized by an aura of variable duration that may involve nausea, vomiting, and visual scotomas or even hemianopsia and speech abnormalities; followed by a severe throbbing unilateral headache that lasts for a few hours to 1-2 days . "Common" migraine lacks the aura phase, but the headache is similar. Although the symptom pattern varies among patients, the severity of migraine headache justifies vigorous therapy in the great majority of cases. 5-HT1D/1B Agonists Almotriptan Eletriptan Frovatriptan Naratriptan Rizatriptan Sumatriptan Zolmitriptan The efficacy of triptan 5-HT1 agonists in migraine is equal to or greater than that of other acute drug treatments, eg, parenteral, oral, or rectal ergot alkaloids. Most adverse effects are mild and include altered sensations (tingling, warmth, etc), dizziness, muscle weakness, neck pain, for parenteral sumatriptan chest pain has been reported, probably because of the ability of these drugs to cause coronary vasospasm. They are therefore contraindicated in patients with coronary artery disease and in patients with angina. Another disadvantage is the fact that their duration of effect (especially that of almotriptan, sumatriptan, rizatriptan, and zolmitriptan is often shorter than the duration of the headache. zolmitriptan Contraindications Known or suspected ischemic heart disease (e.g., angina pectoris, myocardial infarction, silent ischemia) coronary vasospasm (e.g., Prinzmetal variant anginaother serious underlying cardiovascular disease (e.g., uncontrolled hypertension), or cerebrovascular syndromes (e.g., stroke syndromes, transient ischemic attacks). Treatment within the previous 24 hours with another 5-HT1 receptor agonist or with an ergot alkaloid (e.g., ergotamine, dihydroergotamine, methysergide). Concurrent or recent (within 2 weeks) treatment with a monoamine oxidase-A (MAO-A)(stop here) As a result, several doses may be required during a prolonged migraine attack, but their adverse effects limit the maximum safe daily dosage. Naratriptan and eletriptan are contraindicated in patients with severe hepatic or renal impairment or peripheral vascular syndromes; frovatriptan is contraindicated in patients with peripheral vascular disease zolmitriptan is contraindicated in patients with Wolff-Parkinson-White syndrome SEROTONIN-RECEPTOR ANTAGONISTS 1- Cyproheptadine - 5-HT2-blocker (STIMULATE APPETITE & CARCINOID SYNDROME) - H1-receptor-blocker - causes sedation - antimuscarinic effects - use in carcinoid tumor in GIT - serotonin syndrome 5-HT3 ANTAGONISTS 1- Selective 5-HT3-receptor antagonists have potent antiemetic 2- mediated mainly through central 5-HT3-receptor blockade in the vomiting center and chemoreceptor trigger zone 3- blockade of peripheral 5-HT3 receptors on extrinsic intestinal vagal and spinal afferent nerves. 4- The antiemetic action of these agents is restricted to emesis attributable to vagal stimulation (eg, postoperative) and chemotherapy 5-HT3 ANTAGONISTS 1- ondansetron 2- granisetron 3- dolasetron (prolong QT interval) These three drug used once daily by oral or intravenous routes. . 4- palonosetron. It is a newer intravenous agent that has greater affinity for the 5-HT3 receptor and a long serum half-life of 40 hours. - All of them do not inhibit dopamine or muscarinic receptors - They do not have effects on esophageal or gastric motility Therapeutic uses 5-HT3 ANTAGONISTS 1- CHEMOTHERAPY-INDUCED NAUSEA AND VOMITING • 5-HT3-receptor antagonists prevent of acute chemotherapy-induced nausea and emesis • The drugs are most effective when given as a single dose by intravenous injection 30 minutes prior to administration of chemotherapy • Or oral dose given 1 hour before chemotherapy • When used alone, these drugs have little or no efficacy for the prevention of delayed nausea and vomiting (ie, occurring > 24 hours after chemotherapy • the efficacy is enhanced by combination therapy with a corticosteroid (dexamethasone) and Neurokinin-1 receptor antagonist (Aprepitant ). 2- POSTOPERATIVE AND POSTRADIATION NAUSEA AND VOMITING ADVERSE EFFECTS OF 5-HT3 ANTAGONISTS 1- All three agents cause a small but statistically significant prolongation of the QT interval, but this is most pronounced with dolasetron. it should not be administered to patients with prolonged QT or in conjunction with other medications that may prolong the QT interval. THE ERGOT ALKALOIDS 1- Ergot alkaloids are produced by Claviceps purpurea, a fungus that infects storage conditions. 2- The accidental ingestion of ergot alkaloids in contaminated grain lead to : - dementia with florid hallucinations - prolonged vasospasm, which may result in gangrene - stimulation of uterine smooth muscle, which in pregnancy may result in abortion. 1- Bromocriptine dopamine agonist 2- Ergonovine (uterine contraction) 3- Ergotamine (MIGRAINE) 4- Lysergic acid diethylamide (LSD) dopamine agonist 5- Methysergide 2- Ergonovine (uterine contraction) Ergonovine is more selective than other ergot alkaloids in affecting the uterus and is the agent of choice in obstetric (prevent bleeding after delivery) MECHANISM OF ACTIONS 1- 5-HT2 partial agonist (Vasoconstriction) 2- α1- Adrenoceptor Agonist. (vasoconstriction) In very small doses, ergot preparations can evoke rhythmic contraction and relaxation of the uterus. 3- Ergotamine Ergotamine and related compounds potently constrict blood vessels for prolonged period - USE IN MIGRAINE HEADACHE 4- Lysergic acid diethylamide (LSD) dopamine agonist 5- Methysergide (mixed actions on the previous receptors CLINICAL USES OF ERGOTAMINE 1- MIGRAINE - Triptan drugs are preferred by most clinicians and patients, - traditional therapy with ergotamine can also be quite effective when given during the prodrome of an attack it becomes progressively less effective if delayed. Ergotamine tartrate is combined with caffeine (100 mg caffeine for each 1 mg ergotamine tartrate) to facilitate absorption of the ergot alkaloid CLINICAL USES OF ERGOTAMINE - The vasoconstriction induced by ergotamine is long-lasting and cumulative when the drug is taken repeatedly - Therefore, patients must be carefully informed that no more than 6 mg of the oral preparation may be taken for each attack and no more than 10 mg per week. - Dihydroergotamine, 0.5-1 mg intravenously, is favored by some clinicians for treatment of intractable migraine. CLINICAL USES OF ERGONOVINE POSTPARTUM HEMORRHAGE Oxytocin is the preferred agent for control of postpartum hemorrhage - but if OXYTOCIN agent is ineffective, ergonovine maleate, 0.2 mg usually given intramuscularly It is usually effective within 1-5 minutes and is less toxic than other ergot derivatives for this application. It is given at the time of delivery of the placenta or immediately afterward if bleeding is significant CLINICAL USES OF BROMOCRIPTINE HYPERPROLACTINEMIA - Increased serum levels of the anterior pituitary hormone prolactin are associated with: * secreting tumors of the gland * with the use of centrally acting dopamine antagonists, especially the D2blocking antipsychotic drugs Because of negative feedback effects, hyperprolactinemia is associated with amenorrhea and infertility in women as well as galactorrhea in both sexes. Bromocriptine is extremely effective in reducing the high levels of prolactin that result from pituitary tumors and has even been associated with regression of the tumor in some cases The usual dosage of bromocriptine is 2.5 mg two or three times daily. Eicosanoids: Prostaglandins, Thromboxanes, Leukotrienes, & Related Compounds Eicosanoids: Prostaglandins, Thromboxanes, SYNTHESIS OF EICOSANOIDS Products of Prostaglandin Endoperoxide Synthases (Cyclooxygenases) Two unique COX isozymes convert arachidonic acid into prostaglandin endoperoxide. PGH synthase-1 (COX-1) is expressed constitutively in most cells. Eicosanoids: Prostaglandins, Thromboxanes, In contrast, PGH synthase-2 (COX-2) is inducible its expression varies markedly depending on the stimulus. COX-2 is an immediate early-response gene product that is markedly up-regulated by shear stress, growth factors, tumor promoters, and cytokines Eicosanoids: Prostaglandins, Thromboxanes, Physiological role COX-1 generates prostanoids such as gastric epithelial cytoprotection whereas COX-2 is the major source of prostanoids in inflammation and cancer. For example, endothelial COX-2 is the primary source of vascular prostacyclin whereas renal COX-2-derived prostanoids are important for normal renal development and maintenance of function. (maintain blood flow) Eicosanoids: Prostaglandins, Thromboxanes, Physiological role COX-3 Mainly centrally FEVER AND PAIN and can be inhibited by PARACETAMOL prostaglandins and prostaglandin analogs currently in clinical use. PHARMACOLOGY OF EICOSANOIDS Effects of Prostaglandins & Thromboxanes The prostaglandins and thromboxanes have major effects on four types of smooth muscle: 1- vascular 2- gastrointestinal 3- airway 4- eye ( smooth muscle) 5- reproductive PHARMACOLOGY OF EICOSANOIDS Effects of Prostaglandins & Thromboxanes 1- platelets and monocytes 2- kidneys 3- the central nervous system, autonomic presynaptic nerve terminals 4- sensory nerve endings 5- endocrine organs PHARMACOLOGY OF EICOSANOIDS A. SMOOTH MUSCLE 1. Vascular TXA2 is a potent vasoconstrictor TXA2 is a smooth muscle cell mitogen The mitogenic effect is potentiated by exposure of smooth muscle cells to testosterone PHARMACOLOGY OF EICOSANOIDS INTESTINE Lubiprostone: Amitiza® USES Lubiprostone is a PGE1 derivative used for the Tretament of chronic idiopathic constipation and irritable bowel syndrome with constipation. PHARMACOLOGY OF EICOSANOIDS INTESTINE Lubiprostone: Amitiza® Mechanism of action: Lubiprostone stimulates chloride channels (ClC-2) in the luminal cells of the intestinal epithelium, thereby increasing intestinal fluid secretion. The increased chloride concentration within the intestinal lumen softens the stool and increases intestinal motility. PHARMACOLOGY OF EICOSANOIDS INTESTINE Lubiprostone: Amitiza® b. Side eff ects: Nausea is the most common side eff ect of lubiprostone which can be decreased if taken with food. diarrhea is the second most reported adverse reaction, followed by headache and abdominal pain. PHARMACOLOGY OF EICOSANOIDS INTESTINE Lubiprostone: Amitiza® Pharmacokinetics Absorption: Minimal absorption, action is primarily in GI tract. Distribution: Minimal systemic distribution. Contraindicated : Mechanical gastrointestinal obstruction PHARMACOLOGY OF EICOSANOIDS INTESTINE Lubiprostone: Amitiza® Pharmacokinetics Absorption: Minimal absorption, action is primarily in GI tract. Distribution: Minimal systemic distribution. Contraindicated : Mechanical gastrointestinal obstruction PHARMACOLOGY OF EICOSANOIDS A. SMOOTH MUSCLE 2. PGF2a is also a vasoconstrictor but is not a mitogen for smooth muscle cells. the isoprostane 8-iso-PGF2a (iPF2aIII) which is also a vasoconstrictor may act via the TP receptor. In patients with cirrhosis 8-iso-PGF2a is produced in large amounts in the liver and is thought to play a pathophysiologic role as an important vasoconstrictor substance in the hepatorenal syndrome. PHARMACOLOGY OF EICOSANOIDS Vasodilator prostaglandins 1- PGI2 and PGE2, promote vasodilation by increasing cAMP and decreasing smooth muscle intracellular calcium 2- Vascular prostacyclin is synthesized by both smooth muscle and endothelial cells, 3- In the microcirculation, PGE2 is a vasodilator produced by endothelial cells. PHARMACOLOGY OF EICOSANOIDS Vasodilator prostaglandins ILOPROST (VENTAVIS) ILOPROST, a synthetic analog of prostacyclin (PGI2), is a vasodilating agent and a plateletaggregation inhibitor. Indications Pulmonary arterial hypertension leading to Improved exercise capacity USE BY INHALATION PHARMACOLOGY OF EICOSANOIDS Vasodilator prostaglandins ILOPROST (VENTAVIS) MECHANISM OG ACTION Dilates pulmonary and arterial vasculature. These actions are mediated through activation of the IP receptors (prostacyclin receptors) Leading to an increase in the production of intracellular cyclic adenosine monophosphate PHARMACOLOGY OF EICOSANOIDS Vasodilator prostaglandins ILOPROST (VENTAVIS) TXA2 production is also inhibited Contraindicated in: Hypersensitivity Systolic BP <85 mm Hg Lactation: Lactation. Use Cautiously in: • PHARMACOLOGY OF EICOSANOIDS Vasodilator prostaglandins ILOPROST (VENTAVIS) Contraindicated in: Systolic BP <85 mm Hg lactation PHARMACOLOGY OF EICOSANOIDS Vasodilator prostaglandins ILOPROST (VENTAVIS) Use Cautiously in: Concurrent use of drugs or coexisting medical conditions that may risk of syncope COPD, asthma, or acute pulmonary infection (may risk of bronchospasm) Hepatic impairment (may need to dosing interval) OB: Use only if maternal benefit outweighs fetal risk. PHARMACOLOGY OF EICOSANOIDS Vasodilator prostaglandins ILOPROST (VENTAVIS) •. Common Adverse Effects Cough headache vasodilation (flushing) Hypotension, syncope and palpitations hemoptysis due to thrombaxe-A2 inhibition PHARMACOLOGY OF EICOSANOIDS Vasodilator prostaglandins ILOPROST (VENTAVIS) •.. Interactions risk of hypotension with other vasodilators or diuretics Risk of bleeding may be by anticoagulant PHARMACOLOGY OF EICOSANOIDS 2. . Airways Respiratory smooth muscle - The cysteinyl leukotrienes are bronchoconstrictors. They act principally on smooth muscle in peripheral airways and are a thousand times more potent than histamine both in vitro and in vivo. - They also stimulate bronchial mucus secretion and cause mucosal edema. - Bronchospasm occurs in about 10% of people taking NSAIDs, probably because of a shift in arachidonate metabolism from COX-1 metabolism to leukotriene formation PHARMACOLOGY OF EICOSANOIDS PLATELETS Both PGD2 and PGI2 inhibit aggregation. TXA2 is the major product of platelet COX-1, is a platelet aggregator and amplifies the effects of other more potent platelet agonists such as thrombin. The platelet actions of TXA2 are restrained in vivo by PGI2 which inhibits platelet aggregation by all recognized agonists. PHARMACOLOGY OF EICOSANOIDS PLATELETS Platelets release TXA2 during activation and aggregation. Urinary metabolites of TXA2 increase in patients experiencing a myocardial infarction this Platelet COX-1-derived thromboxane synthesis is irreversibly inhibited by chronic dosing with aspirin in low doses. Macrophage COX-2 appears to contribute roughly 10% of the increment in TX biosynthesis observed in smokers, while the rest is derived from platelets. PHARMACOLOGY OF EICOSANOIDS KIDNEY Both the medulla and the cortex of the kidney synthesize prostaglandins the medulla substantially more than the cortex. The kidney also synthesizes several hydroxyeicosatetraenoic acids, leukotrienes, cytochrome P450 products, and epoxides. These compounds play important autoregulatory roles in renal function PHARMACOLOGY OF EICOSANOIDS KIDNEY by modifying renal hemodynamics and glomerular and tubular function. This regulatory role is especially important in marginally functioning kidneys, as shown by the decline in kidney function caused by COX inhibitors in elderly patients and those with renal disease. The major eicosanoid products of the renal cortex are PGE2 and PGI2 PHARMACOLOGY OF EICOSANOIDS KIDNEY PGE2 and PGI2 increase glomerular filtration through their vasodilating effects. These prostaglandins also increase water and sodium excretion. PHARMACOLOGY OF EICOSANOIDS KIDNEY Loop diuretics, eg, furosemide, produce some of their effect by stimulating COX activity. In the normal kidney, this increases the synthesis of the vasodilator prostaglandins. Therefore, patient response to a loop diuretic is diminished if a COX inhibitor is administered concurrently PHARMACOLOGY OF EICOSANOIDS KIDNEY The normal kidney synthesizes only small amounts of TXA2. However, in renal conditions involving inflammatory cell infiltration (such as glomerulonephritis and renal transplant rejection) the inflammatory cells (monocytemacrophages) release substantial amounts of TXA2 PHARMACOLOGY OF EICOSANOIDS REPRODUCTIVE ORGANS 1. Female reproductive organs PGF2-alpha, together with oxytocin, is essential for the onset of parturition - Uterine muscle is contracted by PGF2a, TXA2 PHARMACOLOGY OF EICOSANOIDS CENTRAL AND PERIPHERAL NERVOUS SYSTEMS 1. Fever PGE2 increases body temperature Exogenous PGF2a and PGI2 induce fever pyrogens release interleukin-1, which in turn promotes the synthesis and release of PGE2. This synthesis is blocked by paracetamol and other antipyretic compounds PHARMACOLOGY OF EICOSANOIDS EYE PGE and PGF derivatives lower intraocular pressure. (eye drops in glaucoma) The mechanism of this action is unclear but probably involves increased outflow of aqueous humor from the anterior chamber via the uveoscleral pathway PHARMACOLOGY OF EICOSANOIDS Glaucoma Latanoprost bimatoprost, travaprost, and unoprostone 1- a stable long-acting PGF2a derivatives 2- first prostanoid used for glaucoma 4- administered as drops into the conjunctival sac once or twice daily. 5- Adverse effects include irreversible brown pigmentation of the iris and eyelashes, drying of the eyes, and conjunctivitis PHARMACOLOGY OF EICOSANOIDS AGENTS THAT ENHANCE MUCOSAL DEFENSE Misoprostol Misoprostol (15-deoxy-16-hydroxy-16-methyl-PGE1; CYTOTEC) is a synthetic analog of prostaglandin E1. The degree of inhibition of gastric acid secretion by misoprostol is directly related to dose; oral doses of 100 to 200 mg significantly inhibit basal acid secretion (up to 85% to 95% inhibition) or foodstimulated acid secretion (up to 75% to 85% inhibition). The usual recommended dose for ulcer prophylaxis is 200 mg four times a day. PHARMACOLOGY OF EICOSANOIDS AGENTS THAT ENHANCE gastric MUCOSAL DEFENSE Mechanism of Action; Pharmacology. Prostaglandin E2 (PGE2) and prostacyclin (PGI2) are the major prostaglandins synthesized by the gastric mucosa. - PGE2 also can prevent gastric injury by cytoprotective effects that include stimulation of mucin and bicarbonate secretion and increased mucosal blood flow. Since NSAIDs diminish prostaglandin formation by inhibiting cyclooxygenase, synthetic prostaglandin analogs offer a logical approach to reducing NSAID-induced mucosal damage PHARMACOLOGY OF EICOSANOIDS AGENTS THAT ENHANCE GASTRIC MUCOSAL DEFENSE Analog of prostaglandin E1 Misoprostol Misoprostol (15-deoxy-16-hydroxy-16-methyl-PGE1; CYTOTEC) is a synthetic analog of prostaglandin E1. The degree of inhibition of gastric acid secretion by misoprostol is directly related to dose; oral doses of 100 to 200 mg significantly inhibit basal acid secretion (up to 85% to 95% inhibition) or foodstimulated acid secretion (up to 75% to 85% inhibition). The usual recommended dose for ulcer prophylaxis is 200 mg four times a day. CLINICAL OF EICOSANOIDS analogues 1- Dinoprostone, a synthetic preparation of PGE2 is administered vaginally for oxytocic use. it is approved for inducing abortion in the second trimester of pregnancy for missed abortion, and for ripening of the cervix for induction of labor in patients at or near term CLINICAL OF EICOSANOIDS analogues Dinoprostone also directly affects the collagenase of the cervix, resulting in softening For abortifacient purposes, the recommended dosage is a 20-mg dinoprostone vaginal suppository repeated at 3- to 5-hour intervals depending on the response of the uterus. For softening of the cervix at term, the preparations used are either a single vaginal insert containing 10 mg PGE2 or a vaginal gel containing 0.5 mg PGE2 administered every 6 hours. The softening of the cervix for induction of labor substantially shortens the time to onset of labor and the delivery time. CLINICAL OF EICOSANOIDS analogues PGF2alpha 1- This drug, carboprost tromethamine (15-methyl-PGF2a; the 15-methyl group prolongs the duration of action) 2-used to induce second-trimester abortions and to control postpartum hemorrhage that is not responding to conventional methods of management. 3- Vomiting and diarrhea occur commonly, probably because of gastrointestinal smooth muscle stimulation. Transient elevations in temperature are seen in approximately one eighth of patients. Modulators of the pathways NSAID’s and Corticosteroids (COX Inhibitors) Lipoxygenase Inhibitors (Zileuton) TXA2 Synthase Inhibitors LTD4 Receptor Antagonist (MontelukastSINGULAIR) Pharmacological/Physiological Effects 2. Platelets ARACHIDONIC ACID COX -1 Platelet TXA2 Vasoconstriction Platelet Aggregation _ _ ASPIRIN COX -2 Endothelial PGI2 Vasodilation Anti-Platelet Aggregation Therapeutic Uses of Modulators NSAID’s Corticosteroids: anti-inflammatory analgesic, antipyretic. Montelukast (LTD4 BLOCKER) in asthma Mean % of Days 33% 32% 19% Placebo Montelukast Beclometh. % of patients without asthma attacks Montelukast in Asthma Mean % days patients experienced sustained asthma control Beclomethasone NS Montelukast Placebo Time after randomization (days) Time to first asthma attack BIOGENIC PEPTIDES ANGIOTENSIN Synthesis & metabolism Angiotensinogen (plasma substrate) to angiotensin I, by renin that is released during renal ischemia. Angiotensin I to II by angiotensin converting enzyme (ACE, Kinase II) in the pulmonary endothelium. Renin inhibited by propranolol Angiotensin II metabolized by angiotensinases in plasma and tissues. BIOGENIC PEPTIDES ANGIOTENSIN Effects Causes profound vasoconstriction Increases peripheral vascular resistance Increases blood pressure Directly stimulates heart Facilitates epinephrine and aldosterone release Increases Na reabsorption in kidney tubules Releases ADH (vasopressin) to restore blood volume Losartan and valsartan blocks angiotensin (AT1) receptors Captopril & enalapril inhibit ACE (congest. heart fail.). BIOGENIC PEPTIDES BRADYKININ Actions Comes under the group “kinins” This mediates nociception (pain) Regulates BP (vasodilator) Increases capillary permeability Balances electrolytes and fluid, Contracts gut slowly and stimulates prostaglandin synthesis Produced by tissue damage, viral infection, allergic reaction & inflammation Contracts various other smooth muscles BIOGENIC PEPTIDE BRADYKININ Synthesis Prekallikrein (Plasma) 1. Kininogen (alpha-2 globulins High and low m.w. Kallikrein Hagman factor (activated by collagen) (plasma & tissues) Bradykinin, Kallidin Kallikrein (nonapeptide)(decapeptide) (Activated by peptidases) Kallidin, a decapeptide, with same actions is also produced along with bradykinin. Both are metabolized to inactive agents by kinases II and ACE BIOGENIC PEPTIDES BRADYKININ & KALLIDIN Receptors B1 mediates vasoconstriction, sensitive to metabolites. B2 mediates vasodilatation, permeability, smooth muscle contraction, pain B3 mediates guinea pig tracheal contraction, not antagonized by B1 or B2 antagonists. SMALL PROTEINS CYTOKINES (TNFa, IL-1,IL-6) 1. TNF (Tumor Necrosis Factor ) A mediator of endotoxic shock Released by macrophages when exposed to endotoxin Triggers wide endotoxemic symptoms Elicits production of other cytokines, eicosanoids & humoral factors Stimulates degradation and adherence of neutrophils to endothelium Symptoms of septicemia at high concentration Can be neutralized by TNF antiserum Chronic exposure leads to cachexia (TNF was formerly known as cachectin) SMALL PROTEINS CYTOKINES 2. Interleukin-1 (IL-1) Known as lymphocyte activating factor ( T-cell responses) Activates endogenous pyrogen (induces fever) Interacts synergestically with TNF synthesis & release of IL-2 by interacting with antigen stimulated T-cells Activates B-cells and antibody synthesis arachidonic acid metabolism, inflammatory proteins neutrophil chemoattraction & fibroblast proliferation PGI2 synthesis in endothelial cells SMALL PROTEINS CYTOKINES 3. Interleukin-6 (IL-6) A phosphoglycoprotein Produced and secreted by macrophates, monocytes, fibroblasts Inflammatory stimuli causes production by endothelium, T lymphocytes, mast cells IL-6 induces production of hepatic fibrinogen for protection against microorganisms Endotoxin, TNF alpha and IL-1 increase the levels of IL-6 Does not cause tissue injury or vascular thrombosis Platelet Activating Factor (PAF) General Information • Hanson (1971) identified a platelet aggregating agent • Same substance (BP) was found in adrenal medulla • Phospholipase A2 releases AA & lysoPAF from membrane • LysoPAF is acetylated by acetyl coenzyme A to PAF (catalyzed by acetyl transferase) • Antigen-antibody reaction, chemotactic peptides, thrombin, collagen and other autacoids synthesis. • Platelets, neutrophils, monocytes, mast cells, eosinophils, renal medullary cells and endothelium synthesize PAF PLATELET ACTIVATING FACTOR Physiological functions • • PAF in amniotic fluid derived from fetal lung. It is a potent bronchoconstrictor (long lasting) & causes pulmonary edema • Simulates G proteins that are on the cell surface • This activates phospholipase C & A2 • Forms inositol phosphate, diacylglycerol and arachidonates • Thus, PAF and TXA leads to the formation of PGs, LT PAF Pharmacological actions • Increases platelet aggregation • Potent vasodilator, PVR and BP • Constricts pulmonary vessels • Microvascular permeability • Releases eicosanoids, generates superoxides • Contracts nonvascular smooth muscles, • Increases respiratory secretions, forms • pulmonary edema Decreases renal flow Nitric Oxide (NO) Known as endothelium derived relaxing factor (EDRF) • Organic nitrates (amyl nitrate, nitroglycerin, nitroprusside) release NO • Nitric Oxide Synthase (NOS) antagonists counteracts vascular relaxation EFFECTS OF NO • Physiological vasodilator • eNOS blockers increase BP by vasoconstriction • Macrophages kill microorganisms by NO It causes: involved in penile erection PHARMACOLOGIC MANIPULATION OF NITRIC OXIDE Inhibitors of Nitric Oxide Synthesis • • In many disorders, such as inflammation and sepsis inhibition of the iNOS isoform is NEEDED SEPTIC SHOCK increased urinary excretion of nitrate, the oxidative product of NO, is a feature of gramnegative bacterial infection. • • • • Lipopolysaccharide components from the bacterial wall induce synthesis of iNOS, resulting in exaggerated hypotension, shock, and, in some cases, death. This hypotension is reversed by NOS inhibitors such as L-NMMA in humans A similar reversal of hypotension is produced by compounds that prevent the action of NO (such as methylene blue) as well as by scavengers of NO (such as hemoglobin). Nitric Oxide Donors 1. Organic nitrates Nitroglycerin • metabolized to NO by mitochondrial aldehyde reductase, an enzyme enriched in venous smooth muscle, . 2. Sodium nitroprusside generates NO in response to light as well as chemical or enzymatic mechanisms in cell membranes. 3. Hybrid NO donors A new strategy involves the incorporation of NO-donating moieties onto currently available cardiovascular drugs. captopril. SNOCap, which incorporates a nitrosothiol moiety on captopril, is currently being examined for its efficacy in cardiovascular disorders • Nitric Oxide Donors 6. Alternate strategies Sildenafil (VIAGRA) - inhibitor of type 5 phosphodiesterase - results in prolongation of the duration of NOinduced cGMP elevations in a variety of tissues Endothelin potent vasoconstrictor peptides that were first isolated from aortic endothelial cells. Receptors: ETB causes transient drop in BP ETA causes prolonged increases in BP endothelin receptor antagonists Bosentan is a nonselective antagonist Endothelin ANTAGONIST Bosentan is currently approved for use in pulmonary hypertension ADVERSE EFFECTS OF Bosentan 1-Bosentan has been associated with fatal hepatotoxicity patients taking this drug must have monthly liver function tests. 2- Negative pregnancy test results are required for women of childbearing age to take this drug