* Your assessment is very important for improving the workof artificial intelligence, which forms the content of this project
Download Reactions of Hydrocarbons & their functional groups
Elias James Corey wikipedia , lookup
Woodward–Hoffmann rules wikipedia , lookup
Aromaticity wikipedia , lookup
Cracking (chemistry) wikipedia , lookup
Marcus theory wikipedia , lookup
Tiffeneau–Demjanov rearrangement wikipedia , lookup
Wolff rearrangement wikipedia , lookup
Aromatization wikipedia , lookup
Hofmann–Löffler reaction wikipedia , lookup
Asymmetric induction wikipedia , lookup
George S. Hammond wikipedia , lookup
Ring-closing metathesis wikipedia , lookup
Baylis–Hillman reaction wikipedia , lookup
Physical organic chemistry wikipedia , lookup
Ene reaction wikipedia , lookup
Petasis reaction wikipedia , lookup
Wolff–Kishner reduction wikipedia , lookup
Hydroformylation wikipedia , lookup
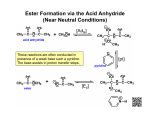
CHAPTER 2: REACTIONS OF ORGANIC COMPOUNDS MAIN TYPES OF REACTIONS in Organic Chem She called it a Blood Bath! 1) 2) 3) 4) 5) 6) 7) 8) Addition Substitution Elimination Oxidation Reduction Condensation Hydrolysis Combustion Look! I think she’s using it to blow her nose I wonder why she wrote it in Japanese Types of Substitution Reactions Whew! That was easy! 1) ADDITION REACTION •Atoms added to a double or triple bond •Alkene or Alkyne undergoes addition reaction to break a double or triple bond •Example: Reactant XY added to alkene makes alkane To recognize: Two reactants make 1 product 1) ADDITION REACTIONS Common atoms that can be added to an alkene or alkyne •H and OH (from H2O ) •H and X (from H-X) where X= Cl , Br, or I •X and X from (X2) where X= Cl , Br, or I •H and H (from H2) EXAMPLES: Addition Reactions 1) 2) ADDITION REACTIONS: ALKENES • Symmetrical molecule reacts with asymmetrical molecule to give one product. Symmetrical Asymmetrical RULES FOR ADDITION • Two asymmetrical molecules react to give two products. Example: + Asymmetrical or Asymmetrical Which product is favoured ? “MARKOVNIKOV’S” Rule • "the rich get richer" • The carbon atom with the largest number of carbon atoms gets the X (halogen) or OH bind to it • Therefore 2- bromobutane is favoured + 2-bromobutane Major product 1-bromobutane Minor Product ADDITION REACTIONS: ALKYNES • Also follow Markovnikov’s rule when asymmetrical Asymmetrical 1,1,2,2-tetrabromopropane ADDITION REACTIONS: ALKYNES • May occur as two addition reactions: + + 2) SUBSTITUTION REACTION • A hydrogen atom or functional group is replaced by a different atom or functional group • To recognize: two compounds react to form two products. 2-bromobutane 2-butanamine 2) SUBSTITUTION REACTION 1) CH3CH2-OH + HI ethanol 2) 3) CH3CH2-I + H2O iodoethane SUBSTITUTION REACTION Aromatics • Aromatics can only undergo substitution reactions SUBSTITUTION REACTION Alcohols • Halogens in HCl, HBr or HI can substitute the OH group of alcohol or the reverse. • For Ex: CH3-CH2-OH + HCl CH3-CH2Cl + H2O • The reverse reaction: CH3-CH2Cl + OHCH3-CH2-OH + Cl(from water) 3) ELIMINATION REACTION • atoms are removed form a molecule to form double bonds. • Reverse of addition • To recognize: One reactant breaks into two products ELIMINATION REACTION: Alcohol • undergo elimination when heated in presence of strong acids, for example: H2SO4 Example: ELIMINATION REACTION: Alkyl Halides • Undergo elimination to produce alkenes Bromoethane ethene hydrobromic acid Elimination • If an asymmetrical molecule undergoes an elimination reaction, constitutional isomers can form example #3 • General rule: H atom most likely to be removed from C atom with most C-C bonds • “The poor get poorer!” – opposite of Markovnikov’s Rule – Called Zaitsev’s rule Elimination (major product) (minor product) 4) OXIDATION & 5) REDUCTION REACTIONS • Change in the number of H or O atoms bonded to C • Always occur together • One reactant is oxidized while the other is reduced • For now, lets focus on reactant only… 4) OXIDATION • Carbon atom forces more bonds to Oxygen or less to Hydrogen • For example: formation of C=O bond • Occurs in presence of oxidizing agents [O] such as KMnO4, K2Cr2O7, and O3 • For now, focus on organic reactant only 4) OXIDATION: Alcohol • Alcohol oxidation can form an aldehyde or ketone Primary Alcohol Secondary Alcohol Tertiary Alcohols do not oxidize 4) OXIDATION: Aldehyde • Aldehydes undergo oxidation to produce carboxylic acid Example: 5) REDUCTION REACTION • Carbon atom forms fewer bonds to Oxygen or more bonds to Hydrogen • Aldehydes, ketones and carboxyliic acids can be “reduced” to alcohols • Alkenes and alkynes can be reduced to become alkanes • Occurs in the presence of reducing agents such as LiAlH4, and H2/Pt where Hydrogen [H] is added 5) REDUCTION: Alkene 5) REDUCTION: Aldehyde/Ketone H O O + C R1 Aldehyde or ketone [H] R1 C R2 Reducing agent H alcohol R2 6) CONDENSATION • two molecules combine to form a single, bigger molecule. • Water is usually produced in this reaction • A carboxylic acid and alcohol can condense to form an ester – called “ esterification” • A carboxylic acid and amine can condense to form an amide Condensation O R2 C R1 O + H carboxylic water acid H O N R2 C R3 R1 amine N + R3 amide H H O • • • • • 7) HYDROLYSIS water adds to a bond splitting it into two Reverse of a condensation reaction Water can add to an ester or amide bond Ester + water makes a carboxylic acid and alcohol Amide + water makes a carboxylic acid and amine 1-propanol 8) Combustion • Type of reaction in which a compound reacts with oxygen to produce the oxides of elements that make up the compound • 2 types: 1) Complete combustion: an excess of oxygen reacts with a hydrocarbon and produces carbon dioxide and water vapour, and releases energy 2) Incomplete combustion: reaction that occurs when insufficient oxygen is present; all elements in the fuel will not combine with oxygen to the greatest extent possible Combustion • Example #1 CxHy + O2(g) CO2(g) + H2O(g) + energy • Example #2 HC + O2(g) C(s) + CO(g) + CO2(g) + H2O(g) + energy POLYMERS • very long molecules made by linking small molecules called monomers • Example: -PET(Polyethylene terephthalate ) polymers - Plastics are polymers that can be heated and moulded into specific shapes and forms -Polyethene is made from monomer of POLYMERS can be synthetic or natural • Synthetic polymers – man made polymer like plastics and polyester • Natural polymers – found in nature like glucose and silk ADDITION Polymerization • Monomers added together through multiple addition reactions • Examples: • Examples Pg 83: Table 2.1 CONDENSATION Polymerization • monomers are joined together by the formation of ester or amide bond • Water created as a side product • Example: • Polyesters contain many ester bonds • Nylon (polyamide) contains many amide bonds