* Your assessment is very important for improving the work of artificial intelligence, which forms the content of this project
Download JV Poster Barcelona 2012
Discovery and development of antiandrogens wikipedia , lookup
Drug discovery wikipedia , lookup
Pharmaceutical industry wikipedia , lookup
Prescription costs wikipedia , lookup
Discovery and development of beta-blockers wikipedia , lookup
Polysubstance dependence wikipedia , lookup
5-HT3 antagonist wikipedia , lookup
5-HT2C receptor agonist wikipedia , lookup
Drug interaction wikipedia , lookup
Pharmacognosy wikipedia , lookup
Nicotinic agonist wikipedia , lookup
Toxicodynamics wikipedia , lookup
Theralizumab wikipedia , lookup
Cannabinoid receptor antagonist wikipedia , lookup
Discovery and development of angiotensin receptor blockers wikipedia , lookup
NK1 receptor antagonist wikipedia , lookup
Psychopharmacology wikipedia , lookup
NMDA receptor wikipedia , lookup
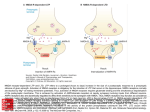
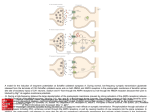
MHeNS, School for Mental Health and Neuroscience Simultaneous Versus Solitary Pharmacological Manipulation of NMDA- and AMPAreceptors: Effects of New Drugs on Contextual Learning and its Extinction Vignisse JulieA,B, Steinbusch H.W.M.A, Griegoriev V.C, Bettendorff L.B, Bolkunov A.C, Nunes J.D, Bachurin S.C and Strekalova T.A,D D CONCLUSIONS Study design o A concomitant low affinity NMDA receptor blockade and AMPA stimulation enhance memory The effects of new drugs on NMDA receptors were studied on isolated cortical neurons that contained a population of NMDA receptors; NMDA solutions of 1-100 μΜ containing 7 μΜ of glycine were applied to activate the receptor. The effects of tested compounds on the stimulation of AMPA receptors were investigated on isolated Purkinje neurons using partial receptor agonist kainic acid (KA), which induces AMPA-receptor mediated currents while evoking relatively low receptor desensitization. The effects of IPAC 1-3 and 5, dimebon, positive modulator of AMPA receptor QXX and memantine on learning were studied in (A) the step-down avoidance task and in (B) a fear conditioning paradigm. Dimebon has been reported to have pro-cognitive effects in these models2,4 and was used as an independent positive control. o Mono-drugs replicate the properties of bifunctional drugs at ten-fold higher doses o Multi-target memory enhancers have a high potential for clinical implications BACKGROUND The development of novel memory enhancers for treating cognitive deficits associated with neurological and psychiatric disorders remains a primary focus of study in central nervous system research. Glutamatergic system has been identified as a central element in this purpose. Indeed, low-affinity blockade of NMDA-receptors or potentiation of AMPA-receptors have been reported to result in memory enhancement but the administration of many of them presents problems with their side-effects1. Therefore, developping molecules that combine procognitive effects of NMDA lowaffinity receptor blocker and AMPA positive modulator while decreasing their adverse effects is of the most importance. Bifunctional drug therapy targeting distinct receptor signalling systems can generate increased efficacy at lower concentrations compared to monofunctional therapy. 0 (A) Step-down Avoidance Administration of drug or vehicle 0 (B) Extinction of Fear Conditioning + 15 min +1h + 24 h Training Recall session 1 Recall session 2 + 24 h + 3 min + 10 min Extinction procedure Training Recall Administration session of drug/vehicle Recall of extinction The present study aimed to compare the mnemotropic potency of molecules that have bifunctional activity (a concomitant effect on the NMDA- and AMPA–receptors) against monofunctional drugs: the low-affinity NMDA receptor blocker memantine and the positive AMPA receptor modulator QQX. New bio-isosteric analogues of MK-801 (IPAC 1-5) were synthesized and designed to have bifunctional activity. Low-affinity NMDA blockade was achieved by introducing greater flexibility into the molecule, and AMPA receptor stimulation was produced by introduction of a sulfamidecontaining derivative of isothiourea in the structure. Dimebon, a weak antagonist (IC50=10 µM) of NMDA-receptor2 and a positive modulator of AMPA receptors at low concentrations (1-20 µM)3, has revealed memory enhancing properties in in vivo studies2,4 which are suggested to be due to its interaction with other neuromediatory systems and mechanisms in additional to glutamatergic2. 1) IPAC 5 was used as a drug of superior concomitant effects on both NMDA and AMPA receptors (A) O N N H H3C + H2N QQX S N H3C CH NH 3 CH3 N H2O N H Cl H3C N N H S N H N CH3 H3C CH MeOH, 4050°C NH 3 CH3 S N N CH3 H3C CH NH 3 CH3 Non competitive blockade of NMDA-R S N 2 (B) R1-N=C=S + R 2 R N H 3 Et2O, r.t. R 1 H N S Potentiation of AMPA-R in % from control, dose of maximal effects and range of effective doses N N H H N CH3 3 H3C CH NH 3 CH3 R4Hal acetone, r.t. R 1 R N N R 4 S R 3 IPAC-2-5 [email protected] www.unimaas.nl/mhens QXX 5 Dim IPAC-1 IPAC-2 IPAC-3 IPAC-5 # # 100 # * * * 50 0 Figure 2. Effects of bio-isosteric analogues of MK-801 on contextual learning in the step-down avoidance test. Mice treated with memantine at a dose of 5 mg/kg or dimebon or IPAC-5 revealed significant increase in the latency of step down 1 h (*p<0.05) and 24 h after training (#p<0.05) as compared with respective control groups, suggesting that the administration of these drugs enhances both short-term and long-term memories in this task. No such effects were observed in animals treated by memantine at a dose of 1 mg/kg, QXX, IPAC-1, IPAC-2 or IPAC-3. Each column represents the mean ± SEM. Experimental groups: Con: saline-treated control; DMSO: DMSO-treated control; Mem 1 or 5: memantine (1 mg/kg or 5 mg/kg); Dim: dimebon (1 mg/kg); QXX- (5 mg/kg), IPAC-1- (1 mg/kg), IPAC-2- (0.5 mg/kg), IPAC-3- (1 mg/kg), IPAC-5 (0.5 mg/kg)- treated group. 3) IPAC 5 (0.5 mg/kg) and QXX (5 mg/kg), but not memantine (5 mg/kg) facilitate extinction of contextual memory (A) Extinction of Contextual Fear Conditioning 80 60 * 40 * * 20 IPAC-3 Con IPAC-5 Mod ↓ High ↓↓ High ↑↑ High ↑↑ Mod ↓ Mod ↑ High ↓↓ Mod ↑ 1.2±0.15 n.a. 21.4±1.58 3.2±0.47 0.8±0.11 High ↓↓ High ↑↑ 0.4±0.07 and 21.2±2.15 n.a. 230% 0.01-0.1 µM 150 % 0.5 µM 148 % 0.5 µM 1050% 30 µM 1.10-5-0.01 µM 0.001– 1.0 µM 0.5 – 1.0 µM 0.02 - 1.0 µM 1.0 – 30 µM Table 1 : Magnitudes of electrophysiological mono- and bi-functional effects of QXX, Memantine and new bio-isoteric analogs of MK-801 (IPAC 1-5) on AMPA- and NMDA-receptors. Freshly isolated neurons from 9-16-day-old rat pups were used for the patch-clamp technique. NMDA-receptor mediated currents were studied in cortical slices. AMPA-receptor mediated currents were studied in Purkinje neurons of the cerebellum3. Arrows indicate the effects of drugs on NMDA- and AMPA-receptors: „high‟ (↓↓) or „moderate‟ (↓) blockade and „high‟ (↑↑) or „moderate‟ (↑) positive modulation. REFERENCES 1 Gonda, Curr. Pharm Des. 2012;18(12):1558-67. 2 Bachurin & al., Ann N Y Acad Sci. 2001;939:425-35. 3 Grigoriev, Dranyi & Bachurin, Bull Exp Biol Med. 2003;136(5):474-7. 4 Vignisse & al., Prog Neuropsychopharmacol Biol Psychiatry 2011;35(2):510-22. School for Mental Health and Neuroscience Div. Neuroscience T +3143 388 4110 F +3143 367 1096 80 60 40 20 0 Con 210% 0.001 µM DMSO Mem 5 QXX 1 QXX 5 Dim IPAC-1 IPAC-2 IPAC-3 IPAC-5 (B) Contextual Freezing Before Dosing 100 HHal Figure 1 : The synthesis of five tested bio-isosteric analogues of MK-801. (A) Routes of synthesis of IPAC-1; (B) Routes of synthesis of IPAC-2-5 compounds (radicals in the formulas: IPAC-2: R1 = Ph, R2 = Bn, R3 = Me, R4 = Bn, Hal = Br. IPAC-3: R1 = Bn, R2 = H, R3 = H, R4 = 2-Cyclohexenyl, Hal = Br; IPAC-5: R1 = All, R2 = Bn, R3 = H, R4 = Et, Hal = I). Correspondence to: Dr Tatyana Strekalova IPAC-2 IPAC-1 2 R Mem 5 150 Potency to affect NMDA and/or AMPA receptors Blockade of NMDA–R: IC50 (µM) Cl NaBH4 R N IPAC-1 Memantine Cl CH3 Mem 1 0 Potentiation of AMPA-R H3C DMSO 100 RESULTS Cl Con + 48 h INTRODUCTION H3C 200 Percent of time spent with freezing, s C Percent of time spent with freezing, % B B 1h 24 h B 1h 24 h B 1h 24 h B 1h 24 h B 1h 24 h B 1h 24 h B 1h 24 h B 1h 24 h B 1h 24 h B 1h 24 h Department of Neurosciences, MHeNS, Maastricht University, Netherlands Bioenergetics And Cerebral Excitability Unit, GIGA-Neurosciences, University of Liege, Liège, Belgium IPAC, Russian Academy of Sciences, Chernogolovka/Moscow , Russia Center of Environmental Biology, Faculty of Sciences, Lisbon University, Lisbon, Portugal; Latency of step down, s A 2) IPAC 5 (0.5 mg/kg) and Memantine (5 mg/kg), but not QQX (5 mg/kg) enhance the acquisition of the step-down avoidance task DMSO Mem 5 QXX 1 QXX 5 Dim IPAC-1 IPAC-2 IPAC-3 IPAC-5 Figure 3: Effects of bio-isosteric analogues of MK-801 on extinction of contextual freezing in a fearconditioning paradigm. (A) During recall of memory extinction trials, the percentage of time spent in freezing was decreased in mice treated with QXX at a dose of 5 mg/kg, with IPAC-5 and with dimebon (versus respective control group, *p<0.05) suggesting that these drugs facilitated fear memory extinction. Groups treated with memantine (5 mg/kg), QXX at a dose of 1 mg/kg, IPAC-1, IPAC-2, and IPAC-3 showed no changes in freezing behaviour during a recall of extinction session. (B) During a recall session of fear conditioning, before dosing, all groups showed similar freezing rates. Each column represents the mean ± SEM. Symbols are as in Figure 2 except for QXX 1 or 5: QQX (1 or 5 mg/kg) - treated group. AKNOWLEDGEMENTS This study was supported by Internationale Stichting Alzheimer Onderzoek (ISAO) grant N 09501 to T.S. (International Foundation on Alzheimer’s disease research, the Netherlands), by the Royal Netherlands Academy of Arts and Sciences (KNAW) and by the Fonds pour la formation à la Recherche dans l'Industrie et dans l'Agriculture (FRIA) to J.V. Authors are grateful to Dr Alexei Proshin for providing us new compounds used in this study. Maastricht University - address P.O. Box 616 6200 MD Maastricht, The Netherlands