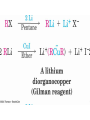* Your assessment is very important for improving the workof artificial intelligence, which forms the content of this project
Download Chemdraw B&W - Pennsylvania State University
George S. Hammond wikipedia , lookup
Enantioselective synthesis wikipedia , lookup
Elias James Corey wikipedia , lookup
Discodermolide wikipedia , lookup
1,3-Dipolar cycloaddition wikipedia , lookup
Kinetic resolution wikipedia , lookup
Ene reaction wikipedia , lookup
Hofmann–Löffler reaction wikipedia , lookup
Ring-closing metathesis wikipedia , lookup
Stille reaction wikipedia , lookup
Tiffeneau–Demjanov rearrangement wikipedia , lookup
Aldol reaction wikipedia , lookup
Wolff rearrangement wikipedia , lookup
Strychnine total synthesis wikipedia , lookup
Petasis reaction wikipedia , lookup
Baylis–Hillman reaction wikipedia , lookup
Wolff–Kishner reduction wikipedia , lookup
Asymmetric induction wikipedia , lookup
Chapter 19. Aldehydes and Ketones:
Nucleophilic Addition Reactions
Based on McMurry’s Organic Chemistry, 6th edition
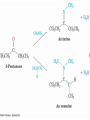
Aldehydes and Ketones
• Aldehydes and ketones are characterized by the the
carbonyl functional group (C=O)
• The compounds occur widely in nature as
intermediates in metabolism and biosynthesis
• They are also common as chemicals, as solvents,
monomers, adhesives, agrichemicals and
pharmaceuticals
19.1 Naming Aldehydes and
Ketones
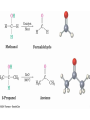
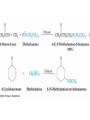
• Aldehydes are named by replacing the terminal -e of
the corresponding alkane name with –al
• The parent chain must contain the CHO group
– The CHO carbon is numbered as C1
• If the CHO group is attached to a ring, use the suffix
See Table 19.1 for common names
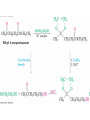
19.2 Preparation of Aldehydes and
Ketones
• Preparing Aldehydes
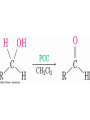
• Oxidize primary alcohols using pyridinium
chlorochromate
• Reduce an ester with diisobutylaluminum hydride
(DIBAH)
Preparing Ketones
• Oxidize a 2° alcohol (see Section 17.8)
• Many reagents possible: choose for the specific
situation (scale, cost, and acid/base sensitivity)
Ketones from Ozonolysis
• Ozonolysis of alkenes yields ketones if one of the
unsaturated carbon atoms is disubstituted (see
Section 7.8)
Aryl Ketones by Acylation
• Friedel–Crafts acylation of an aromatic ring with an
acid chloride in the presence of AlCl3 catalyst (see
Section 16.4)
Methyl Ketones by Hydrating
Alkynes
• Hydration of terminal alkynes in the presence of Hg2+
(catalyst: Section 8.5)
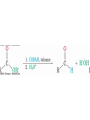
19.3 Oxidation of Aldehydes and
Ketones
• CrO3 in aqueous acid oxidizes aldehydes to carboxylic
acids efficiently
• Silver oxide, Ag2O, in aqueous ammonia (Tollens’
reagent) oxidizes aldehydes (no acid)
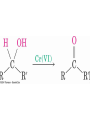
Hydration of Aldehydes
• Aldehyde oxidations occur through 1,1-diols
(“hydrates”)
• Reversible addition of water to the carbonyl group
• Aldehyde hydrate is oxidized to a carboxylic acid by
usual reagents for alcohols
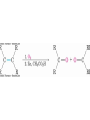
Ketones Oxidize with Difficulty
• Undergo slow cleavage with hot, alkaline KMnO4
• C–C bond next to C=O is broken to give carboxylic
acids
• Reaction is practical for cleaving symmetrical ketones
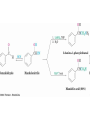
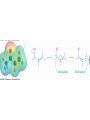
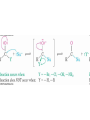
19.4 Nucleophilic Addition Reactions of
Aldehydes and Ketones
• Nu- approaches 45° to the plane of C=O and adds to
C
• A tetrahedral alkoxide ion intermediate is produced
Nucleophiles
• Nucleophiles can be negatively charged ( : Nu) or
neutral ( : Nu) at the reaction site
• The overall charge on the nucleophilic species is not
considered
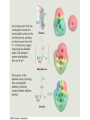
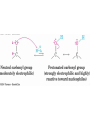
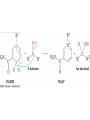
19.5 Relative Reactivity of
Aldehydes and Ketones
• Aldehydes are generally more reactive than ketones in
nucleophilic addition reactions
• The transition state for addition is less crowded and
lower in energy for an aldehyde (a) than for a ketone (b)
• Aldehydes have one large substituent bonded to the
C=O: ketones have two
Electrophilicity of Aldehydes and
Ketones
• Aldehyde C=O is more polarized than ketone C=O
• As in carbocations, more alkyl groups stabilize +
character
• Ketone has more alkyl groups, stabilizing the C=O
carbon inductively
Reactivity of Aromatic
Aldehydes
• Less reactive in nucleophilic addition reactions than
aliphatic aldehydes
• Electron-donating resonance effect of aromatic ring
makes C=O less reactive electrophilic than the
carbonyl group of an aliphatic aldehyde
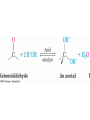
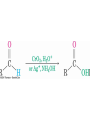
19.6 Nucleophilic Addition of H2O:
Hydration
• Aldehydes and ketones react with water to yield 1,1diols (geminal (gem) diols)
• Hyrdation is reversible: a gem diol can eliminate water
Relative Energies
• Equilibrium generally favors the carbonyl compound
over hydrate for steric reasons
– Acetone in water is 99.9% ketone form
• Exception: simple aldehydes
– In water, formaldehyde consists is 99.9% hydrate
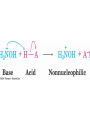
Base-Catalyzed Addition of
Water
• Addition of water is catalyzed by
both acid and base
• The base-catalyzed hydration
nucleophile is the hydroxide ion,
which is a much stronger
nucleophile than water
Acid-Catalyzed Addition of
Water
• Protonation of C=O makes it
more electrophilic
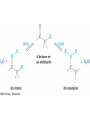
Addition of H-Y to C=O
• Reaction of C=O with H-Y, where Y is electronegative,
gives an addition product (“adduct”)
• Formation is readily reversible
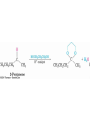
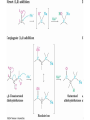
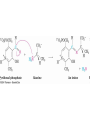
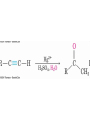
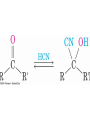
19.7 Nucleophilic Addition of HCN:
Cyanohydrin Formation
• Aldehydes and unhindered ketones react with HCN to
yield cyanohydrins, RCH(OH)CN
Mechanism of Formation of
Cyanohydrins
• Addition of HCN is reversible and base-catalyzed,
generating nucleophilic cyanide ion, CN
• Addition of CN to C=O yields a tetrahedral
intermediate, which is then protonated
• Equilibrium favors adduct
Uses of Cyanohydrins
• The nitrile group (CN) can be reduced with LiAlH4 to
yield a primary amine (RCH2NH2)
• Can be hydrolyzed by hot acid to yield a carboxylic
acid
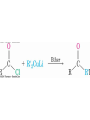
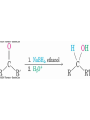
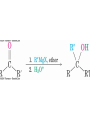
19.8 Nucleophilic Addition of Grignard Reagents
and Hydride Reagents: Alcohol Formation
• Treatment of aldehydes or ketones with Grignard
reagents yields an alcohol
– Nucleophilic addition of the equivalent of a carbon anion, or
carbanion. A carbon–magnesium bond is strongly polarized, so a
Grignard reagent reacts for all practical purposes as R : MgX +.
Mechanism of Addition of Grignard
Reagents
2+,
• Complexation of C=O by Mg Nucleophilic addition of R : ,
protonation by dilute acid yields the neutral alcohol
• Grignard additions are irreversible because a carbanion is
not a leaving group
Hydride Addition
• Convert C=O to CH-OH
• LiAlH4 and NaBH4 react as donors of hydride ion
• Protonation after addition yields the alcohol
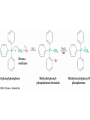
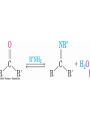
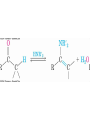
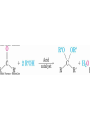
19.9 Nucleophilic Addition of Amines:
Imine and Enamine Formation
RNH2 adds to C=O to form imines, R2C=NR (after loss of
HOH)
R2NH yields enamines, R2NCR=CR2 (after loss of HOH)
(ene + amine = unsaturated amine)
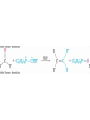
Mechanism of Formation of
Imines
• Primary amine adds to C=O
• Proton is lost from N and adds to O to yield a neutral
amino alcohol (carbinolamine)
• Protonation of OH converts into water as the leaving
group
• Result is iminium ion, which loses proton
• Acid is required for loss of OH – too much acid blocks
RNH2
Note that overall reaction is substitution of RN for O
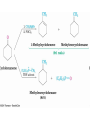
Imine Derivatives
• Addition of amines with an atom containing a lone pair
of electrons on the adjacent atom occurs very readily,
giving useful, stable imines
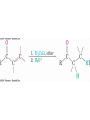
• For example, hydroxylamine forms oximes and 2,4dinitrophenylhydrazine readily forms 2,4dinitrophenylhydrazones
– These are usually solids and help in characterizing liquid
ketones or aldehydes by melting points
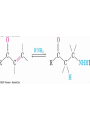
Enamine Formation
• After addition of R2NH, proton is lost from adjacent
carbon
R R
O
O
C
H
+ R2NH
H
C
NH
HO
H+
N
C
C
N
H
H
C
H
R
N
H2O
C
+ H3O+
C
C
H
R
R R
R R
C H
H
C
H
19.11 Nucleophilic Addition of
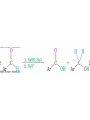
Alcohols: Acetal Formation
• Two equivalents of ROH in the presence of an acid
catalyst add to C=O to yield acetals, R2C(OR)2
• These can be called ketals if derived from a ketone
Formation of Acetals
• Alcohols are weak nucleophiles but acid promotes
addition forming the conjugate acid of C=O
• Addition yields a hydroxy ether, called a hemiacetal
(reversible); further reaction can occur
• Protonation of the OH and loss of water leads to an
oxonium ion, R2C=OR+ to which a second alcohol adds
to form the acetal
Uses of Acetals
• Acetals can serve as protecting groups for aldehydes
and ketones
• It is convenient to use a diol, to form a cyclic acetal
(the reaction goes even more readily)
19.12 Nucleophilic Addition of Phosphorus
Ylides: The Wittig Reaction
• The sequence converts C=O is to C=C
• A phosphorus ylide adds to an aldehyde or ketone to
yield a dipolar intermediate called a betaine
• The intermediate spontaneously decomposes through
a four-membered ring to yield alkene and
triphenylphosphine oxide, (Ph)3P=O
• Formation of the ylide is shown below
A Note on the Word “Betaines”
•
The term “betaines” is an extension from a specific substance
(betaine) that has permanent + and – charges (as in a zwitterion) that
cannot be neutralized by proton transfers (as in normal amino acids).
Webster's Revised Unabridged Dictionary lists: Betaine \Be"ta*ine\,
n. [From beta, generic name of the beet.] (Chem.) A nitrogenous
base, {C5H11NO2}, produced artificially, and also occurring naturally
in beet-root molasses and its residues. The listed pronunciation
indicates it has the exact same emphasis as “cocaine”.
• Cocaine \Co"ca*ine\, n. (Chem.) A powerful alkaloid, {C17H21NO4},
obtained from the leaves of coca
• So – if you say “co-ca-een” (as this dictionary suggests) then you
would also say “bee-ta-een”. If you sat “co-cayn” then say “beetayn”.
• Whatever you say, the “beta” in “betaine” refers to beets and not a
letter in the Greek alphabet. There have been a lot of wagers on this
over the years.
RK
Uses of the Wittig Reaction
• Can be used for monosubstituted, disubstituted, and
trisubstituted alkenes but not tetrasubstituted alkenes
The reaction yields a pure alkene of known structure
• For comparison, addition of CH3MgBr to
cyclohexanone and dehydration with, yields a mixture
of two alkenes
Mechanism of the Wittig
Reaction
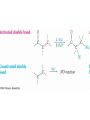
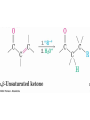
19.14 Conjugate Nucleophilic Addition to ,bUnsaturated Aldehydes and Ketones
• A nucleophile can
add to the C=C
double bond of an
,b-unsaturated
aldehyde or ketone
(conjugate addition,
or 1,4 addition)
• The initial product
is a resonancestabilized enolate
ion, which is then
protonated
Conjugate Addition of Amines
• Primary and secondary amines add to , b-unsaturated
aldehydes and ketones to yield b-amino aldehydes and
ketones
Mechanism of Alkyl Conjugate
Addition
• Conjugate nucleophilic addition of a diorganocopper
anion, R2Cu, an enone
• Transfer of an R group and elimination of a neutral
organocopper species, RCu
Conjugate Addition of Alkyl Groups:
Organocopper Reactions
• Reaction of an , b-unsaturated ketone with a lithium
diorganocopper reagent
• Diorganocopper (Gilman) reagents from by reaction of
1 equivalent of cuprous iodide and 2 equivalents of
organolithium
• 1, 2, 3 alkyl, aryl and alkenyl groups react but not
alkynyl groups
Enantioselective Synthesis
• When a chiral product is formed achiral reagents, we get
both enantiomers in equal amounts - the transition states
are mirror images and are equal in energy
• However, if the reaction is subject to catalysis, a chiral
catalyst can create a lower energy pathway for one
enantiomer - called an enantionselective synthesis
• Reaction of benzaldehyde with diethylzinc with a chiral
titanium-containing catalyst, gives 97% of the S product and
only 3% of the R
Summary
•
•
•
•
•
•
•
•
•
•
Aldehydes are from oxidative cleavage of alkenes, oxidation of 1°
alcohols, or partial reduction of esters
Ketones are from oxidative cleavage of alkenes, oxidation of 2°
alcohols, or by addition of diorganocopper reagents to acid
chlorides.
Aldehydes and ketones are reduced to yield 1° and 2° alcohols ,
respectively
Grignard reagents also gives alcohols
Addition of HCN yields cyanohydrins
1° amines add to form imines, and 2° amines yield enamines
Reaction of an aldehyde or ketone with hydrazine and base yields an
alkane
Alcohols add to yield acetals
Phosphoranes add to aldehydes and ketones to give alkenes (the
Wittig reaction)
b-Unsaturated aldehydes and ketones are subject to conjugate
addition (1,4 addition)