* Your assessment is very important for improving the workof artificial intelligence, which forms the content of this project
Download Chapter 20 reactions of carbonyls
Kinetic resolution wikipedia , lookup
Ring-closing metathesis wikipedia , lookup
Discodermolide wikipedia , lookup
Tiffeneau–Demjanov rearrangement wikipedia , lookup
Organosulfur compounds wikipedia , lookup
Elias James Corey wikipedia , lookup
Metal carbonyl wikipedia , lookup
Ene reaction wikipedia , lookup
Enantioselective synthesis wikipedia , lookup
Aldol reaction wikipedia , lookup
Baylis–Hillman reaction wikipedia , lookup
1,3-Dipolar cycloaddition wikipedia , lookup
Wolff rearrangement wikipedia , lookup
Stille reaction wikipedia , lookup
Petasis reaction wikipedia , lookup
Asymmetric induction wikipedia , lookup
Hydroformylation wikipedia , lookup
Strychnine total synthesis wikipedia , lookup
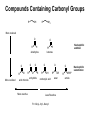
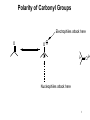
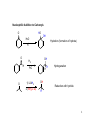
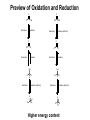
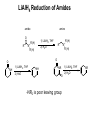
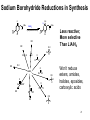
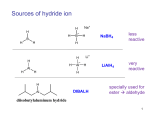
Chapter 20 Carbonyl compounds Introduction to carbonyls Reductions and oxidations Addition of organometallics (Rli, RMgX, R2CuLi) to carbonyls 1 Compounds Containing Carbonyl Groups R R OH NH2 More reduced O O H R R aldehydes O O R More oxidized Cl R acid chloride More reactive ketones O O anhydride O O R Nucleophilic addition R R OH carboxylic acid OR R ester Less Reactive R = Alkyl, Aryl, Akenyl O R Nucleophilic substitution NR'R" amide Polarity of Carbonyl Groups Electrophiles attack here O O O+ - Nucleophiles attack here 3 General Reactions of Carbonyl Compounds Nucleophilic addition: Aldehydes & ketones Nu R + R Nu +H+ O O - Nucleophilic substitution: Nu OH esters, acid chlorides, acids, anhyrides, amides Nu Nu + Z O - Nu O Z O Reactivity to Nucleophilic Addition Nucleophilic substitution: esters, acid chlorides, acids, anhyrides, amides Nu Nu + Z O - Nu O O Z 5 Nucleophilic Addition to Carbonyls O HO H2O OH Hydration (formation of hydrate) H+ O H O OH H2 PtC 1) LiAlH4 2) NH4Cl aq. H H Hydrogenation OH Reduction with hydride H 6 Reactivity to Nucleophilic Substitution Nucleophilic substitution: esters, acid chlorides, acids, anhyrides, amides Nu Nu O O - + Z Nu O Z Better the leaving group, Z, the more reactive the carbonyl O Cl Cl R acid chloride > O O O R > > NR2 O O R anhydride OR R O O OH, R OH carboxylic acid R O OR R ester NR'R" amide more reactive How does pKa change with Z? 7 Nucleophilic substitution: Not with aldehydes and ketones Nu Nu + O- H -H Nu O O Z pKa of H2? Nu Nu + R O - -H O Nu O R pKa of R ? 8 Nucleophilic Substitution on Carbonyls O H+ CH3 OH H2O O Acid catalyzed hydrolysis of ester O OH O O Cl Acetylation of an alcohol O Pyridine O O O O NH2 HN Pyridine Acetylation of an amine to form an amide 9 Preview of Oxidation and Reduction O O R OH Reduction oxidation R OH Reduction oxidation (difficult) O O R H Reduction R oxidation R Reduction oxidation OH R H Reduction OH H oxidation (difficult) R H Reduction H R oxidation (difficult) H H R H R H R Higher energy content Oxidation and Reduction of Carbonyl Compounds • The three most useful oxidation and reduction reactions of carbonyl starting materials can be summarized as follows: Reductive Addition to Aldehydes and Ketones Reduction of ketones to secondary alcohols OH O [H] Reduction of Aldehydes to primary alcohols O R OH [H] R H H H R R R R H Reductive Addition to Carboxylic acids and their derivatives O R [H] R Z OH O [H] H aldehyde R H H primary alcohol Oxidation of Aldehydes O R O O] H R OH 11 Reduction of carbonyls by hydrogenation O HO H2 H PtC O O H 1 equiv.H2 C=C reduction is faster than C=O reduction PtC O H HO excess H2 PtC H H H Metal hydrides are an alternative 12 Alternative Reducing Agents Aluminum hydrides and borohydrides H Li H H Li Al H H H LiAlH4 B H H Na H H B H H RO Al OR OR NaBH4 LiBH4 Neutral boranes and aluminanes O BH BH3-THF O diborane or Borane H Li catecholborane Al H DiBAl-H Carbanionic-organometallics R Li organolithium R MgX Grignard R R Cu Li Cuprate 13 Reduction of Aldehydes and Ketones with hydride reagents O R OH LiAlH4 2) aqueous H R H R H H Li H + Al H H - OH O LiAlH4 R H H Al H H H H Al H H Li H O O RO OR Al OR OH H O H H H Al H O H O + A(OH)3 R H O LiAlH4 H R 2) aqueous Lithium Aluminum Hydride OH OH OH R R-H H H H OH OH O RCO2H R O H R-X R NH2 R R H H O C N LiAlH4 R R OH R NO2 R O R NH2 R O O NHR' R R No unactivated alkenes R H Cl OR' OH R NHR' OH R H R H H H Strong reducing agent. Not very selective 15 LiAlH4 Reduction Mechanism for esters and acid chlorides H H Al H O H2O Li Li O O O R' O H H Al H H R R H O R' R H R H H Al H H H H OH H O H HO OH Al OH O R H HO OH OH Al H O H R H H R H O H H 16 H H H H Al H O R O H O R Al H O R H O Li H H Al H O H2O Li O H H Al H R O H H Al H H O R H O H H Al H O R H Al H O H H H H Al H H R H H OH H O H HO OH Al OH O R H HO OH OH Al H O H R H H R H O H H Aldehyde intermediate is more reactive than carboxylic acid and is immediately reduced to alcohol 17 LiAlH4 Reduction of Amides amine amide O R N 1) LiAlH4, THF R'(H) R 2) H2O R'(H) N R'(H) R'(H) O O NH 1) LiAlH4, THF 2) H2O NH NR O -NR2 is poor leaving group 1) LiAlH4, THF 2) H2O NR Mechanism for reduction of amides with LiAlH4 H H pKa 25 Li O O R H Al N H R H N O H R H2 H H Al H N H N H H Al R O H H H Al H H H pKa 35 H H Al H H H N H H Al R O H H imine NH NH R H H Al H H H R H H HA NH2 R H H Imine is rapidly reduced 19 Sodium Borohydride Reductions in Synthesis O R OH H NaBH4 2) aqueous work-up R H H O R OH R' NaBH4 2) aqueous work-up H R' secondary alcohols primary alcohols Less reactive; More selective Than LiAlH4 R Won’t reduce esters, amides, halides, epoxides, carboxylic acids 20 Sodium Borohydride Reductions in Synthesis O H OMe O O OH OMe NaBH4 O 2) aqueous work-up O Br Less reactive; More selective Than LiAlH4 Br NR NR OH R O RCO2H O R H H H R-X NR O NaBH4 R R OH O R NR R O NHR' O R R Cl R H Won’t reduce esters, amides, halides, epoxides, carboxylic acids OR' OH NR R H H 21 Sodium Borohydride Reductions with CeCl3 Luche reduction O H O O NaBH4/CeCl3 H OH 2) aqueous work-up Only reduces ketones, not aldehydes 22 Stereochemistry of Carbonyl Reduction • Hydride converts a planar sp2 hybridized carbonyl carbon to a tetrahedral sp3 hybridized carbon. 23 Hydroboration Chemistry 1) BH3 THF R R 2) NaOH, H2O2 1) BH3 THF R R 2) X2, NaOMe 1) BH3 THF R 2) R'NH2, NaOCl R 1) BH3 THF OH R 2) NaOH, H2O2 H 1) BH3 THF X R R 2) X2, NaOMe 1) BH3 THF NHR' R O R R 2) CO X O C R 3) NaOH, H2O2 Better regio control with hindered boranes: H B H thexylborane BH BH 9-BBN H B O BH O diisoamylborane catecholborane 24 reductions with BH3 w/ NaBH4 as catalyst OH R H H H H OH OH fast NR OH OH O RCO2H slow R O H R-X R slow NH2 R slow R H H O C N BH3 R slow R OH R NO2 R O NR R R R OR' NHR' R 2) H+ O O NHR' R R H Cl R H OH slow R H H slow NR Allows carboxylic acids to be reduced in the presence of aldehydes or ketones Enantioselective Carbonyl Reductions • Selective formation of one enantiomer over another can occur if a chiral reducing agent is used. • A reduction that forms one enantiomer predominantly or exclusively is an enantioselective or asymmetric reduction. • An example of chiral reducing agents are the enantiomeric CBS reagents. 26 CBS Reducing Agents • CBS refers to Corey, Bakshi, and Shibata, the chemists who developed these versatile reagents. • One B–H bond serves as the source of hydride in this reduction. • The (S)-CBS reagent delivers (H:−) from the front side of the C=O. This generally affords the R alcohol as the major product. • The (R)-CBS reagent delivers (H:−) from the back side of the C=O. This generally affords the S alcohol as the major product. 27 S CBS isomer H N N B H N O H Lewis acid RS Ph RL B Ph Ph O B H3B R' nucleophile R' O Ph BH3 B R' Ph S H Ph Ph O RS Ph S RS B N O H B H H Ph RL N H2O H RL B R' B H O H R alcohol RL opposite sides of ring; reduction cannot occur H Ph R' O RS B B H N H R' O O O H Ph Ph Ph R' O Ph N B H RS B HO H H 28 RL Enantioselectivity of CBS Reagents • These reagents are highly enantioselective. • For example, treatment of propiophenone with the (S)-CBS reagent forms the R alcohol in 97% enantiomeric excess (ee). 29 Enantioselective Reductions in Synthesis • Enantioselective reductions are key steps in the synthesis of several widely used drugs, including salmeterol, a long-acting bronchodilator. Figure 20.3 30 Biological Reductions • Biological reductions that occur in cells always proceed with complete selectivity, forming a single enantiomer. • In cells, the reducing agent is NADH. • NADH is a coenzyme—an organic molecule that can function only in the presence of the enzyme. 31 Mechanism of NADH Reductions • The active site of the enzyme binds both the carbonyl substrate and NADH, keeping them in close proximity. • NADH then donates H:− in much the same way as a hydride reducing agent. 32 Enantioselectivity of NADH Reduction • The reaction is completely enantioselective. • For example, reduction of pyruvic acid with NADH catalyzed by lactate dehydrogenase affords a single enantiomer with the S configuration. • NADH reduces a variety of different carbonyl compounds in biological systems. • The configuration of the product (R or S) depends on the enzyme used to catalyze the process. 33 NAD+ —Biological Oxidizing Agent • NAD+, the oxidized form of NADH, is a biological oxidizing agent capable of oxidizing alcohols to carbonyl compounds (it forms NADH in the process). • NAD+ is synthesized from the vitamin niacin. 34 Other Metal Hydride Reducing Agents: Al Less reactive and more selective than LiAlH4 1) isobutyl groups are bulky 2) trivalent Al is not as reactive of a H- donor H diisobutylaluminum hydride DIBAL-H 1) Reaction with aldehydes and ketones O R H DIBAL-H R(H) 2) H2O O R R(H) H 2) Reduction of acid chlorides (Z = Cl), esters (Z = OR'), amides (Z = NR'R") to aldehydes O R Z DIBAL-H 2) H2O O R H Use only 1 equiv. DiBAL-H to avoid over reduction LiAlH4 reduces all the way to alcohols. 35 DIBAL-H Reduction of acid chloride to aldehyde no reaction except with LiAlH4 or BH3 reduction to primary alcohol O SOCl2 OH -HCl and SO2 O DIBAL-H Cl 2) H2O O H LiAlH4 or BH3 OH 36 Reductions with DiBAL-H OH NR NR NR OH OH O RCO2H R O H R-X R O R H R C N H H O Al H R R OH R NO2 R O NR R O O NHR' R R Cl OR' O R R H NR O H R H @ low temperature O OH O DiBAl-H O 37 Reduction of Esters • In the reduction of an acid chloride, Cl− comes off as the leaving group. • In the reduction of the ester, CH3O− comes off as the leaving group, which is then protonated by H2O to form CH3OH. 38 DIBAL-H Reduction of an Ester Figure 20.4 The DIBAL-H reduction of an ester to an aldehyde in the synthesis of the marine neurotoxin ciguatoxin CTX3C 39 Other Metal Hydride Reducing Agents: Li O Al Less reactive and more selective than LiAlH4 1) t-BuO groups are bulky 2) Inductive electron withdrawing with three oxygens makes (RO)3AlH less of a negative hydride O H O (t-BuO)3AlH tri-t-butyloxyaluminum hydride 1) Reaction with aldehydes and ketones H O R(H) R O (t-BuO)3AlH 2) H2O R R(H) H 2) Reduction of acid chlorides (Z = Cl), esters (Z = OR') to aldehydes O R Z (t-BuO)3AlH 2) H2O O R H must use only 1 equivalent to avoid reduction of aldehyde 40 O O R Cl R OH O R H R OH O R R R O O OH O R OR' R OH R NR'R" R CN R HO LiAlH4 R X R H OH R R OH R R NR'R" NH2 R R R NO2 R R R N N NR NR NR NR NR NR R HO NaBH4 NR R NR OH R NR NR NR NR NR NR R HO NaBH4/CeCl3 BH3 NR NR R HO Slow OH R NR R Slow R R NR Fast OH R NR R OH R O Slow O * R R H R H low Temp O HO (t-BuO)3AlH R NR O * HO DIBAL-H NR H R OH R R NR R R NR NR NR NR Slow Slow NR NR NR O * O NR NR NR H R H R R B NR B R i-Bu * NR NR NR NR NR R H NR NR NR H HO H2/Cat difficult R OH R R difficult difficult * HO2C i-Bu Al R CO2Me difficult R NH2 R NH2 R R OH with > 2 equivalents of hydride ? HO2C OH 41 O O R Cl R O H O R R R O O OH R O OR' R NR'R" R CN R HO LiAlH4 OH R OH R R X R H OH R R OH R OH R R NR'R" NH2 R R NO2 R R R R N N NR NR NR NR NR NR R HO NaBH4 NR R NR OH R NR NR NR NR NR NR R HO NaBH4/CeCl3 BH3 NR NR R Slow OH R NR R HO Slow R R NR Fast OH R NR R OH O Slow O * R R H R H low Temp H R OH R R NR R R NR NR NR NR Slow Slow NR NR NR O * O NR NR NR H R H R R B NR B R i-Bu Al i-Bu O HO (t-BuO)3AlH R R NR O * HO DIBAL-H NR * NR NR NR NR NR R H NR NR NR H HO H2/Cat difficult R OH R R difficult difficult * R difficult R NH2 R NH2 R R OH with > 2 equivalents of hydride O OH ? HO2C HO2C 42 O O R Cl R O H O R R R O O OH R O OR' R NR'R" R CN R HO LiAlH4 OH R OH R R X R H OH R OH R R OH R R NR'R" NH2 R R R NO2 R R R N N NR NR NR NR NR NR R HO NaBH4 NR R NR OH R NR NR NR NR NR NR R HO NaBH4/CeCl3 BH3 NR NR R Slow OH R NR NR R HO Slow R R Fast OH R NR R OH O Slow O * R R R H low Temp H O HO (t-BuO)3AlH R R NR O * HO DIBAL-H NR H R OH R R NR R R NR NR NR NR Slow Slow NR NR NR O * O NR NR NR NR NR NR R H H R H R R B NR B R i-Bu * NR NR NR NR NR H HO H2/Cat difficult R OH R R difficult difficult * R difficult NH2 R R NH2 R R OH with > 2 equivalents of hydride O HO2C i-Bu Al O HO 43 Oxidation of Aldehydes • A variety of oxidizing agents can be used, including CrO3, Na2Cr2O7, K2Cr2O7, and KMnO4. • Aldehydes can also be oxidized selectively in the presence of other functional groups using silver(I) oxide in aqueous ammonium hydroxide (Tollen’s reagent). • Since ketones have no H on the carbonyl carbon, they do not undergo this oxidation reaction. 44 Organometallic Reagents • Li, Mg, and Cu are the most common organometallic metals. • Other metals found in organometallic reagents are Sn, Si, Tl, Al, Ti, and Hg. • General structures of common organometallic reagents are shown: 45 Reactivity of Common Organometallic Compounds • Since both Li and Mg are very electropositive metals, organolithium (RLi) and organomagnesium (RMgX) reagents contain very polar carbon-metal bonds and are therefore very reactive reagents. • Organomagnesium reagents are called Grignard reagents. • Organocopper reagents (R2CuLi), also called organocuprates, have a less polar carbon–metal bond and are therefore less reactive. • Although they contain two R groups bonded to Cu, only one R group is utilized in the reaction. • In organometallic reagents, carbon bears a − charge. 46 Preparation of Organolithium Compounds Old School 2 Li Br R R Li + LiBr Now: Commercially available organolithium as solutions in hexanes or pentane Li Li Li H3C Li Li pKa of conjugate acid: pKa 70 pKa 65 pKa 62 pKa 60 pKa 46 More basic, more reactive Metal-Halogen exchange Br LI Br THF, -78 °C Li Down hill reaction by 70-65 = 5 orders of magnitude • Crystalline solids, pyrophoric. Sold and used as solution in pentanes. Most reactive of common carbanionic reagents. • Low temperature prevents beta elimination (E2) to afford alkenes instead of metal halogen exchange • primary butyl lithiums can react readily as nucleophiles • t-BuLi never reacts as nucleophile. Only as hindered base or metal halogen exchange • At room temperature BuLi will attack THF 47 organolithiums in SN2 reacctions primary R Li R' R' R X X = Cl, Br, I, OTos Br Li Li -78 °C (dry ice-acetone) Hexane H Br H Li H Br 1) 2eq. t-BuLi, THF, -78 °C Br R 2) RX 48 Organolithiums and carbonyyls OH R O R Li THF, -78 °C 2) aq. work-up O OH O O 2 R R Li THF, -78 °C R 2) aq. work-up R O O Li + H 49 R CO2H R 2 equiv. RLi CO2 R' O R' O R' X OH R R' OH X O R' R Lewis acid R' R" H R, Li R O O R' R' OR" O R' R" OH R R R' OH R R' OH R" R R' 50 Grignard Reagents add to electrophile HO O R' Grignard reagent Mg(0) R X R' R(or Z = H, alkyl, Aryl) R Z Z = H, alkyl, aryl, OR", NR2, Cl R' R MgBr R ether solvent X = Cl, Br, I R' Y Y = Cl, Br, I, OTs R = Alkyl, aryl, alkenyl O No ether (diethyl ether, THF , glyme), no reaction R' R R' OH Slow, cautious addition of < 5% RX to Magnesium in refluxing ether Once reaction starts exotherming, then slow addition of remainder of RX DANGER: addition of all of RX to Mg can cause runaway reaction and 51 explosion Grignard reactions R H R Acidic protons R' R' NHR" R' R R' NR" X R' OH R R' O R MgBr Lewis acid R' R HC(OEt)3 or O R' O R'CN R' OR" R' R' ° H O Me2N O H R" OH R R R' R' O R H OH R" R R' 52 Preparation of Organocuprate Compounds • Organocuprates are prepared from organolithium reagents by reaction with a Cu+ salt, often CuI. Less reactive, more selective than organolithiums 53 Cuprates allow reactions that are not possible with organolithiums or Grignards R R CuLi R' X R R' X = Cl, Br, I, OTos R' = 1°, 2° alkyl, aryl, alkenyl R R CuLi O O R' Cl R R' acidic work-up R' = alkyl, aryl, H 54 R' R R R' X X R R CuLi R R' O R' R' Cl R' X R'' O R' O R R R' O R' R' Only 1,4 addition X R' X OH Direct substitution reactions with alkenyl and aryl halides R' R' R Retention of stereochemistry R'' O R' R 2° halides without E2 R No double addition to afford alcohols 55 Convert aryl bromides into nucleophilic carbanions O Br2 FeBr3 Br 2 eq. t-BuLi OH Li THF, -78 °C 2) aq. H2O, H+ 0.5 eq. CuBr O CuLi 2 O 2) aq. H2O, H+ 56 Preparation of Acetylide Ions • Acetylide ions are another example of organometallic reagents. • Acetylide ions can be thought of as “organosodium reagents”. • Since sodium is even more electropositive than lithium, the C–Na bond of these organosodium compounds is best described as ionic, rather than polar covalent. pKa 35 pKa 25 57 Preparation of Lithium Acetylides • An acid–base reaction can also be used to prepare sp hybridized organolithium compounds. • Treatment of a terminal alkyne with CH3Li affords a lithium acetylide. • The equilibrium favors the products because the sp hybridized C–H bond of the terminal alkyne is more acidic than the sp3 hybridized conjugate acid, CH4, that is formed. 58 Alcohols Formed by Organometallic Addition • This reaction is used to prepare 1°, 2°, and 3° alcohols. 59 Synthesis of Ethynylestradiol Figure 20.5 60 Retrosynthetic Analysis of Grignard Products • To determine what carbonyl and Grignard components are needed to prepare a given compound, follow these two steps: 61 Retrosynthetic Analysis of 3-pentanol 62 Synthesis of 3-pentanol • Writing the reaction in the synthetic direction—that is, from starting material to product—shows whether the synthesis is feasible and the analysis is correct. • Note that there is often more than one way to synthesize a 2° alcohol by Grignard addition. 63 Limitations of Organometallic Reagents • Addition of organometallic reagents cannot be used with molecules that contain both a carbonyl group and N–H or O–H bonds. • Carbonyl compounds that also contain N–H or O–H bonds undergo an acid–base reaction with organometallic reagents, not nucleophilic addition. 64 Use of Protecting Groups Solving this problem requires a three-step strategy: [1] Convert the OH group into another functional group that does not interfere with the desired reaction. • This new blocking group is called a protecting group, and the reaction that creates it is called “protection”. [2] Carry out the desired reaction. [3] Remove the protecting group. • This reaction is called “deprotection”. • A common OH protecting group is a silyl ether. 65 General Protecting Group Strategy Figure 20.7 • In Step [1], the OH proton in 5-hydroxypentanone is replaced with a protecting group, written as PG. • Because the product no longer has an OH proton, it can now undergo nucleophilic addition. • In Step [2], CH3MgCl adds to the carbonyl group to yield a 3o alcohol after protonation with water. • Removal of the protecting group in Step [3] forms the desired product. 66 Preparing Silyl Ethers • tert-Butyldimethylsilyl ethers are prepared from alcohols by reaction with tert-butyldimethylsilyl chloride and an amine base, usually imidazole. • The silyl ether is typically removed with a fluoride salt such as tetrabutylammonium fluoride (CH3CH2CH2CH2)4N+F−. 67 Preparing Silyl Ethers • The use of tert-butyldimethylsilyl ether as a protecting group makes possible the synthesis of 4-methyl-1,4-pentanediol by a three-step sequence. 68 Organometallic Reactions with Esters and Acid Chlorides • Both esters and acid chlorides form 3° alcohols when treated with two equivalents of either Grignard or organolithium reagents. 69 Organocuprates—a Less Reactive Organometallic • To form a ketone from a carboxylic acid derivative, a less reactive organometallic reagent—namely an organocuprate— is needed. • Acid chlorides, which have the best leaving group (Cl−) of the carboxylic acid derivatives, react with R’2CuLi to give a ketone as the product. • Esters, which contain a poorer leaving group (−OR), do not react with R’2CuLi. 70 Grignard Reaction with CO2 • Grignards react with CO2 to give carboxylic acids after protonation with aqueous acid. • This reaction is called carboxylation. • The carboxylic acid formed has one more carbon atom than the Grignard reagent from which it was prepared. 71 Organometallic Reactions with Epoxides • Like other strong nucleophiles, organometallic reagents— RLi, RMgX, and R2CuLi—open epoxide rings to form alcohols. 72 Organometallic Reactions with Epoxides • The reaction follows the same two-step process as opening of epoxide rings with other negatively charged nucleophiles— that is, nucleophilic attack from the back side of the epoxide, followed by protonation of the resulting alkoxide. • In unsymmetrical epoxides, nucleophilic attack occurs at the less-substituted carbon atom. 73 Organometallic Reactions with ,Unsaturated Carbonyl Compounds • ,-Unsaturated carbonyl compounds are conjugated molecules containing a carbonyl group and a C=C separated by a single bond. • Resonance shows that the carbonyl carbon and the carbon bear a partial positive charge. 74 ,-Unsaturated Carbonyl Compounds • This means that ,-unsaturated carbonyl compounds can react with nucleophiles at two different sites. 75 1,2-Addition Mechanism • The steps for the mechanism of 1,2-addition are exactly the same as those for the nucleophilic addition of an aldehyde or a ketone—that is, nucleophilic attack, followed by protonation. 76 77 1,2 vs. 1,4-Addition Products 78 1,2 vs. 1,4-Addition Products O OH LiAlH4 1,2- reduction of enone 2) aq. H2O, H+ Li O BH 3 2) aq. H2O, H+ O 1,4- reduction of enone Summary of Organometallic Reactions [1] Organometallic reagents (R–M) attack electrophilic atoms, especially the carbonyl carbon. 80 Summary of Organometallic Reactions [2] After an organometallic reagent adds to the carbonyl group, the fate of the intermediate depends on the presence or absence of a leaving group. [3] The polarity of the R–M bond determines the reactivity of the reagents: • RLi and RMgX are very reactive reagents. • R2CuLi is much less reactive. 81 Synthesis Practice • Synthesize 1-methylcyclohexene from cyclohexanol and any organic alcohol. • Begin with Retrosynthetic Analysis: • Form double bond from alcohol dehydration. • Make the 3o alcohol by Grignard addition. • Prepare the Grignard from methanol. 82 Synthesis Practice • Four steps are required to accomplish the synthesis. • Convert methanol to the Grignard reagent by forming the alkyl halide, followed by reaction with Mg. • Add the Grignard reagent to cyclohexanone, followed by protonation, to form the alcohol. • Acid-catalyzed elimination of water forms the desired product as the major product. 83