* Your assessment is very important for improving the work of artificial intelligence, which forms the content of this project
Download THE ELECTRODE-TISSUE INTERFACE DURING RECORDING
Neuropsychology wikipedia , lookup
Neuroplasticity wikipedia , lookup
Optogenetics wikipedia , lookup
Neural modeling fields wikipedia , lookup
Neuroanatomy wikipedia , lookup
Development of the nervous system wikipedia , lookup
Cortical cooling wikipedia , lookup
Neuropsychopharmacology wikipedia , lookup
Holonomic brain theory wikipedia , lookup
Patch clamp wikipedia , lookup
Nervous system network models wikipedia , lookup
Metastability in the brain wikipedia , lookup
Biological neuron model wikipedia , lookup
Neural engineering wikipedia , lookup
Evoked potential wikipedia , lookup
Transcranial direct-current stimulation wikipedia , lookup
Microneurography wikipedia , lookup
Neuroprosthetics wikipedia , lookup
Multielectrode array wikipedia , lookup
Neurostimulation wikipedia , lookup
THE ELECTRODE-TISSUE INTERFACE DURING RECORDING AND
STIMULATION IN THE CENTRAL NERVOUS SYSTEM
by
SCOTT FRANCIS LEMPKA
Submitted in partial fulfillment of the requirements
For the degree of Doctor of Philosophy
Dissertation Advisor: Dr. Cameron C. McIntyre
Department of Biomedical Engineering
CASE WESTERN RESERVE UNIVERSITY
May, 2010
CASE WESTERN RESERVE UNIVERSITY
SCHOOL OF GRADUATE STUDIES
We hereby approve the thesis/dissertation of
Scott Francis Lempka
_____________________________________________________
Ph.D.
candidate for the ______________________degree
*.
Dominique M. Durand
(signed)_______________________________________________
(chair of the committee)
Cameron C. McIntyre
________________________________________________
Jerrold L. Vitek
________________________________________________
Robert F. Kirsch
________________________________________________
Uziel Landau
________________________________________________
Dawn M. Taylor
________________________________________________
March 18, 2010
(date) _______________________
*We also certify that written approval has been obtained for any
proprietary material contained therein.
DEDICATION
I would like to dedicate this dissertation to Puffy.
Without her sacrifice this work would not have been possible
TABLE OF CONTENTS
TITLE PAGE ....................................................................................................................... I
SIGNATURE PAGE ..........................................................................................................II
DEDICATION.................................................................................................................. III
TABLE OF CONTENTS.................................................................................................. IV
LIST OF TABLES............................................................................................................ IX
LIST OF FIGURES ........................................................................................................... X
ACKNOWLEDGMENTS ...............................................................................................XII
LIST OF ABBREVIATIONS..........................................................................................XV
ABSTRACT.....................................................................................................................XX
1
CHAPTER 1: INTRODUCTION ............................................................................... 1
1.1
Introduction......................................................................................................... 1
1.1.1
Recording applications – brain machine interfaces .................................... 1
1.1.2
Stimulation applications – deep brain stimulation...................................... 6
1.2
Problem statement............................................................................................... 9
1.2.1
Recording applications – intracortical microelectrode recordings ............. 9
1.2.2
Stimulation applications – deep brain stimulation.................................... 10
1.3
Hypotheses........................................................................................................ 11
1.3.1
Recording applications – intracortical microelectrode recordings ........... 12
1.3.2
Stimulation applications – deep brain stimulation.................................... 12
1.4
Project goals...................................................................................................... 13
1.4.1
Recording applications – intracortical microelectrode recordings ........... 13
1.4.2
Stimulation applications – deep brain stimulation.................................... 13
iv
2
CHAPTER 2: THE ELECTRODE-TISSUE INTERFACE ..................................... 15
2.1
Definition .......................................................................................................... 15
2.2
The foreign-body reaction at the ETI................................................................ 15
2.2.1
2.3
Effect of the foreign-body reaction on neural recording and stimulation. 20
Impedance models of the ETI ........................................................................... 21
2.3.1
2.4
ETI characterization with EIS........................................................................... 27
2.4.1
Basic methods and principles of EIS ........................................................ 27
2.4.2
Published examples of ETI characterization using EIS............................ 31
2.5
3
Constant phase element............................................................................. 25
Summary ........................................................................................................... 33
CHAPTER 3: THEORETICAL ANALYSIS OF CHRONIC INTRACORTICAL
MICROELECTRODE RECORDINGS............................................................................ 34
3.1
Introduction....................................................................................................... 34
3.2
Methods............................................................................................................. 37
3.2.1
Cortical recording model .......................................................................... 38
3.2.2
Noise models............................................................................................. 42
3.2.3
Electrode impedance spectroscopy ........................................................... 48
3.3
Results............................................................................................................... 50
3.3.1
Signal amplitude estimation...................................................................... 50
3.3.2
Noise estimation and SNR analysis .......................................................... 50
3.4
Discussion ......................................................................................................... 54
3.4.1
Recording amplitude................................................................................. 54
3.4.2
Noise levels............................................................................................... 55
v
3.4.3
Comparison to Experimental Results........................................................ 60
3.4.4
Study limitations ....................................................................................... 61
3.4.5
Future directions ....................................................................................... 66
3.5
4
Conclusion ........................................................................................................ 67
CHAPTER 4: IMPEDANCE OF DEEP BRAIN STIMULATION
ELECTRODES… ............................................................................................................. 68
4.1
Introduction....................................................................................................... 68
4.2
Materials and methods ...................................................................................... 70
4.2.1
Electrode impedance spectroscopy ........................................................... 70
4.2.2
Equivalent circuit models ......................................................................... 76
4.2.3
Parameter estimation................................................................................. 80
4.3
Results............................................................................................................... 81
4.3.1
Model analysis .......................................................................................... 81
4.3.2
Changes in electrode impedance after implantation ................................. 81
4.3.3
Stimulation-induced changes in electrode impedance.............................. 85
4.4
Discussion ......................................................................................................... 88
4.4.1
Characterizing DBS electrode impedance ................................................ 88
4.4.2
Model limitations ...................................................................................... 93
4.4.3
Experimental limitations........................................................................... 94
4.4.4
Clinical relevance of results...................................................................... 95
4.5
Conclusion ........................................................................................................ 97
vi
5
CHAPTER 5: CURRENT-CONTROLLED DEEP BRAIN STIMULATION
REDUCES IN VIVO VOLTAGE FLUCTUATIONS OBSERVED DURING
VOLTAGE-CONTROLLED STIMULATION ............................................................... 98
6
5.1
Introduction....................................................................................................... 98
5.2
Materials and Methods...................................................................................... 99
5.2.1
Stimulation and recording protocols......................................................... 99
5.2.2
Surgical procedure and DBS electrode implantation.............................. 104
5.2.3
Voltage changes after DBS electrode implantation ................................ 104
5.2.4
Voltage changes during voltage-controlled DBS ................................... 105
5.2.5
Voltage-controlled v. current-controlled DBS........................................ 105
5.3
Results............................................................................................................. 106
5.4
Discussion ....................................................................................................... 112
CHAPTER 6: DISCUSSION AND CONCLUSION ............................................. 119
6.1
Contributions of the research .......................................................................... 119
6.1.1
Neural recording – intracortical microelectrodes ................................... 119
6.1.2
Neurostimulation - deep brain stimulation ............................................. 120
6.2
Implications of the research and future directions.......................................... 122
6.2.1
Neural recording – intracortical microelectrodes ................................... 122
6.2.2
Neurostimulation – deep brain stimulation............................................. 123
6.3
Limitations ...................................................................................................... 125
6.3.1
Neural recording – intracortical microelectrodes ................................... 125
6.3.2
Neurostimulation – deep brain stimulation............................................. 126
6.4
Final conclusion .............................................................................................. 127
vii
REFERENCES ............................................................................................................... 128
viii
LIST OF TABLES
Table 3.1. Variability in noise estimates........................................................................... 58
ix
LIST OF FIGURES
Figure 1.1. Various types of electrodes used in BMI technology....................................... 3
Figure 1.2 Examples of standard intracortical microelectrode arrays ................................ 5
Figure 1.3. Components of an implanted DBS system....................................................... 7
Figure 2.1. Histological examples of the ETI for microelectrodes and macroelectrodes. 19
Figure 2.2. Components of the ETI. ................................................................................. 21
Figure 2.3 Standard electrode-electrolyte interface models. ............................................ 23
Figure 2.4. Standard tissue impedance models................................................................. 25
Figure 2.5. Example of an ideal capacitor and CPE on the complex plane...................... 26
Figure 2.6. Electrode impedance spectroscopy methodology. ......................................... 28
Figure 2.7. Two- and three-electrode cell configurations................................................. 30
Figure 2.8. Correlation of EIS measurements with histological measurements. .............. 32
Figure 3.1. Overall recording model infrastructure. ......................................................... 38
Figure 3.2. Coupled neuron-FEM model for extracellular neural recording.................... 42
Figure 3.3. Impedance model for thermal noise estimates. .............................................. 51
Figure 3.4. Signal recording amplitude estimation and biological noise model............... 53
Figure 3.5. Simulated extracellular recordings and noise................................................. 54
Figure 4.1. Surgical planning and electrode implantation. ............................................... 74
Figure 4.2. EIS of the implanted DBS electrodes............................................................. 75
Figure 4.3. Equivalent circuit models. .............................................................................. 79
Figure 4.4. Evolution of DBS electrode impedance after implantation............................ 83
Figure 4.5. Model parameters after electrode implantation.............................................. 84
Figure 4.6. Effect of clinically-relevant stimulation on DBS electrode impedance. ........ 87
x
Figure 5.1. In vivo microelectrode recordings examining the temporal evolution of the
voltages generated in the brain during DBS. .................................................................. 103
Figure 5.2. Effect of the foreign-body reaction on the voltages generated in the brain
during voltage-controlled DBS. ...................................................................................... 107
Figure 5.3. Temporal voltage fluctuations observed in the brain during voltage-controlled
DBS................................................................................................................................. 109
Figure 5.4. Temporal voltage changes observed during current-controlled and voltagecontrolled DBS................................................................................................................ 111
xi
ACKNOWLEDGMENTS
I would like to thank all of the people who have made this dissertation possible. It
has been a trying and interesting time in my life and I thank all of you for your support.
I would like to thank all members of the Vitek lab (past and present): Erin
Bynum, Melissa Jedlicka, Sohaib Khalid, Jenny Minnich, Garry Russo, Feng Weng,
Weidong Xu, and Jianyu Zhang. Thanks for sharing your space, helping with
experiments, and covering for me when I was out of town.
I would like to thank all of my labmates (past and present) who have helped me
along the way: Chris Butson, Ashu Chaturvedi, Tom Foutz, Phil Hahn, J.L. Lujan, and
Angela Noecker. Thanks for making Thursday lab lunches so interesting.
I would like to thank Mike Moffitt for the hard work he put into the original
development of the cortical recording model described in Chapter 3. I would also like to
thank Kevin Otto and Matt Johnson for sharing the in vivo impedance spectroscopy data
that was used for the model anlaysis in Chapter 3.
I also want to thank Jeff Weisgarber for sharing his electronics expertise.
Thanks to all of the wonderful friends and people I have met over the years. A
couple of people in particular have helped me tremendously. D. Mike thanks for knowing
everything and for being willing to share your knowledge with me. L. thanks for being
such an amazing friend and for helping me through all of this.
Dr. B. thanks for being a great advisor and friend. I have learned a lot from you
and I appreciate that you still help me to this day. Maybe one day we can actually
estimate cell membrane capacitance.
xii
I would, of course, like to thank my committee members. Dr. Kirsch thanks for
stepping up when I needed you and for helping me with all of the annoying graduate
requirements over the years. Dr. Landau thanks for helping spark my interest in
electrochemisty, even though I am not good at it. I really enjoyed your class. Dawn
thanks for your willingness to help and for sharing your wealth of knowledge in the area
of microelectrode recording. Dr. Durand thanks for scaring me into working harder. Dr.
Vitek thanks for helping a fellow mid-westerner and for sharing your lab. I wish you luck
in Minnesota.
There are two people who were instrumental in my graduate career and I
definitely would not been able to do this work without them. Svjetalana Miocinovic is
one of the smartest and most generous people I know. She did a lot of the dirty work and
I just happened to come along at the right time and take advantage of her efforts. The
other person is Matt Johnson. Matt is a true academic and loves science. He shared this
passion with me and was willing to spend countless hours helping with experiments and
helping me learn. I owe so much to the two of you. Thank you.
I would like to thank Cameron. He has been an amazing boss who has been more
patient with me than I deserved. I have learned so much from him about the art of being a
scientist. Thanks for picking up a grad student who needed a place to work. I can’t
imagine it having turned out any better (for me).
Last, and most importantly, I would like to thank my amazing family. Katelin Jo
thanks for keeping it weird. I am proud of you and I miss you. Matty, thanks for teaching
me what it means to be truly selfless. Dad, thanks for teaching me the meaning of family.
xiii
Mom, thanks for showing me unconditional love. I love you all more than you know and
everyday I try to make you proud. Sorry I have been away from home for so long.
This work was supported by the National Institutes of Health (R01 NS047388 and
R01 NS037019), a United States Department of Education Graduate Assistance in the
Areas of National Need (GAANN) fellowship, and a grant from the Ohio Biomedical
Research and Technology Transfer Partnership.
xiv
LIST OF ABBREVIATIONS
3D
– three-dimensional
α
– phase factor of the CPE (0 ≤ α ≤1)
αe
– phase factor for the CPE representing the electrode-electrolyte interface
αt
– phase factor for the CPE representing the tissue capacitance
AE
– auxiliary electrode
Ag|AgCl – silver-silver chloride
Am
– membrane area scaling term (cm2)
BMI
– brain machine interface
Cdl
– Helmholtz double layer capacitance
cm
– specific membrane capacitance (1 µF / cm2)
cm
– centimeter (10-2 meter)
CE
– counter electrode
CNP
– cortical neural prosthetic
CNS
– central nervous system
CPE
– constant phase element
DBS
– deep brain stimulation
ºC
– degrees Celsius
DC
– direct current
∆R
– difference between R0 and R∞ (i.e. R0 - R∞)
Ea
– potential applied to an electrochemical cell
ECM – extracellular matrix
EEG
– electroencephalography
xv
EIS
– electrode impedance spectroscopy
ETI
– electrode-tissue interface
f
– ordinary frequency (in hertz)
gm
– specific membrane conductance (0.3 mS / cm2)
GP
– globus pallidus
Hz
– hertz (1 / second)
Hsig
– transfer function of the recording band-pass filter
i
– current between two electrodes for EIS measurements
I(f)
– electrode current at a specific frequency
IPG
– implantable pulse generator
iR-drop – ohmic overpotential between two electrodes
Isource – ideal point current source, load condition to solve recording FEM
I/V
– current-voltage
J
– matrix containing the transmembrane currents for each model compartment
k
– Boltzmann’s constant (1.3807 x 10-23 joules / kelvin)
K
– degrees kelvin
K
– magnitude scaling factor for CPE (Ω s-α)
K
– vector containing the voltages generated at the model recording electrode for a
unit current at the position of each model neuron compartment
Ke
– magnitude scaling factor for CPE representing the electode-electrolyte interface
kHz
– kilohertz (103 hertz)
kΩ
– kilohm (103 ohm)
Kt
– magnitude scaling factor for CPE representing the tissue capacitance
xvi
LFP
– local field potential
M
– molarity (moles/liter)
MEMS – micro-electromechanical systems
MΩ
– megaohm (106 ohm)
µA
– microamp (10-6 amp)
µF
– microfarad (10-6 farad)
µm
– micrometer (10-6 meter)
µs
– microsecond (10-6 second)
µV
– microvolt (10-6 volt)
mg
– milligram (10-3 gram)
mL
– milliliter (10-3 liter)
mm
– millimeter (10-3 meter)
mS
– millisiemens (10-3 siemens)
mV
– millivolt (10-3 volt)
ω
– angular frequency (radians / second)
Ω
– ohm
p
– p-value
PD
– Parkinson’s disease
φ
– phase angle of a CPE
Φ
– vector containing the model voltages recorded at each time step
pF
– picofarad (10-12 farad)
pH
– potentiometric hydrogen ion concentration
R0
– resistance measured at DC
xvii
RC
– compensated resistance in a three-electrode cell configuration
Rct
– Faradaic (charge transfer) resistance
Ren
– encapsulation resistance
Rex
– resistance of extracellular space
R∞
– resistance measured at an infinite frequency
ROI
– region-of-interest
rS
– Spearman rank-order correlation coefficient
RS
– resistance of bulk solution
RU
– uncompensated resistance in a three-electrode cell configuration
S(ω)
– thermal noise spectral density
σ
– conductivity of the conducting medium (siemens / cm)
SNR
– signal-to-noise ratio
STN
– subthalamic nucleus
SV(ω) – unfiltered thermal noise spectral density derived from the Johnson-Nyquist
formula
T
– absolute temperature
UIEA – Utah Intracortical Electrode Array
V
– volt
V(f)
– electrode voltage at a specific frequency
W
– Warburg impedance element
WE
– working electrode
Z
– electrode impedance
ZCPE
– impedance of a CPE
xviii
Zmodel – equivalent circuit model impedance
xix
The Electrode-Tissue Interface during Recording and Stimulation in the Central Nervous
System
Abstract
by
SCOTT FRANCIS LEMPKA
It is commonly assumed that the long-term functionality of electrodes used for
recording and stimulation in the central nervous system (CNS) is highly dependent on the
composition of the electrode-tissue interface (ETI). The ETI consists of various ions,
proteins, and cells adhered directly to the electrode contact with a surrounding
encapsulation layer. However, there is a lack of quantitative details and experimentallyvalidated models describing the ETI. This project exploited both experimental and
theoretical techniques to provide a more detailed description of the ETI for recording and
stimulation applications in the CNS.
In this study, the ETI was characterized for chronic recording with intracortical
microelectrode arrays that are typically used in brain machine interface (BMI)
applications. This study utilized a detailed cortical recording model incorporating a
volume conductor model with an explicit representation of the recording microelectrode
and a neural source model. Thermal and biological noise sources were also incorporated
into the modeling infrastructure. Model analysis showed electrode design along with a
number of other factors (e.g. recording bandwidth, neural density, neural firing rate, etc.)
can significantly affect the recording quality.
xx
This study also examined changes at the ETI of chronically implanted deep brain
stimulation
(DBS)
electrodes
with
electrode
impedance
spectroscopy
(EIS)
measurements. Microelectrode voltage recordings showed that changes in the
composition of the ETI significantly affected the voltage distributions generated in the
brain during voltage-controlled stimulation. However, the effect of these interface
changes was minimized during current-controlled stimulation.
This study produced a detailed characterization of the complex environment of
the ETI for chronic recording and stimulation applications in the CNS. The details of this
complex environment are often oversimplified or neglected; however, the results of this
study show that consideration of these details is necessary to understand the confounding
factors that can limit the success of recording and stimulation applications in the CNS.
This study provides a significant step towards improving the technologies and therapies
for BMI and DBS applications and the results of the study can also be applied to the
general fields of neural recording and stimulation.
xxi
1
CHAPTER 1: INTRODUCTION
1.1
Introduction
It is commonly assumed that the long-term functionality of electrodes used for
recording and stimulation in the central nervous system (CNS) is highly dependent on the
composition of the electrode-tissue interface (ETI). The ETI consists of various ions,
proteins, and cells adhered directly to the electrode contact with a surrounding
encapsulation layer. However, there is a lack of quantitative details and experimentallyvalidated models that describe the ETI. This project addressed some of these
shortcomings by exploiting both experimental and theoretical techniques to provide a
more detailed description of the ETI. The results of this study will allow for improved
cortical microelectrode designs for recording applications and provide a better
understanding of the effect of ETI composition on the voltage distributions generated
during stimulation in the CNS.
1.1.1
Recording applications – brain machine interfaces
The therapeutic goal of a brain machine interface (BMI) is to extract signals
directly from the brain to aid in the restoration of sensory or motor function lost by
disease or injury. Recent advances in neuroscience and neurotechnology have enabled the
development of the first clinically relevant BMIs for human patients. Current examples
include controlling cursor movement on computer screens for brain stem stroke,
amyotrophic lateral sclerosis, or tetraplegic patients (Kennedy and Bakay, 1998; Wolpaw
and McFarland, 2004; Hochberg et al., 2006). These diseases affect nearly two million
1
people in the United States and millions more worldwide (Wolpaw et al., 2002). These
millions of people are the driving force behind the development of BMI technology so
that one day lost sensory or motor functions may be restored and these patients can enjoy
an improved quality of life.
BMI technology can be divided into a number of categories, but there are three
general components of all BMI systems: data acquisition module, data interpretation
module, and data output module. The job of the data acquisition module is to capture
neural signals that represent the control signal for the BMI. The neural signals can be
captured using a variety of methods such as scalp electrodes, conductive bone screws,
subdural electrode grids, or intracortical microelectrodes (Fig. 1.1) (Kennedy et al., 2004;
Hochberg et al., 2006; Vaughan et al., 2006; Wilson et al., 2006). All of these electrode
types record neural signals at different resolutions. Scalp electrodes record gross regional
field potentials generated from centimeters of cortex while intracortical microelectrodes
can be used to monitor local field potentials (LFP) on the submillimeter scale or the firing
activity of single neurons. The second major component of a BMI is the data
interpretation module. The neural signal captured by the data acquisition module is sent
to the data interpretation module via a telemetric communication or direct wire contact.
The function of the data interpretation module is to transform the digitized signal into a
code that best represents the desired action. The last step in a BMI is the generation of a
command signal from the data interpretation module and production of the desired output
of the BMI. Some of the typical outputs involve the movement of a cursor on a computer
screen, movement of a robotic arm, or control of a functional electrical simulation device
2
designed to activate paralyzed muscles (Lauer et al., 1999; Taylor et al., 2003; Hochberg
et al., 2006).
1.1.1.1 Cortical neural prosthetics
Cortical neural prosthetics (CNP) are a subclass of BMIs that can be used to
overcome some of the limitations of electroencephalography (EEG)-controlled BMIs at
the cost of being more invasive (Friehs et al., 2004). CNPs use intracortical
microelectrodes to monitor the neural signals (inset, Fig. 1.1). The measured signals can
either be LFPs or single-unit action potentials. The LFPs recorded using these cortical
microelectrodes represent a smaller population of neurons relative to recordings from
EEG-based BMIs. Single-unit recordings represent the firing patterns of single cells and
can be modulated rapidly. This ability to rapidly modulate signals expands the bandwidth
of CNPs and allows them to be used for controlling more complex movements (Wessberg
et al., 2000; Serruya et al., 2002; Taylor et al., 2002).
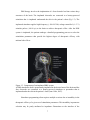
Figure 1.1. Various types of electrodes used in BMI technology.
Shown are scalp EEG electrodes, conductive bone screws, a subdural electrode grid, and
an intracortical microelectrode array. (Image courtesy of DM Taylor, Cleveland Clinic)
3
1.1.1.2 Microelectrode technology
There are many types of intracortical microelectrodes available to monitor cortical
signals. One popular type of intracortical microelectrodes is microwires (Fig. 1.2D-E)
(Williams et al., 1999; Taylor et al., 2002). The advantage of using microwires is the
simplicity of fabrication and durability. However, microwires do not allow for detailed
geometric knowledge of the relative position of the recording electrodes to each other or
the overall cortical layering architecture.
Other
popular
types
of
cortical
microelectrodes
are
silicon-substrate
microelectrodes fabricated through the use of micro-electromechanical systems (MEMS)based technology (Jones et al., 1992; Wise et al., 2004). The two most popular types of
microelectrode arrays have been developed at the University of Utah, termed the Utah
Intracortical Electrode Array (UIEA), and at the University of Michigan. The UIEA
consists of 100 needle electrodes of ~1-1.5 mm in length over a 4x4 mm grid, allowing
for high density recording over a small cortical area (Fig. 1.2A-B). A major drawback of
the UIEA is that each electrode has only one contact and this contact is located at the tip
of the electrode shank. This contact location prevents recording from multiple cortical
layers along the same cortical column and excludes the possibility for developing true
three-dimensional (3D) electrode arrays.
The planar silicon-substrate microelectrodes fabricated at the Center for Neural
Communication Technology at the University of Michigan and at NeuroNexus
Technologies (Ann Arbor, MI) can be used to overcome some of the limitations of
microwires and the UIEA (Fig. 1.2C). These probes are being developed for acute and
chronic recording and/or stimulation of the CNS. The single- and four-shank designs now
4
have a number of design variations including holes through the shanks for tissue
anchoring and/or seeding with pharmacological agents, and distributed contact sites.
However, the design flexibility of the Michigan probes presents users with the question
of “What electrode is best for my application?”, and more importantly “Why?” These
questions have been largely ignored primarily because experimental trial-and-error
techniques to answer these questions are costly and time consuming.
Figure 1.2 Examples of standard intracortical microelectrode arrays
A) Silicon-based 100-channel microelectrode array developed at the University of Utah
and fabricated by Cyberkinetics Neurotechnology Systems (Foxborough, MA). B) View
of the metal recordings sites at the tip of each shank for the array shown in (A). C)
Silicon-based 16-channel microelectrode array developed at the University of Michigan
and fabricated by NeuroNexus Technologies. Four metal contacts can be seen at the end
of each of the four electrode shanks. D) Microwire microelectrode array fabricated by
Tucker-Davis Technologies (Alachua, FL). E) View of the recording sites of the
microwire array shown in (D) (modified from Ward et al., 2009).
5
1.1.1.3 Human applications and limitations
Recently, CNPs have been used to restore function in human patients (Kennedy
and Bakay, 1998; Hochberg et al., 2006). However, the translation of CNPs into human
applications has suffered from the poor long-term performance of the cortical
microelectrodes (Schwartz, 2004; Hochberg et al., 2006). The poor chronic recording
capabilities of the microelectrode arrays are typically caused by device failure (e.g. lead
wire fractures, electrical shorting due to insulation degradation) or the inability to
sufficiently isolate neuronal populations (i.e. low signal-to-noise ratio).
1.1.2
Stimulation applications – deep brain stimulation
Deep brain stimulation is a widely accepted surgical intervention for the treatment
of various movement disorders such as essential tremor, Parkinson’s disease (PD), and
dystonia (Limousin and Martinez-Torres, 2008; Lyons and Pahwa, 2008; Ostrem and
Starr, 2008). DBS is also being investigated for the treatment of several other
neurological disorders such as obsessive-complusive disorder, Tourette’s syndrome,
depression, and epilepsy (Hodaie et al., 2002; Gabriels et al., 2003; Mayberg et al., 2005;
Ackermans et al., 2008). DBS is typically selected for patients who are no longer
responding appropriately to medication and it has largely become as an alternative to
treatment with lesioning or ablating of target brain structures. DBS represents a low-risk
alternative to brain lesioning because it is reversible and the stimulation can be fine tuned
to maximize its therapeutic efficacy. The success of DBS therapies has resulted in
thousand of patients being implanted with DBS systems every year.
6
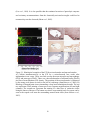
DBS therapy involves the implantation of a four-electrode lead into various deep
structures of the brain. The implanted electrodes are connected to a battery-powered
stimulator that is implanted underneath the skin in the patient’s chest (Fig 1.3). The
implanted stimulator applies high-frequency (~100-185 Hz) voltage-controlled (~1-3 V)
stimulus pulses (~60-90 µs) to the brain to achieve therapeutic effect. After the DBS
system is implanted, the patients undergo a detailed programming process to select the
stimulation parameters that provide the highest degree of therapeutic efficacy with
minimal side effects.
Figure 1.3. Components of an implanted DBS system.
A DBS stimulation lead is permanently implanted in the desired area of the brain and the
four electrodes are connected to a battery-powered stimulator or pacemaker that is
implanted under the skin in the patient’s chest (from Gulie, 2007).
Stimulator programming often requires multiple sessions due to instability in the
therapeutic efficacy of a given set of stimulation parameters. This instability in parameter
selection may be partly attributed to impedance fluctuations at the interface of the
7
stimulating electrode. For example, most clinical centers find it is necessary to wait 3-4
weeks after electrode implantation before beginning the process of therapeutic
stimulation parameter selection to ensure that disease symptoms stabilize from any
micro-lesioning effects induced in the operating room and to allow time for the foreignbody reaction to stabilize (Deuschl et al., 2006). If patient programming is started within
the initial 3-4 weeks after implantation, there is often a need to frequently adjust the
parameter settings to maintain therapeutic benefit while minimizing unwanted side
effects.
Following the initial electrode stabilization period, stimulation parameters
(amplitude, pulse width, frequency) are selected by an experienced clinical programmer,
to be delivered through an electrode contact(s) that maximizes therapeutic benefit while
minimizing any stimulation-induced side effects. However, after a couple hours of
stimulation, unwanted side effects can appear (Volkmann et al., 2002). The appearance of
these unwanted side affects may be attributed to a decrease in electrode impedance
induced by the stimulation (Hemm et al., 2004; Lempka et al., 2009). The overall
decrease in electrode impedance that occurs due to the applied stimulation would result in
a larger area of tissue being stimulated for the same stimulation parameters (Butson et al.,
2006). Thus, as the impedance decreases over time, supra-threshold stimulation can reach
regions of the brain implicated in the appearance of various side effects (e.g. sustained
muscle contractions, dyskinesias, paresthesias, etc.).
The instability in stimulation parameters and variability in patient outcomes show
that although DBS for the treatment of movement disorders has been one of the most
successful clinical applications of neurostimulation, there is a need to further improve
8
and optimize the therapy. In addition, while the clinical applications of DBS have been
widely accepted the therapeutic mechanisms of DBS still remain unclear. In order to
optimize DBS therapies, we need to develop a better understanding of the effects of
stimulation on the surrounding neural tissue.
1.2
Problem statement
It is commonly assumed that the long-term functionality of electrodes used for
recording and stimulation in the central nervous system (CNS) is highly dependent on the
composition of the complex environment surrounding an implanted electrode; however,
there is a lack of quantitative details and experimentally-validated models describing the
ETI that limits the ability to design successful chronic recording and stimulation systems.
1.2.1
Recording applications – intracortical microelectrode recordings
Translation of CNPs into human applications has suffered from the poor long-
term performance of the cortical microelectrodes (Schwartz, 2004; Hochberg et al.,
2006). It is believed that the development of these chronic recording electrodes is the
single biggest remaining challenge in the development of CNPs (Schwartz, 2004). With
current microelectrode technology, an electrode chronically implanted in monkey cortex
only has a 40 - 60 % chance of recording unit activity and these recordings typically
deteriorate after a few months (Schwartz, 2004). In a recent publication using a human
CNP, over 50% of the electrodes malfunctioned within the first year after implantation
(Hochberg et al., 2006). There are many possible causes for electrode failure. However, if
9
these electrodes are to be used in human applications they must be able to perform
reliably for years.
One of the major reasons for poor long-term performance of these cortical
microelectrodes is a low recording signal-to-noise ratio (SNR). A low SNR can be caused
by a variety of factors such as the formation of a fibrous capsule around the recording
electrode or the firing of a large number of neurons in the surrounding biological media.
Fibrous encapsulation of the electrode is due to the foreign body reaction and results in
the formation of a high impedance layer around the electrode (Grill and Mortimer, 1994;
Turner et al., 1999; Szarowski et al., 2003). This high impedance layer effectively
isolates the electrode from neurons in the surrounding gray matter and can lead to an
increase in thermal noise levels (Johnson et al., 2005; Ludwig et al., 2006; Otto et al.,
2006). The firing of several neurons in the surrounding biological media can also lead to
the inability to preferentially record from a single cell (i.e. biological noise) (Ludwig et
al., 2006).
1.2.2
Stimulation applications – deep brain stimulation
As described above, clinical DBS systems typically apply voltage-controlled
stimulus pulses to the brain to achieve their therapeutic effect. A consequence of applying
voltage-controlled sitmulation is that the voltage-distributions generated in the brain
during DBS are dependent upon the composition of the ETI and the corresponding
electrode impedance (Gimsa et al., 2005; Wei and Grill, 2005; Butson et al., 2006;
Miocinovic et al., 2008).
10
In the days and weeks after surgical implantation of an electrode into peripheral
or central nervous system tissue, electrode impedances typically increase due to the
foreign-body reaction in which proteins and cells attach directly to the electrode and an
encapsulation layer develops around the implanted device (Grill and Mortimer, 1994; Xu
et al., 1997; Haberler et al., 2000; Szarowski et al., 2003; Moss et al., 2004; Biran et al.,
2005; Johnson et al., 2005; Williams et al., 2007). After several weeks, the foreign body
reaction and the electrode-tissue impedance typically stabilize (Grill and Mortimer, 1994;
Lempka et al., 2009), but this stability can be perturbed with electrical stimulation
(Johnson et al., 2005; Otto et al., 2006; Lempka et al., 2009). Clinical measurements
have also shown reversible decreases in DBS electrode impedance following electrical
stimulation (Hemm et al., 2004).
Because commercial DBS systems utilize voltage-controlled stimulation, these
changes in ETI composition will have an effect on the amplitude and shape of the
stimulation that reaches the target neural tissue and the corresponding volume of tissue
activated by stimulation (Butson et al., 2006; Miocinovic et al., 2008). Moreover,
fluctuations in the DBS electrode impedance may be responsible for some clinical
observations relevant to complications in patient programming.
1.3
Hypotheses
This project utilized theoretical and experimental techniques to investigate how the
ETI affects electrode performance during chronic recording and stimulation applications
in the CNS. This project was designed to answer the hypotheses described below.
11
1.3.1
Recording applications – intracortical microelectrode recordings
This research project produced a modeling infrastructure to analyze numerous
factors influencing the quality of chronic extracellular microelectrode recordings (e.g.
microelectrode design, recording noise). Although this theoretical framework can be used
to address many potential issues or problems, this study was directed at answering the
following hypothesis:
• Microelectrode design (i.e. contact size) can be theoretically optimized to
improve long-term recording capabilities.
1.3.2
Stimulation applications – deep brain stimulation
This project produced a detailed description of changes in the ETI composition of
DBS electrodes that occur in vivo and the effect of these changes on the voltage
distributions generated in the brain during stimulation. These studies were guided by
three major hypotheses:
1.) After electrode implantation, the foreign-body reaction leads to electrode
encapsulation and the generation of lower voltage amplitudes in the brain during
(voltage-controlled) DBS.
2.) Application of continuous stimulation disrupts the stability of the ETI,
allowing for higher voltage amplitudes to be generated in the brain during (voltagecontrolled) DBS.
3.) The voltages generated in the brain during current-controlled DBS are less
dependent upon the composition of the ETI relative to voltage-controlled DBS.
12
1.4
Project goals
The fundamental goal of this project was to provide a more detailed description of
the ETI and investigate how its composition affects electrode performance during chronic
recording and stimulation in the CNS. The proposed project addressed some of these
shortcomings by exploiting both experimental and theoretical techniques to provide a
more detailed description of the ETI.
1.4.1
Recording applications – intracortical microelectrode recordings
A fundamental goal of this study was to develop new techniques to optimally
design cortical microelectrodes. This goal included the utilization of experimental and
theoretical techniques to devise an overall modeling infrastructure to study arbitrary
electrode designs and numerous factors affecting the quality of intracortical
microelectrode recordings. Specifically, the goal of this project was to investigate the
effect of microelectrode contact size on recording quality and account for the major noise
sources during intracortical microelectrode recordings (i.e. biological and thermal noise).
Optimization of microelectrode design with this type of theoretical analysis could help
produce BMI technology with the long-term performance required for human
applications.
1.4.2
Stimulation applications – deep brain stimulation
The major goal of this study was to provide a detailed description of the
impedance of chronically-implanted DBS electrodes and investigate the effects of
ETI composition on the voltage distributions generated in the brain during
13
stimulation. Specifically, this project investigated changes in the impedance of DBS
electrodes after implantation and during the start of prolonged stimulation. This project
then considered how these impedance changes affected the voltage distributions
generated in the brain during both voltage-controlled and current-controlled stimulation.
Understanding the changes that occur at the ETI and their functional significance could
assist in the definition of new or alternative programming paradigms to improve the DBS
patient experience.
14
2
2.1
CHAPTER 2: THE ELECTRODE-TISSUE INTERFACE
Definition
The electrode-tissue interface (ETI) of a recording or stimulating electrode can be
defined as the electrode-electrolyte interface and the tissue immediately surrounding the
electrode. When an electrode is implanted in vivo a complicated series of events, called
the foreign body reaction, lead to major changes in the environment surrounding the
electrode. This reaction can significantly affect the performance of the electrode in its
desired application (i.e. recording or stimulation). For many years, the ETI has been
characterized with histological techniques to examine the composition of the ETI and its
evolution over time. More recently, detailed impedance spectroscopy measurements
applied to various impedance models have also been considered as a means to study the
behavior and composition of the ETI.
2.2
The foreign-body reaction at the ETI
After an electrode is implanted into the central nervous system (CNS), there is a
response from cells in the surrounding tissue in an attempt to remove the object or
effectively isolate it from the remaining bulk tissue. This response is termed the foreign
body reaction. The foreign body reaction involves a number a steps and can be
considered to have an acute phase and a chronic phase. This reaction is dynamic and lasts
for the entire lifetime of the electrode.
There are two major effector cell types considered to be involved in the foreign
body reaction of the CNS: astrocytes and microglia. The first cell type, astrocytes, serve
15
numerous functions in the CNS and make up approximately 30-65% of the total number
of glial cells (Glees, 1955; Nathaniel and Nathaniel, 1981). Astrocytes provide
mechanical support for neuronal circuits and help control the chemical environment
surrounding neurons through the buffering of neurotransmitters and ions. Astrocytes also
establish end feet structures at capillary walls that aid in the transfer of nutrients across
the blood-brain barrier and similar structures that make up the glia limitans that form the
boundary between CNS and non-CNS structures. When injury occurs in the CNS,
asctrocytes can become activated and make a transition from a normal to a “reactive”
phenotype. This phenotype is characterized by hypertrophy, phagocytosis, proliferation,
migration, increased extracellular matrix (ECM) production, and production of
neurotrophic and inflammatory factors (Polikov et al., 2005). Astrocyte activation also
leads to a large increase in the production of glia fibrillary acid protein (GFAP) that
allows astrocyte activation to be quantified in histological analyses with GFAP staining.
Microglia are the second effector cell type to play a major role in the foreign body
reaction of the CNS and make up 5-10% of the total number of glial cells (Glees, 1955;
Ling, 1981). Microglia are the resident macrophages of the CNS and can serve as either
cytotoxic cells or phagocytes that secrete proteolytic enzymes to degrade cellular debris
or damaged ECM after injury or during normal cell turnover. Microglia are normally in a
resting or ramified state with a cell geometry characterized by long processes. However,
CNS injury can lead to activation of microglia in which a more compact or amoeboid
phenotype is adopted. These activated microglia attempt to phagocytose foreign material
and also increase production of proteolytic enzymes to aid in the degradation of foreign
objects. Activated microglia secrete numerous soluble factors such as chemokines to
16
recruit macrophages and activated microglia, cytokines to promote the inflammatory
response, neurotrophic factors to promote neuronal survival and growth, and cytotoxic
and neurotoxic factors (Polikov et al., 2005).
The first phase of the foreign body reaction occurs after the initial trauma of
electrode implantation. Insertion of the electrode in the brain causes damage to
vasculature, extracellular matrix (ECM), and neural and non-neuronal cells at the
implantation site. Damage to the local vasculature leads to a number of results: fluid
accumulation at the implantation site, release of serum and blood cells, and activation of
platelets, clotting factors, and the complement cascade to aid in the recruitment of
macrophages and tissue repair (Polikov et al., 2005; Stroncek and Reichert, 2008).
Activated and proliferating macrophages begin to appear at the implantation site as soon
as one day after implantation and play a major role during the acute phase of the foreign
body reaction that persists for 1-3 weeks after electrode implantation (Polikov et al.,
2005). During the first week after implantation, the accumulation of fluid and cellular
debris is reduced due to the action of the activated microglia and through reabsorption
(Stensaas and Stensaas, 1976). The activation and migration of astrocytes is also seen
during the acute phase of the foreign body reaction.
The foreign body reaction to the implanted electrode persists and the chronic
phase of the foreign body reaction is characterized by the presence of activated microglia
and astrocytes. Over time the distribution of astrocytes become more compact and forms
a stable encapsulation layer around the electrode. At 6-12 weeks after implantation,
histology has shown that this encapsulation layer extends approximately 50-100 µm from
the electrode (Szarowski et al., 2003). This encapsulation layer is similar to the fibrotic
17
encapsulation layer that is observed in the foreign body reaction outside the CNS. Inside
the encapsulation layer, there is typically a 1-2 cell thick layer of microglia in which a
number of microglia can attach directly to the electrode (Kim et al., 2004; Anderson et
al., 2008). The microglia attempt to phagocytose the electrode and also secrete various
proteolytic enzymes in an attempt to degrade the foreign object. The microglia can
undergo “frustrated” phagocytosis that will lead them to form multi-nucleated “giant”
cells similar to the foreign-body giant cells seen in the foreign body reaction outside the
CNS (Polikov et al., 2005).
Although the glial scar in the CNS is often simply discussed in terms of astrocytes
and microglia, there are numerous other cell types and components that have been shown
to form part of this encapsulation layer. In addition to microglia and astrocytes, an
increased number of basal lamina and ECM molecules are often found along with several
types of collagen, fibronectin, and laminin (Kim et al., 2004). The presence of connective
tissue is similar to the ECM encapsulation seen outside the CNS. The presences of
meningeal fibroblasts at the electrode interface has also been suggested (Kim et al.,
2004).
An additional consequence of the foreign body reaction in the CNS is a decreased
neural density near the electrode. The size of this neural “kill zone” is highly variable and
has been reported to be between 1 µm and more than 100 µm (Fig 2.1A) (Biran et al.,
2005; Polikov et al., 2005). This decrease in neural density is often considered a result of
the initial tissue damage induced by electrode insertion. However, it is possible that a
lower neural density is a result of glial scar formation in which the accumulation of
microglia and astrocytes at the electrode could displace neurons away from the electrode
18
(Liu et al., 1999). It is also possible that the continued secretion of proteolytic enzymes
and excitatory neurotransmitters from the chronically-activated microglia could lead to
neurotoxicity near the electrode (Biran et al., 2005).
Figure 2.1. Histological examples of the ETI for microelectrodes and macroelectrodes.
A) Cellular immunoreactivity at the ETI for a microelectrode four weeks after
implantation in rat cortex. There was little overlap between microglia and macrophages
(ED1) and astrocytes (GFAP). There was a decreased number of neuronal bodies (NeuN)
and loss of neurofilament (NF) density. The position of the microelectrode is illustrated
by the orange oval (drawn to scale) to the left of each image (from Biran et al., 2005). B)
Histology of human brain tissue surrounding a deep brain stimulation electrode. The top
row represents GFAP staining of astrocytes at two separate magnifications (left and right
columns). The second row represents the staining of a thin layer of connective tissue
lining the lumen of the tract. The bottom row shows axon staining and a few axons can be
seen at the capsule wall near the surrounding normal brain tissue (from Nielsen et al.,
2007).
19
2.2.1
Effect of the foreign-body reaction on neural recording and stimulation
The foreign body reaction in the CNS is considered to be the main reason behind
the failure of microelectrode systems for chronic recording of neural activity in the brain.
The dynamic foreign body response causes instability in the recording quality of
microelectrodes and often leads to the inability to effectively record neural activity over
an extended period of time. The glial scar consists of a dense cellular layer with
connective tissue that results in a higher tissue resistivity around the electrode (Grill and
Mortimer, 1994; Otto et al., 2006; Williams et al., 2007). This increased tissue resisitivity
is thought to isolate the electrode and prevent it from recording the electrical activity of
neurons in the surrounding bulk brain tissue.
In addition to this high resistivity encapsulation layer, the foreign-body reaction
also creates a low neural density around the electrode. This neural kill zone can extend
more than 100 µm from the electrode surface. Theoretical models and experimental
measurements predict that to effectively identify the electrical activity of individual
neurons, a neuron must be located within a distance between 50 and 100 µm of the
electrode recording site (Rall, 1962; Henze et al., 2000; Moffitt and McIntyre, 2005).
Because the foreign body reaction can lead to a large decrease in neural density that
extends beyond a distance of 100 µm from the electrode, it severely decreases the
probability of recording neural activity with chronic microelectrode arrays.
Although much of the literature examining the foreign body reaction at the ETI
deals with microelectrode recording applications, these interface changes need to be
considered
for
neurostimulation
applications
with
both
microelectrodes
and
macroelectrodes. It is possible that changes at the ETI of stimulating electrodes can affect
20
the voltage distributions generated in the brain during stimulation and the corresponding
volume of tissue activated for a given set of stimulation parameters (Butson et al., 2006;
Miocinovic et al., 2009). Application of electrical stimulation has also been shown to
cause dramatic changes in the composition of the ETI (Keese et al., 2004; Otto et al.,
2006; Lempka et al., 2009). Changes at the ETI as part of the foreign body reaction and
from electrical stimulation could therefore lead to instability in therapeutic stimulation
levels.
2.3
Impedance models of the ETI
In order to develop a better theoretical understanding of the ETI and characterize its
behavior, impedance models of the ETI are often considered. The electrical properties of
the ETI can be modeled with spatially-lumped and spatially-distributed circuit elements.
These impedance models consist of two main components: 1) electrode-electrolyte
interface impedance, and 2) impedance of the biological tissue surrounding the electrode
(Fig. 2.2). These impedance models are based on physical properties and empirical data.
Figure 2.2. Components of the ETI.
Impedance models of the ETI typically consist of two main components that are
connected in series. The first component represents the impedance of the electrodeelectrolyte interface and the second component represents the impedance of the
surrounding biological tissue.
21
The first component of the impedance models of the ETI is the electrodeelectrolyte interface. The electrode-electrolyte interface is the region where electrical
charge from the metal electrode is transduced into ionic charge in the surrounding
electrolyte (or vice versa depending on the direction of the current). Charge transduction
at the electrode-electrolyte interface can occur via two different mechanisms: Faradaic
and non-Faradaic current. Faradaic current consists of charge transfer through reductionoxidation reactions in which electrons are exchanged between the metal atoms of the
electrode and species in the electrolyte. Non-Faradiac currents are capacitive in nature
and involve charge redistribution at the electrode and in the surrounding electrolyte. This
capacitive current typically consists of a double layer of charge accumulation and
separation, called the Helmholtz double layer, that resembles the charge build up at a
parallel-plate capacitor.
Models of the electrode-electrolyte interface typically consist of a resistance
representing the Faradaic impedance (Rct) and a capacitor representing the Helmholtz
double layer (Cdl) (Fig. 2.3A). Additional impedance models have been proposed in an
attempt to decribed the behavior of the electrode-electrolyte interface measured
experimentally. For example, a Randles equivalent circuit consists of a Warburg
impedance element in series with a charge transfer resistance (Fig 2.3B) (McAdams et
al., 1995; Geddes, 1997). The Warburg impedance element accounts for the diffusion of
ions to/from the electrode interface during the Faradaic reactions. The Faradaic branch of
the Randles equivalent circuit is connected in parallel to a double layer capacitance. In
many applications the electrode-electrolyte interface is assumed to be purely capacitive
22
and can be represented with a simple capacitor or a constant phase element (CPE) (Fig
2.3C) (see ‘2.3.1’).
The models shown in Fig. 2.3 are idealized models of the electrode-electrolyte
interface. In reality, the electode-electrolyte interface displays complex behavior that can
not always be accurately characterized with the simple impedance models shown in Fig.
2.3. When the impedance models of Fig. 2.3 are used to describe the behavior of the
electrode-electrolyte interface under a wide range of operating conditions, the model
parameters are not constant but can be a function of several parameters (e.g. currentdensity, potential, frequency) (Schwan, 1968; Dymond, 1976; Geddes, 1997).
Figure 2.3 Standard electrode-electrolyte interface models.
A) Standard model of the electrode-electrolyte interface consisting of a Faradaic charge
transfer resistance (Rct) and a capacitor representing the Helmholtz double layer (Cdl). B)
Randles equivalent circuit with a Warburg impedance element (W) to account for mass
transfer limitations. C) In several stimulation and recording applications, the electrodeelectrolyte interface is assumed to be purely capacitive and can be represented with a
simple capacitor or a constant-phase element (CPE).
23
The impedance of the biological tissue surrounding the electrode can be
represented by a number of impedance models (Fig. 2.4). Biological tissue has both
resistive and capacitive properties. One of the classical impedance models was originally
adopted by Lapique and included a resistance (R∞) in series with a parallel combinations
of an additional resistance (∆R) and a capacitance (Fig 2.4A) (Lapicque, 1907; McAdams
and Jossinet, 1995). Another impedance model has been used in the literature to describe
the impedance of microelectrodes chronically implanted in the neocortex that is similar to
the Lapicque model but contains cellular and extracellular compartments (Otto et al.,
2006; Williams et al., 2007). The cellular compartment represents the impedance of
membranes from cells in the immediate vicinity of the electrode and includes a specific
membrane conductance (gm) and capacitance (cm) multiplied by a membrane area scaling
term (Am) (Fig. 2.4B). The extracellular compartment (Rex) represents the resistance of
the extracellular pathways. Another standard tissue impedance model is a modified
Lapicque model that represents the tissue capacitance with a CPE that can improve the
accuracy of the impedance model in reproducing experimental measurements (Fig. 2.4C)
(McAdams and Jossinet, 1995; Lempka et al., 2009).
24
Figure 2.4. Standard tissue impedance models
A) Classical Lapicque equivalent circuit model. B) Tissue impedance model with cellular
and extracellular branches. The cellular branch represents the impedance of cells in the
vicinity of the electrode and consists of a specific membrane conductance (gm) and
capacitance (cm) multiplied by a membrane area scaling term (Am). C) Modified Lapicque
equivalent circuit model in which the tissue capacitance is modeled using a CPE.
2.3.1
Constant phase element
The electrode-electrolyte interface capacitance and tissue capacitance are often
represented with an empirically-derived constant phase element (CPE) impedance that
accounts for the pseudo-capacitive behavior of the electrode-electrolyte interface and
biological tissue (McAdams and Jossinet, 1995; McAdams et al., 1995). A CPE follows
the equation below:
25
Z CPE =
K
( jω )α
(2.1)
with a magnitude scaling factor (K) and a phase factor (α) defined for 0 ≤ α ≤1. For an
ideal capacitor, α = 1 and represents a line at 90 degrees relative to the abscissa on the
complex plane (Fig. 2.5A). For solid metal electrodes, experimental measurements have
shown that the phase angle is actually less than 90 degrees (Fig. 2.5B). This non-ideal
capacitive behavior has been attributed to surface roughness and specific adsorption
effects (Pajkossy, 1994; McAdams et al., 1995). Many investigators have tried to
determine the origin of this capacitance dispersion (i.e. α < 1) and have attempted to
explain it through a distribution of relaxation times at the electrode, transmission lines,
and fractal electrode geometry (McAdams, 1989a, b, 1990; Pajkossy, 1994). However,
these theoretical interpretations have been met with limited success. Pajkossy (1994)
presented strong evidence that the origin of this capacitance dispersion is purely an
interfacial phenomenon and is attributed to surface disorder and anion adsorption at the
electrode interface.
Figure 2.5. Example of an ideal capacitor and CPE on the complex plane.
A) Example of the 90 degree phase angle of an ideal capacitor on the complex plane. B) Example
of a CPE that has a phase angle of less than 90 degrees (φ = 90α). RS is representative of a series
or access resistance from in vitro measurements that produces a resistive shift along the abscissa.
26
2.4
2.4.1
ETI characterization with EIS
Basic methods and principles of EIS
The impedance of the ETI is most commonly characterized using impedance
measurements at a single frequency of 1 kHz. However, the behavior of the ETI is highly
frequency-dependent and this behavior can not be adequately described with impedance
measurements at a single frequency (Otto et al., 2006; Williams et al., 2007; Lempka et
al., 2009). Electrode impedance spectroscopy (EIS) is the method of measuring the
electrode impedance at multiple frequencies using small amplitude voltage-controlled or
current-controlled signals. Because EIS measures the ETI impedance at multiple
frequencies, the behavior and composition of the ETI can be assessed with
parameterization of the impedance models described in Figs. 2.2-4.
EIS measurements can be performed with the application of voltage-controlled
(potentiostatic) or current-controlled (galvanostatic) signals. The most common method
to perform EIS measurements involves application of a small amplitude sinusoid between
two electrodes and measurement of the corresponding output signal. If the amplitude of
the sinusoidal perturbation is kept sufficiently low, the electrode will be operating under
linear conditions and the electrode impedance at a specific frequency can simply be
calculated using Ohm’s law (Fig. 2.6). There are numerous methods and types of
equipment that can be used to perform EIS measurements in either the time domain or
frequency domain (e.g. audio frequency bridges, direct measurements with an
oscilloscope, phase-sensitive detection with a lock-in amplifier, and automated frequency
response analysis) (Barsoukov and Macdonald, 2005).
27
Figure 2.6. Electrode impedance spectroscopy methodology.
The figure above illustrates the principles of EIS measurements. In this figure, a small
amplitude sinusoidal voltage is applied at the desired frequency and the current output is
measured. If the amplitude of the applied signal is small, the electrode will be operating
under linear conditions (i.e. gray line tangent to the theoretical I/V curve) and the
electrode impedance at the specified frequency can be calculated with Ohm’s law
(adapted from http://www.ecochemie.nl/download/content/Appl011.pdf).
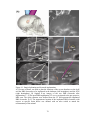
EIS measurements can be performed in multiple cell configurations: two-, three-,
or four-electrode cell configurations. The most commonly used cell configurations are
two-electrode and three-electrode cell configurations. In a two-electrode cell
configuration, the control signal is applied between a counter electrode and a working
electrode and therefore the interfacial potential of the working electrode is measured
relative to the interfacial potential of the counter electrode (Fig 2.7A-B). The working
electrode is the electrode being characterized (i.e. the neurostimulation or recording
electrode). Because the interfacial potential of the working electrode is measured relative
to the interfacial potential of the counter electrode, it is vital the interfacial potential of
the counter electrode remains largely constant (i.e. independent of the current density).
Otherwise, changes in the interfacial potential of the counter electrode will contaminate
28
the impedance measurements attempting to characterize the impedance of the working
electrode. To minimize potential changes at the counter electrode, the counter electrode
typically has a large surface area relative to the surface area of the working electrode and
helps ensure the current density at the counter electrode remains low. Another possible
limitation of the two-electrode cell configuration is a large ohmic overpotential at
nonzero current conditions (i.e. iR drop) that can develop across the bulk electrolyte
and/or tissue separating the two electrodes (Fig 2.7C). To minimize the iR drop, the
current levels between the counter and working electrodes should be kept low and/or the
conductivity of the electrolyte high (e.g. supported electrolyte).
A three-electrode cell configuration is often utilized to avoid or minimize the
limitations of the two-electrode cell configuration. In a three-electrode cell configuration,
the current is applied between the working electrode and an auxiliary electrode (Fig.
2.7D). A third electrode (i.e. reference electrode) is used to estimate the interfacial
potential of the working electrode. Because current only passes between the working
electrode and auxiliary electrode, there will be no change in the interfacial potential of
the reference electrode that would contaminate the impedance measurements. The
reference electrode is also placed close to the working electrode to minimize the effects
of ohmic overpotentials (Fig. 2.7F).
29
Figure 2.7. Two- and three-electrode cell configurations.
A) Primitive controlled-potential apparatus illustrating the principles of a two-electrode
cell configuration. B) Potential gradients in a two-electrode cell configuration for a zero
current (i = 0). C) Potential gradients in a two-electrode cell configuration for i ≠ 0. In the
two-electrode cell configuration a large ohmic overpotential (i.e. iRs) is possible. (CE =
counter electrode, WE = working electrode, Ea = potential applied to the cell, Rs =
resistance of bulk solution). D) Primitive controlled-pontential apparatus illustrating the
principles of a three-electrode cell configuration. E) Potential gradients in a threeelectrode cell configuration for i = 0. F) Potential gradients in a three-electrode cell
configuration for i ≠ 0. In a three-electrode cell configuration, a reference electrode is
placed close to the working electrode to minimize the effects of the ohmic overpotential.
The reference electrode can compensate for a portion of the ohmic overpotential (i.e. iRC)
but a portion of the ohmic potential will still contaminate the impedance measurements
(i.e. iRU) (RE = reference electrode, AE = auxiliary electrode, Ea = desired potential
difference between the RE and WE, RC = compensated resistance, and RU =
uncompensated resistance of the bulk solution) (adapted from Kissinger and Heineman,
1996).
30
2.4.2
Published examples of ETI characterization using EIS
EIS measurements can be used to characterize electrodes for a wide variety of
applications and there are many instances in the literature of using EIS to characterize the
ETI of electrodes for biomedical applications (both in vitro and in vivo applications). For
example, in vitro EIS measurements and parameterization of impedance models have
been used to estimate the sealing resistance of neural cells cultured on arrays of
stimulating electrodes (Buitenweg et al., 1998). Merrill and Tresco (2005) cultured
various cell types in vitro directly on silicon-substrate microelectrodes typically used for
intracortical recordings and performed EIS measurements to investigate the cell types
responsible for the increased tissue resistivity observed in vivo. Frampton et al., (2007)
developed 3D in vitro hydrogel cultures seeded with various neural densities of astrocytes
to model the changes in tissue impedance around microfabricated neural probes and
quantified these changes with EIS measurements.
31
Figure 2.8. Correlation of EIS measurements with histological measurements.
The figure above displays the impedance spectra for individual microwire electrodes
implanted in the rat cortex. Measurements are shown for 0, 2, 4, and 6 days after
implantation. Each curve represents the impedance spectrum of an individual electrode.
The electrodes were divided into two groups based on histological analysis: low degree
of tissue response at the electrode site (blue spectra) and a large degree of tissue response
at the electrode site (red spectra). By looking at the figure above it is clear that within 6
days after implantation, electrodes determined to have a large degree of tissue response
showed a corresponding increase in the tissue impedance (i.e. development of
semicircular arcs in the high frequency range of the impedance spectra) (from Williams
et al., 2007).
EIS measurements have also been used as a way to study the ETI in vivo.
Williams et al. (2007) performed EIS measurements and histological staining on
microwire electrode arrays that had been implanted in the cortex of adult rats. This study
reported a correlation between the degree of tissue response at the ETI assessed by
standard histological techniques and the ETI changes detected with EIS measurements
(Fig. 2.8). Johnson et al., (2005) and Otto et al., (2006) investigated changes in recording
quality that occurred after the application of brief large-amplitude voltage pulses. These
32
studies showed a significant increase in recording SNR that was correlated to the
stimulation-induced decrease in tissue impedance of the ETI. McConnell et al., (2009)
also examined impedance changes at the ETI of microwire electrode arrays chronically
implanted in the cortex of adult rats and compared the EIS measurements to results
obtained with immunohistochemistry. This study suggests astrocytes are the predominant
cell type affecting the ETI impedance and the observed increase in electrode impedance
was correlated with the high density of astrocytes within the first 100 µm of the electrode.
2.5
Summary
The ETI is the complex system of the electrode-electrolyte interface and the
surrounding biological tissue. After an electrode is implanted in the CNS, there is a
complicated sequence of events, collectively known as the foreign body reaction, that
lead to major changes in the composition of this interface. The consequence of these
compositional changes is modification to the electrical behavior (i.e. impedance) of the
ETI that affects the electrode’s recording and/or stimulation capabilities in chronic
applications. The ETI can be characterized with EIS measurements that describe its
frequency-dependent behavior. This impedance data can be used to parameterize
impedance models to study the electrode and tissue components of the ETI that can’t be
resolved with impedance measurements at a single frequency. In summary, EIS
measurements and corresponding model analyses provide information about the longterm recording and stimulating capabilities of electrode systems. This information can
help optimize the design and implementation of neurostimulation and neurorecording
devices.
33
3
CHAPTER
3:
THEORETICAL
ANALYSIS
OF
CHRONIC
INTRA-
CORTICAL MICROELECTRODE RECORDINGS
3.1
Introduction
The therapeutic goal of a brain machine interface (BMI) is to extract signals
directly from the brain to aid in the restoration of sensory or motor function lost by
disease or injury. Recent advances in neuroscience and neurotechnology have enabled the
development of the first clinically relevant BMIs for human patients. Current examples
include controlling cursor movement on computer screens for brain stem stroke,
amyotrophic lateral sclerosis, or tetraplegic patients (Kennedy and Bakay, 1998; Wolpaw
and McFarland, 2004; Hochberg et al., 2006). These diseases affect nearly two million
people in the United States alone and millions more worldwide (Wolpaw et al., 2002).
These millions of people are the driving force behind the development of BMI
technology so that one day lost sensory or motor functions may be restored and these
patients can enjoy an improved quality of life.
Cortical neural prosthetics (CNP) are a subclass of BMIs that can be used to
overcome some of the limitations of electroencephalography (EEG)-controlled BMIs at
the cost of being more invasive (Friehs et al., 2004). CNPs use intracortical
microelectrodes to monitor the neural signals in which the measured signals can either be
local field potentials (LFP) or single-unit action potentials. The LFPs recorded using
these cortical microelectrodes represent a smaller population of neurons relative to
recordings from EEG-based BMIs. Single-unit recordings represent the firing patterns of
single cells and can be modulated rapidly. The ability to modulate signals rapidly
34
expands the bandwidth of CNPs and allows them to be used for controlling more
complex movements (Wessberg et al., 2000; Serruya et al., 2002; Taylor et al., 2002).
There are many types of microelectrodes available to monitor cortical signals
such as microwires and silicon-substrate microelectrodes fabricated through use of microelectromechanical systems (MEMS)-based technology (Jones et al., 1992; Williams et
al., 1999; Taylor et al., 2002; Wise et al., 2004). The advantage of using microwires is
the simplicity of fabrication and durability. However, microwires do not allow for the
detailed geometric knowledge of the relative position of the recording electrodes to each
other or the overall cortical layering architecture. The two most popular types of MEMSfabricated microelectrode arrays have been developed at the University of Utah (termed
the Utah Intracortical Electrode Array (UIEA)) and at the University of Michigan. The
UIEA consists of 100 needle electrodes of ~1-1.5 mm in length over a 4x4 mm grid,
allowing for high density recording over a small cortical area. A major drawback of the
UIEA is that each electrode has only one contact and this contact is located at the tip of
the electrode shank. This contact location prevents recording from multiple cortical layers
along the same cortical column and excludes the possibility for developing true threedimensional (3D) electrode arrays. The planar silicon-substrate microelectrodes
fabricated at the Center for Neural Communication Technology at the University of
Michigan and at NeuroNexus Technologies (Ann Arbor, MI) can be used to overcome
some of the limitations of microwires and the UIEA. These probes are being developed
for acute and chronic recording and/or stimulation of the central nervous system. The
single- and four-shank designs have a number of possible design variations that can
include holes in the electrode the shanks for tissue anchoring and/or seeding with
35
pharmacological agents, recordings sites distributed along the length of the electrode
shank, and multiple contact sizes.
In spite of the technological advances that have allowed for the development of
complex microelectrode arrays for recording neural activity, translation of CNPs into
human applications has suffered from the poor long-term performance of the cortical
microelectrodes (Schwartz, 2004; Hochberg et al., 2006). It is believed that the
development of these chronic recording electrodes is the single biggest remaining
challenge in the development of CNPs (Schwartz, 2004). With current microelectrode
technology, an electrode chronically implanted in monkey cortex only has a 40-60%
chance of recording unit activity and these recordings typically deteriorate after a few
months (Schwartz, 2004). In a recent publication using a human CNP, over 50% of the
electrodes malfunctioned within the first year after implantation (Hochberg et al., 2006).
There are many possible causes for electrode failure; however, if these electrodes are to
be used in human applications they must be able to perform reliably for years.
One of the major reasons for poor long-term performance of these cortical
microelectrodes is a low recording signal-to-noise ratio (SNR). A low SNR can be caused
by a variety of factors such as the formation of a fibrous capsule around the recording
electrode or the firing of a large number of neurons in the surrounding biological media.
Fibrous encapsulation of the electrode is due to the foreign body reaction and results in
the formation of a high impedance layer around the electrode (Grill and Mortimer, 1994;
Turner et al., 1999; Szarowski et al., 2003). This high impedance layer effectively
isolates the electrode from neurons in the surrounding gray matter and can lead to an
increase in thermal noise levels (Johnson et al., 2005; Ludwig et al., 2006; Otto et al.,
36
2006). The firing of several neurons in the surrounding biological media can also lead to
the inability to preferentially record from a single cell (i.e. biological noise) (Ludwig et
al., 2006).
A fundamental goal of this study was to develop new techniques to optimally
design cortical microelectrodes and to investigate variables affecting recording quality.
Specifically, this study utilized a computational model of cortical recording to investigate
the effects of microelectrode contact size and major noise sources on the quality of
chronic intracortical microelectrode recordings of single-unit activity. This type of
analysis would be difficult, if not impossible, to perform experimentally. A previouslydescribed computational model of cortical recording (Moffitt and McIntyre, 2005) was
modified to estimate extracellular microelectrode recordings for a variety of recording
contact sizes for a microelectrode design resembling the microelectrode arrays fabricated
at the University of Michigan and NeuroNexus Technologies. Noise sources were also
incorporated into the overall modeling infrastructure to estimate the thermal and
biological noise levels experienced during chronic intracortical recordings. Preliminary
portions of this work have been presented (Lempka et al., 2006).
3.2
Methods
The general methodology in this study involved models of signal recording
amplitude and recording noise for microelectrodes of different contact sizes. An overall
recording signal-to-noise ratio (SNR) was calculated for each contact size by combining
the model estimates for recording and noise amplitudes (Fig. 3.1). Recording amplitude
was estimated using the coupled finite-element model (FEM) and neuron model
37
described below. Recording noise was estimated by combining model predictions of
thermal and biological noise sources.
Figure 3.1. Overall recording model infrastructure.
A) Method for estimating the recorded neural signal for a given electrode design. B)
Noise models to be included in estimating the signal noise. C) Signal-to-noise ratio
calculation for a given electrode design combining the model estimates for neural
recording amplitude and signal noise.
3.2.1
Cortical recording model
The computational model of cortical recording used in this study included two
major components: an electrical volume conductor and an electrical source model (Fig.
3.2) (Moffitt and McIntyre, 2005). The electrical volume conductor consisted of a FEM
of a rat head with a silicon-substrate microelectrode inserted in the cortex (Fig. 3.2A).
The electrical source model consisted of a multi-compartment cable model of a layer V
cortical pyramidal neuron (Fig. 3.2B) (Mainen et al., 1995; Mainen and Sejnowski, 1996;
Moffitt and McIntyre, 2005).
38
3.2.1.1 Electrical volume conductor model
The volume conductor model contained four concentric spheres representing the
brain (300 Ω-cm), cerebrospinal fluid (CSF) (56 Ω-cm), skull (16,000 Ω-cm), and scalp
(230 Ω-cm) (Fig 3.2A). A 20 µm thick domain of higher resitivity (600 Ω-cm) was
incorporated around the electrode to account for the encapsulation layer that forms as a
result of the foreign body reaction (Grill and Mortimer, 1994; Szarowski et al., 2003).
The electrical parameters of the head model domains were based on previously published
head models (Haueisen et al., 2002). The FEM also included explicit representation of a
silicon-substrate microelectrode. The electrode shank had a width of 107 µm, length of
2000 µm, and a thickness of 15 µm (Fig 3.2A). The microelectrode contacts were circular
with radii of 7.5, 11.5, 15, or 20 µm corresponding to approximate surface areas of 177,
413, 703, or 1250 µm2, respectively. These contact sizes are often used experimentally
and are commercially available from NeuroNexus Technologies. The volume conductor
models were assumed to be purely resistive and were generated in Ansys v8.0 (Ansys,
Inc., Canonsburg, PA). The models were discretized in space using tetrahedral elements
(ANSYS element SOLID98) that consisted of 10 nodes, one node at each of the 4
vertices and bisector nodes on each of the 6 edges. The nodal density was non-uniform
with a higher node density region-of-interest (ROI) consisting of the electrode and brain
tissue in a 500 x 500 x 500 µm3 box surrounding the recording contact. The FEM
consisted of over 660,000 nodes. Load and boundary conditions were necessary to
achieve a model solution. The load condition consisted of an ideal point current (Isource)
located at the electrode contact (reciprocal solution – rationale described below) and the
boundary condition required the voltage attenuate to zero at the bottom of the head. The
39
electrostatic model was solved in ANSYS with an iterative Jacobi conjugate gradient
solver for the Poisson equation:
∇ ⋅ σ∇Φ = − I source
(3.1)
Additional details of the FEM generation can be found in the literature (Moffitt and
McIntyre, 2005).
3.2.1.2 Electrical source model
The electrical source model used in this study was a multi-compartment cable
model of layer V pyramidal cell from the cat visual cortex (Fig. 3.2B) (Mainen et al.,
1995; Mainen and Sejnowski, 1996). The model geometry was derived from a 3D
neuroanatomical reconstruction and the membrane biophysics were based on voltageclamp and current-clamp measurements describing the behavior of the membrane ion
channels. The neuron model was modified according to Moffitt and McIntyre (2005) by
removing the compartments of dendritic processes that intersected the recording electrode
and by increasing the number of axonal compartments. An action potential was generated
in the model by incorporating excitatory synaptic inputs in the apical dendrites (Moffitt
and McIntyre, 2005). Simulations were performed in NEURON v5.8 in order to solve the
transmembrane currents generated in each of the 531 compartments (Hines and
Carnevale, 1997).
3.2.1.3 Model coupling
In the coupled neuron-FEM model, each compartment of the neuron model is
represented as an independent current source (i.e. the time-dependent transmembrane
40
currents computed in NEURON) at the appropriate spatial location in the FEM. The
simulated recorded voltage waveform was then calculated by summing the voltages
generated at the electrode contact by the transmembrane currents of the individual neural
compartments. The fundamental task was to calculate the voltage impressed at the
electrode contact for a given current at an arbitrary point in the volume conductor. This
can be formulated mathematically with the following expression:
Φ = KJ
(3.2)
where Φ is a (1 x t) vector containing the recorded voltages a t instances in time, K is a
(1 x n) vector containing the voltages that would be impressed at the electrode contact for
a unit current at the location of each of the n individual neuron compartments, and J is a
(n x t) matrix containing the transmembrane currents for the individual neuron
compartments at each time step. The J matrix was calculated in NEURON, while each
value in the K vector was derived from the FEM using a reciprocal solution described by
Moffitt and McIntyre (2005). Briefly, this reciprocal solution involved placing a current
source at the electrode contact and solving for the scalar potentials generated at each node
in the volume conductor mesh. By the theorem of reciprocity, this output voltage at a
given node in the mesh can be interpreted as the voltage that would be generated at the
recording electrode for a unit current. Therefore, the contribution of each neural
compartment to the recorded waveform (i.e. individual values in the K vector) could be
calculated using interpolation of the output voltages from the nearest nodes surrounding
the individual neuron compartments.
41
Figure 3.2. Coupled neuron-FEM model for extracellular neural recording.
A) FEM of the rat head with an explicit representation of the cortical microelectrode. B)
Multi-compartment model of layer V pyramidal cell from the cat visual cortex. C)
Simulated extracellular recording for microelectrode contact sizes of 177, 413, 703, and
1250 µm2.
3.2.2
Noise models
3.2.2.1 Thermal noise model
One of the major sources of noise encountered during cortical microelectrode
recordings is thermal noise, also called Johnson noise, generated by the random thermal
motion of electrons in a resistive material. It is present in all circuit elements containing
resistance and is independent of the composition of the resistance. In cortical recording,
the real (i.e. resistive) component of the system impedance is highly dependent on
frequency; therefore, in order to develop an accurate model of thermal noise, impedance
measurements must be taken over the expected recording bandwidth. In order to derive a
parametric equation describing the microelectrode impedance as a function of frequency,
42
in vitro and in vivo impedance spectroscopy measurements were used to parameterize a
previously-published impedance model of cortical microelectrodes (Fig. 3.3A) (Johnson
et al., 2005; Otto et al., 2006; Williams et al., 2007).
In this spatially-lumped impedance model, the electrode-electrolyte interface is
represented by a constant phase element (CPE) with a magnitude scaling factor (K) and a
phase factor (α) defined for 0 ≤ α ≤ 1 (see Fig. 2.5).
Z CPE =
K
( jω )α
(3.3)
The CPE accounts for the pseudo-capacitive behavior of the electrode-electrolyte
interface and is based on empirical data (McAdams et al., 1995). The CPE is connected
in series with an encapsulation resistance, Ren, which represents the electrode
encapsulation formed as part of the body’s immune response (Fig. 3.3A) (Grill and
Mortimer, 1994). The resistance of the extracellular space is represented by Rex and the
cellular tissue component is represented by a parallel combination of a specific
membrane conductance (gm) and capacitance (cm) that is multiplied by a membrane area
scaling term (Am) (Fig 3.3A). The values of gm and cm are 0.3 mS/cm2 and 1 µF/cm2,
respectively (Buitenweg et al., 1998). The shunt capacitance of the electrode shank,
represented by Csh, was found to be < 10 pF and was assigned a value of 10 pF in the
model analysis (Otto et al., 2006). The equation describing the impedance of the entire
model was:
Z model =
1
jωCsh +
1
K ( jω )
−α
+ Ren +
43
Rex
1 + Am ( jω cm + g m )
(3.4)
Average model parameters for the electrode component of the impedance model (K and
α) for each microelectrode contact size were determined by a nonlinear regression of the
in vitro EIS measurements. Average model parameters for the tissue component (Rex, Ren,
and Am) of the impedance model described above were estimated from in vivo EIS
measurements of microelectrode arrays chronically implanted in rats using a previouslydescribed nonlinear regression algorithm (Otto et al., 2006). The parameters Rex, Ren, and
Am were assumed to be independent of electrode contact size. For each microelectrode
contact size, the average parameter values for both the electrode and tissue components
were combined and used to derive an expression for the recording impedance as a
function of frequency according to (3.4). The model fit quality was assessed for the
electrode and tissue parameters by calculating the coefficient of determination for both
the real and imaginary components. If either value was less than 0.98, the individual
recording site was not included in the pool of recording sites used to determine the
average model parameters.
The derived impedance expression was then used to estimate the thermal noise
spectral density using the Johnson-Nyquist formula:
SV (ω ) = 2kT Re {Z (ω )}
(3.5)
where k represents the Boltzmann constant and T is the absolute temperature. The
equation above is valid for any passive, reciprocal network and can be used to describe
the noise voltage across two arbitrary terminals, such as the working and ground
electrodes (Papoulis, 1984). As evident by the impedance model (Fig. 3.3A), at low
frequencies the recording impedance becomes extremely high due to the capacitive
nature of the electrode-electrolyte interface. At high frequencies, the recording
44
impedance becomes small due to the electrode shunt capacitance and the capacitive
nature of both the electrode-electrolyte interface and the membranes of cells in the
surrounding media. However, during single-unit microelectrode recordings the recorded
signal is band-pass filtered (e.g. 450-5000 Hz) (Vetter et al., 2004; Otto et al., 2006). The
effects of the bandpass filtering were accounted for using the following equation:
S (ω ) = SV (ω ) H sig (ω )
2
(3.6)
where Hsig represents the transfer function of the combined filter stages. After estimating
the noise spectral density, the noise variance was predicted by integration. Because the
noise was zero-mean, integration of the autospectral density led directly to the noise
variance.
For the neural recording model, a sampling rate of 40 kHz and a simulation time
of 12 ms were used. The noise spectrum, S(ω), was evaluated over the frequency range of
0-20 kHz, corresponding to the frequency range of the discrete Fourier transform of the
simulated neural recording. Hsig consisted of a two-pole low-pass Butterworth filter with
a cutoff frequency of 5 kHz and a two-pole high-pass Butterworth filter with a cutoff
frequency of 450 Hz in order to simulate a typical recording bandwidth of 450-5000 Hz.
The temperature in (3.5) was set equal to a physiological temperature of 37ºC. All of the
numerical methods described above were performed using Matlab 7.6 (Mathworks,
Natick, MA).
3.2.2.2 Biological noise model
In intracortical microelectrode recordings, biological noise mainly arises from the
firing of several neurons in the tissue surrounding the recording microelectrode and
45
represents a second major source of noise. Biological noise was modeled by placing
several model neurons around the recording microelectrode in the coupled neuron-FEM
model (Fig. 3.4B). The model neurons were the same multi-compartment cable model
described in ‘3.2.1.2’ and were positioned around the recording contact with their somas
located within an approximate 400 x 400 x 200 µm3 ROI. This ROI was translated a
distance of 50 µm normal to the electrode surface. The size of this ROI was based on the
following factors: 1) neural signals in this cortical recording model decay to small values
when the neuron is greater than >150 um away from the recording contact (Moffitt and
McIntyre, 2005), and 2) neurons in the motor cortex are arranged in columns and/or
elongated slabs (relative to the main axis of the precentral gyrus) that are arranged 100300 µm wide (Meyer, 1987). A width of 200 µm is therefore representative of the
dimensions that can be seen physiologically and extending the dimensions of the ROI
would not significantly change the results because neurons greater than 200 µm away
from the recording microelectrode would not contribute significantly to the recorded
signal. 325 model neurons were placed within this ROI resulting in a neuronal density of
9.5 x 106 neurons/cm3 representative of the average neuronal density of 9.9 x 106
neurons/cm3 reported for the motor cortex (Cragg, 1967). Neurons were placed 17 µm
apart in the x-z plane and the individual vertical locations in the y axis were randomly
determined (Fig. 3.4B).
The individual neurons were simulated to generate action potentials at a rate of 20
Hz by periodic excitatory synaptic inputs at the apical dendrites. The transmembrane
currents for each compartment of the neuron model were solved in NEURON for a total
simulation time of one second. The voltage signal recorded at the microelectrode contact
46
was then calculated using the coupled neuron-FEM model described above for each of
the 324 model neurons and the individual results superimposed to determine the overall
“noisy” voltage recording. This recorded signal was then band-pass filtered over a typical
recording bandwidth (i.e. 450-5000 Hz). Neural spikes in the recorded waveform were
detected using a standard threshold detection method (Lewicki, 1998; Vetter et al., 2004;
Ludwig et al., 2006). A threshold for action potential detection was set at three times the
standard deviation of the entire voltage recording. Individual spikes were detected and a
three millisecond window (1 ms before the spike and 2 ms after the spike) was used to
classify the data falling within this window as an action potential and the remaining data
was considered noise. The biological noise was calculated as the standard deviation of the
remaining noise component of the voltage recording.
In order to account for possible variability in the estimated biological noise levels
due to the random placement of the model neurons surrounding the recording
microelectrode and the random firing times for the individual model neurons, 10 different
neuron “meshes” were generated. For each individual mesh, the biological noise was
estimated for 10 separate voltage recordings (i.e. 10 different random firing times for the
individual neurons). This analysis was performed for each microelectrode contact size
using the same neuron meshes and firing times.
3.2.2.3 Total recording noise
The total recording noise (σnoise) for each microelectrode contact size was
determined as the square root of the sum of the squared RMS (i.e. standard deviation)
values of the thermal noise and biological noise:
47
σ noise = σ t2 + σ b2
(3.7)
where σt and represent σb represent the standard deviation of the thermal and biological
noise respectively.
3.2.2.4
SNR analysis
The simulated neural recordings and noise were used to estimate the SNR for
each contact size. The SNR for a given contact size was defined as the peak-to-peak
amplitude of the simulated neural recording divided by twice the standard deviation of
the noise (Nordhausen et al., 1996).
3.2.3
Electrode impedance spectroscopy
Electrode impedance spectroscopy (EIS) measurements were performed for both
in vitro and in vivo conditions using an Autolab potentiostat (PGSTAT12, Eco Chemie,
Utrecht, The Netherlands) with a built-in frequency response analyzer (Brinkmann
Instruments, Westbury, NY). In both situations, a 25 mV (rms) sine wave was applied at
a given frequency and the current output measured. The impedance at a specific
frequency was calculated in the frequency domain using Ohm’s law (i.e. Z(f)=V(f)/I(f)
where f is the specified frequency).
3.2.3.1 In vitro EIS
Cortical microprobes having contact sites of 177, 413, 703, and 1250 µm2 were
obtained from NeuroNexus Technologies and tested using a standard three-electrode cell
configuration. In this three-electrode configuration, a cortical microelectrode contact
48
served as the working electrode, the reference electrode was a saturated calomel
electrode, and a stainless steel (316SS-grade) wire served as the auxiliary electrode. In
vitro EIS measurements were perfomed at 37°C in a beaker filled with 500 mL of 0.1 M
phosphate buffered saline (PBS) (pH=7.4). All in vitro measurements were performed
inside a Faraday cage to help minimize noise that would contaminate the impedance
measurements. EIS measurements were perfomed at 31 logarithmically-spaced
frequencies ranging from 100 Hz to 10 kHz (10 frequency points/decade). These in vitro
EIS measurements were used to estimate the electrode-electrolyte interface parameters (K
and α) in the impedance model described in ‘3.2.2.1’.
3.2.3.2 In vivo EIS
Previously-published in vivo EIS measurements were used to estimate the
encapsulation, cellular, and extracellular components (Ren, Am, and Rex) of the impedance
model described in ‘3.2.2.1’ (Johnson et al., 2005; Otto et al., 2006). These in vivo EIS
measurements were made on six silicon-substrate microelectrode arrays implanted in the
motor cortex of four Sprague-Dawley rats (250-300 g, implanted for 19, 33, 94, and 97
days). The electrode arrays consisted of iridium contacts that were 703 µm2 in size.
Measurements were performed using a two-electrode cell configuration in which the
working electrode was an individual microelectrode contact and the counter electrode
was a stainless steel (316SS-grade) bone screw. Measurements were performed at 11
frequencies ranging from 100 Hz to 10 kHz. A typical impedance spectra measured in
vivo is shown in Fig. 3.3B. Surgical procedures for these implants have been described
previously (Otto et al., 2006).
49
3.3
3.3.1
Results
Signal amplitude estimation
Recording signal amplitude for each microelectrode recording contact size
considered in this study was estimated with the described neuron-FEM model. In this
analysis, a model neuron was placed with its soma located 50 µm normal to the center of
the recording site. The simulated extracellular recordings were also filtered over a typical
recording bandwidth (i.e. 450-5000 Hz). Model analysis produced peak-to-peak signal
amplitudes of 205, 199, 194, and 186 µV for contact surface areas of 177, 413, 703, and
1250 µm2, respectively (Fig. 3.5D).
3.3.2
Noise estimation and SNR analysis
3.3.2.1 Impedance parameter estimation
In vitro EIS measurements were performed in 0.1 M PBS to define the electrode
parameters, K and α. Multiple microelectrode arrays were tested for each contact size (n
= 28, 44, 45, and 47 contacts for contact sizes 177, 413, 703, and 1250 µm2 respectively).
EIS measurements were performed in triplicate and averaged in order to help reduce
variability inherent to the measuring system. The electrode parameters for each contact
site were determined using nonlinear least squares and the average values are shown in
Fig. 3.3D.
The tissue parameters of the impedance model (Ren, Rex, and Am) were determined
from in vivo EIS measurements. Data was obtained from six separate microelectrode
50
arrays implanted in the motor cortex of four different rats (n = 73 recording sites). The
parameter values were estimated using a previously-published nonlinear regression
algorithm and the average values are shown in Fig. 3.3D.
Figure 3.3. Impedance model for thermal noise estimates.
A) Impedance model of chronically-implanted intracortical microelectrodes. B) Example
of an in vivo impedance spectrum for the cortical microelectrodes considered in this study
and the contribution of various model components to the overall electrode impedance
spectra. C) Impedance spectra for the individual contact sizes generated with the average
electrode and tissue model parameters from in vitro and in vivo impedance data. D)
Table of average electrode and tissue parameters.
3.3.2.2 Thermal noise
The thermal noise for each microelectrode contact size was determined by
combining the average tissue component parameters (Ren, Rex, and Am) with the average
electrode parameters (K and α) to estimate the total model recording impedance as
function of frequency according to (3.4). The thermal noise spectral density was
51
estimated from the impedance spectrum with (3.5). Filtering effects were approximated
from the transfer function of the band-pass filter according to (3.6). Integration of the
noise spectral density led directly to the noise variance. The parameters shown in Fig.
3.3D resulted in thermal noise levels with standard deviation of 6.32, 6.23, 6.20, and 6.18
µV for contacts surfaces areas of 177, 413, 703, and 1250 µm2, respectively (Fig. 3.5E).
These values show the expected result of decreasing thermal noise with increasing
contact surface areas; however, the differences between the individual contact sizes were
small. The average thermal noise level (independent of contact size) was 6.23 µV.
3.3.2.3 Biological noise
Biological noise levels were calculated according to the methods described in
‘3.2.2.2’. Biological noise levels of 8.25±0.28, 8.22±0.37, 8.14±0.34, and 8.01±0.23 µV
were estimated for contact surface areas of 177, 413, 703, and 1250 µm2, respectively
(Fig. 3.5E). The biological noise decreased for large contact sizes; however, the
differences between the individual contact sizes were small. The average biological noise
in the simulated extracellular recordings (independent of contact size) was 8.16 µV.
52
Figure 3.4. Signal recording amplitude estimation and biological noise model.
(A) To estimate the signal amplitude for the extracellular recording of single-unit
activity, a model neuron was placed with its soma 50 µm away from the center of the
recording contact. The image in (A) shows a log scale of the transmembrane currents (im)
in nanoamperes for each neural compartment during the peak of the action potential at the
axon hillock. (B) An example of one of the neural distributions used to simulate
biological noise. The neuron highlighted in red represents the position of the neuron in
(A) from which signal amplitudes were estimated. The plot on the far right shows one of
the neural meshes used in the model analysis for a neuronal density of 9.5 x 106
neurons/cm3. Each dot represents the location of the soma for each of the 325 individual
model neurons. As described in ‘3.2.2.2’, the ROI containing the model neurons was
translated a distance of 50 µm normal to the electrode surface.
3.3.2.4 Total recording noise and SNR analysis
The total recording noise was calculated for each recording contact size according
to (3.7) and was estimated as 10.4, 10.3, 10.2, and 10.1 µV for contact surface areas of
177, 413, 703, and 1250 µm2, respectively (Fig. 3.5E). The recording SNR for each
contact size was then calculated as the peak-to-peak recording amplitude divided by
twice the standard deviation of the recording noise: 9.9, 9.7, 9.5, and 9.2 for contact
surface areas of 177, 413, 703, and 1250 µm2, respectively (Fig. 3.5F).
53
Figure 3.5. Simulated extracellular recordings and noise.
(A) Simulated extracellular neural recording for a contact surface area of 703 µm2 shown
with and without the estimated thermal noise. (B) Simulated extracellular neural
recording with and without one example of the estimated biological noise. (C) Simulated
noisy extracellular neural recording with both thermal and biological noise. (D) Action
potential recording amplitude as a function of contact surface area. The signal amplitudes
were estimated with the soma of the neuron model located 50 µm away from the center of
the recording electrode contact. (E) Standard deviations of thermal, biological, and total
recording noise as a function of contact surface area. (F) Recording SNR as a function of
contact surface area combing the estimates of recording amplitude and total noise from
(E) and (F), respectively.
3.4
3.4.1
Discussion
Recording amplitude
The coupled neuron-FEM model returned differences in the estimated recording
amplitude that were ~10% of the total recording amplitude. The range of contact sizes
examined in this study was 177 – 1250 µm2 and corresponded to the range of contact
surface areas of the microelectrodes commercially available from NeuroNexus
54
Technologies. A wider range of contact sizes has been examined previously and a
significant decrease in the recording amplitude was observed for extremely large contacts
(e.g. 10,000 µm2) (Moffitt and McIntyre, 2005). However, the modeling analysis
performed in this study focused on standard contact sizes that are commonly used
experimentally. To perform the EIS measurements for the thermal noise analysis, it was
also vital that microelectrodes with the specified contact sizes were commercially
available for testing.
3.4.2
Noise levels
The model analysis performed in this study accounted for two of the main noise
sources encountered in intracortical microelectrode recordings (i.e. thermal and
biological noise).
3.4.2.1 Thermal noise
Thermal noise estimates exhibited minor differences between the recording
contact sizes examined in this study (~ 2 %). For chronically implanted microelectrodes,
the electrode-electrolyte interface impedance dominates the overall electrode-tissue
interface impedance at low frequencies. Therefore, the major impedance differences
between the individual contact sizes occurred in the low frequency range (i.e. < 1 kHz).
Because these extracellular recordings were band-pass filtered (e.g. 450-5000 Hz), the
low frequency information was attenuated and the impedance differences only resulted in
small changes in thermal noise. A majority of the recording bandwidth consists of higher
frequencies (i.e. > 1 kHz) in order to capture the large amount of high frequency
55
information in extracellular action potential waveforms. Therefore, the thermal noise
levels were dominated by the impedance in the higher frequency range (i.e. the tissue
impedance).
While the theoretical analysis performed in this study provided an estimate to the
thermal noise that may be encountered experimentally (~6 µV), thermal noise levels will
be highly dependent on the impedance of the electrode-tissue interface for each
individual recording site (due to the foreign body reaction) and the selected recording
bandwidth (i.e. a wider recording bandwidth will increase thermal noise levels). To
address some of the possible variability in thermal noise, we considered multiple
impedance conditions and recording bandwidths. The recording bandwidth in the analysis
described
above
was
a
standard
recording
bandwidth
for
silicon-substrate
microelectrodes (i.e. 450-5000 Hz), however, experimentalists frequently use wider pass
bands in microelectrode recordings (Hashimoto et al., 2003; Suner et al., 2005). To look
at the effect of a wider pass band on thermal noise, we performed the same model
analysis described above with a pass band of 0.1 – 10 kHz. The wide pass-band resulted
in an average increase ~35% in thermal noise along with a ~25% increase in the action
potential amplitudes recorded at the microelectrode. The specific changes for a contact
size of 703 µm2 are shown in Table 3.1A. However, no major differences were observed
between the individual contact sizes.
Another factor that can significantly affect the recording thermal noise is the large
variability in the tissue response around individual recording sites that has been shown
with both histological techniques and impedance measurements (Williams et al., 2007).
Variability in the tissue response produce differences in the electrode-tissue interface
56
impedance between various contacts that could lead to corresponding differences in
thermal noise. Therefore, we also investigated the effects of variable impedance
conditions at the electrode-tissue interface. A low electrode-tissue interface impedance
(i.e. low degree of tissue response) showed a large decrease in the thermal noise levels
relative to the average tissue parameter values (27-45%). The largest decrease in thermal
noise was observed for the largest recording site (i.e. 1250 µm2) and showed the
electrode-electrolyte interface impedance contributes more significantly to the thermal
noise when there is a low tissue impedance. A high electrode-tissue interface impedance
(i.e. high degree of tissue response) only produced a small increase in thermal noise
levels relative to the average parameter values determined from the in vivo EIS
measurements (~5%) that was fairly constant for all contact sizes. Table 3.1A shows the
tissue parameter values for the low and high electrode-tissue impedance and the specific
differences in thermal noise levels for a contact size of 703 µm2.
57
Table 3.1. Variability in noise estimates.
A) Thermal noise estimates for variable impedance conditions and recording bandwidths.
Low and high impedance conditions were considered to account for possible variability
in the tissue response as part of the foreign body reaction. Model parameters were Ren =
10 kΩ, Rex = 100 kΩ, and Am = 1x10-5 cm2 for the low impedance condition and Ren =
500 kΩ, Rex = 2MΩ, and Am = 1x10-3 cm2 for the high impedance condition. The tissue
parameters listed above were determined from the range of values produced in the model
fitting of the in vivo EIS data described in ‘3.2.2.1’ and ‘3.2.3.2’. The electrode
parameters (K and α) for all three impedance conditions and the tissue parameters for the
average impedance condition were the average model parameters shown in Fig. 3.3D.
The effect of a wider recording bandwidth on thermal noise was also considered. B)
Biological noise for different neuron densities, firing rates, and recording bandwidth. In
both (A) and (B) the estimated noise standard deviation and the percent change are
shown. All of the values reported in (A) and (B) were for a microelectrode surface area of
703 µm2.
3.4.2.2 Biological noise
The biological noise levels predicted in this study showed a slight decrease in the
biological noise for large recording contacts (~ 3 % decrease for a recording site of 1250
µm2 relative to 177 µm2). This trend was opposite to the common view in the field of
58
neural recording in which it is typically believed large surface area recording sites result
in more neural “hash” because large recording sites are sensitive to neural currents from a
larger area of tissue and therefore a larger number of neurons. However, the decrease in
biological noise for large recording sites was most likely related to the lower electrode
impedance that produces a lower voltage at the recording contact for a given amount of
current (Moffitt and McIntyre, 2005). Low electrode impedance was the same reason
large contacts produced small recording amplitudes (186 µV for a contact surface area of
1250 µm2 relative to 205 µV for a contact surface area of 177 µm2).
Due to the complex anatomical organization and electrical behavior of neurons in
the neocortex, the biological noise encountered during intracortical microelectrode
recordings can be highly variable. Therefore, we investigated the biological noise
expected for different neuronal densities and firing rates. Because the cortex is organized
in cell-dense columns or slabs separated by cell-sparse regions (neuronal density also
varies between cortical areas and individual cortical layers), the neuron density
surrounding the recording microelectrode is highly dependent on the position of the
recording contact. Therefore, we compared the biological noise estimated for the
neuronal density similar to the average motor cortical density reported by Cragg (1967)
(i.e. 9.5 x 106 neurons/cm3) to a low cell density (i.e. 2.4 x 106 neurons/cm3). The low
neuron density produced much lower biological noise at the recording microelectrode
(~62% decrease). Table 3.1B shows the specific biological noise values for a contact size
of 703 µm2. However, no major differences were observed between the individual contact
sizes.
59
Another factor that will likely produce large differences in the observed
biological noise is the complex electrical behavior of neurons in the cortex. In this study,
biological noise was simulated with neurons firing at a rate of 20 Hz, a frequency of
single-unit activity that can be observed in the motor cortex, especially during movement
(Evarts, 1964; Fetz, 1969; Zhang et al., 1997; Johnson et al., 2009). However,
microelectrode recordings in the cortex have also shown that a large number of neurons
often remain quiescent or have a lower firing rate (Goldberg et al., 2002). In order to
simulate decreased neuronal activity, biological noise levels were also estimated for
neurons firing at 5 Hz. This decrease in overall neural activity produced a dramatic
decrease in the biological noise levels (~62% decrease). Table 3.1B shows the specific
biological noise values for a contact size of 703 µm2. However, no major differences
were observed between the individual contact sizes.
The biological noise was also affected by the recording bandwidth. When a wide
recording bandwidth was examined (i.e. 0.1-10 kHz) there was a large increase in the
biological noise (~92% increase). Table 3.1B shows the biological noise with the wide
recording bandwidth for a contact size of 703 µm2.
3.4.3
Comparison to Experimental Results
The average signal recording amplitude, noise levels, and resulting recording
SNR are similar to the experimental values observed in chronic recordings with siliconsubstrate recording microelectrode (Vetter et al., 2004; Otto et al., 2006; Ludwig et al.,
2006). For a contact size of 703 µm2, the recording amplitude, noise, and SNR were 194
µV, 10.2 µV, and 9.5, respectively. For the same contact size in vivo, Otto et al, (2006)
60
and Ludwig et al., (2006) reported average noise levels of 12.1 and 13.1 µV respectively.
Ludwig et al., (2006) also reported an average signal recording amplitude of 113 ± 13 µV
that corresponded to an average SNR of 4.3 ± 1.0. The recording amplitudes (186-205
µV) and the corresponding SNR’s (9.2-9.9) estimated in this study were higher than the
average values that are typically seen experimentally. The recording amplitude and SNR
were only estimated for a neuron located 50 µm from the recording site and represented a
high recording quality within the range that can be seen experimentally (Vetter et al.,
2004). However, single-unit recording amplitude can vary significantly depending on a
number of factors (e.g. neural geometry, position of the neuron relative to the recording
site) and has been shown to vary between 50-800 µV for the type of microelectrode
design considered in this study (Vetter et al., 2004).
The similarities in recording amplitude and noise between the model estimates
and experimental values suggest the model detail was sufficient to capture a number of
factors that contribute to intracortical microelectrode recording quality. However, the
total noise estimates composed of thermal and biological noise models were on the low
end of the noise encountered experimentally and may be a result of ignoring additional
noise sources (e.g. preamplifier noise, shot noise, 1/f noise).
3.4.4
Study limitations
3.4.4.1 Realism of the neural source and biological noise model
The fundamental concepts and theoretical foundation of extracellular neural
recording are well documented (Rall, 1962; Bedard et al., 2004). However, recent
61
advances in both the physiological and anatomical characterization of cortical neurons
have provided the opportunity to critically evaluate the effect of detailed 3D neural
geometries, ion channel distributions, and synaptic currents on extracellular neural
recordings (Holt and Koch, 1999; Moffitt and McIntyre, 2005; Gold et al., 2006).
Unfortunately, neural source models incorporating the level of detail proposed in this
study are hindered by the high degree of uncertainty in many of the model parameters.
Although the neural density of the biological noise model mimics the neural
density experimentally measured in the motor cortex, the density of the cortical layers is
highly variable due to the detailed functional organization of the cortex. The cortical
layers are somatotopically organized in cell-dense columns or elongated slabs that are
separated by cell-sparse regions (Meyer, 1987). The cell numbers, cell types, and cell
sizes can also vary widely between the various cortical layers (Sloper et al., 1979; Meyer,
1987) along with the firing rates and patterns of cortical neurons (Goldberg et al., 2002;
Fetz, 1969; Evarts, 1964). Therefore, the biological noise encountered during intracortical
microelectrode recordings will be highly dependent on the location of the recording
electrode sites relative to the organization of the local cortical area. This complex cortical
organization and neural behavior is the reason we estimated the biological noise under
multiple conditions (i.e. cell densities and firing rates) (Table 3.1B). A major advantage
of this model was its ability to investigate the biological noise for all of these different
conditions and provide a range of biological noise that can be encountered
experimentally.
62
3.4.4.2 Realism of the tissue medium in the volume conductor model
The complex 3D tissue encapsulation and bulk medium that surrounds
chronically-implanted cortical microelectrodes creates a highly tortuous electrical
environment that can affect the signals recorded from cortical neurons. However, our
recording model accounted for this complex environment with simplistic domains of
uniform conductivities. This limitation may be particularly important in the context of the
model encapsulation domains because of the lower conductivity and close proximity to
the electrode (Moffitt and McIntyre, 2005). Incorporation of a more detailed
encapsulation layer could affect the estimated signal and noise amplitudes, however, we
expect the trends would be similar to those reported in this study.
3.4.4.3 Capacitive components of the electrode-tissue interface and bulk tissue
The volume conductor model was assumed to be purely resistive and therefore
underestimated the impedance of the electrode-tissue interface. A more complete model
of the electrode-tissue interface consists of both the resistive and capacitive impedances
(McAdams and Jossinet, 1995; Bedard et al., 2004). However, models of extracellular
neural recordings have shown the simulated recording is largely unaffected by the type of
electrode interface model (e.g. purely capacitive interface or ideal interface with infinite
input impedance) due to the low current densities at the recording electrode and the high
input impedance of standard recording electronics (Moulin et al., 2008). Experimental
impedance measurements have also shown the impedance of cortical gray matter is
relatively frequency independent and can be accurately represented as a purely resistive
volume conductor (Logothetis et al., 2007).
63
3.4.4.4
Thermal noise model
A possible limitation in the thermal noise model was the use of an equivalent
circuit model containing spatially-lumped elements to describe recording in a spatiallydistributed 3D environment. However, this model accurately describes measured
impedance values (Otto et al., 2006; Williams et al., 2007) and allows straight forward
characterization of electrode and tissue components of the measured impedance. A
second limitation in the thermal noise model was the use of in vivo EIS data from
recording sites of only one size (i.e. 703 µm2) to determine the average tissue parameters
of the impedance model. It is possible that the tissue parameters (Ren, Rex, and Am) could
vary as a function of contact size and might affect the thermal noise levels estimated for
each contact size. However, the differences in tissue parameters as a function of contact
size would likely be much smaller than the parameter variability attributed to the large
variations in tissue response observed between individual recording sites in vivo.
It is also important to recognize that the thermal noise model in this study only
accounted for the thermal noise of the electrode and not the thermal noise encountered at
the preamplifier. However, over a biological recording bandwidth (e.g. 450-5000 Hz), the
electrode thermal noise dominates the characteristics of the electrode-preamplifier system
(Najafi, 1994).
3.4.4.5 Impedance measurements
One possible limitation with the measurements performed in this study was the
two-electrode cell configuration used to measure the electrode impedance for the
microelectrode arrays implanted in vivo (in vitro measurements were performed with a
64
three-electrode cell configuration). In a two-electrode cell configuration, if the impedance
of the counter electrode is similar to the impedance of the working electrode, the
measured impedance spectra will contain contributions from both the counter and
working electrodes. However, in these measurements the counter electrode (i.e. stainless
steel bone screw) was extremely large relative to the microelectrode contacts and
therefore the measured impedance spectra should be dominated by the microelectrode
impedance. Another potential problem in the impedance measurements was instability in
the electrode interface of the bone screw. Because the bone screw was chronically
implanted, the interface properties could change over time due to the foreign body
reaction. However, because the measurements were performed several weeks after
implantation, the electrode-tissue interface of the bone screw should have stabilized.
3.4.4.6 Variability in tissue response to implanted electrodes
The most significant limitation for characterizing the ETI of cortical
microelectrodes was the limited sample size available. Individual recording sites can
show substantial variability in the degree of tissue response and an extremely large
sample size would be required to accurately characterize the ETI. However, because such
large variability is observed in the degree of tissue response, it is difficult to assign a
standard or definitive set of average impedance parameters and a corresponding thermal
noise that would be expected experimentally. It is much more reasonable to investigate a
range of impedance conditions that might be encountered experimentally, as performed in
the analysis detailed in Table 3.1A.
65
3.4.5
Future directions
Future studies will help remove some of the model limitations discussed above.
Although we considered multiple factors that affect the recording SNR (e.g. filter
bandwidth, electrode-tissue interface impedance, neuron density, neuron firing rates), a
more detailed parameter sensitivity analysis could be performed to help address the
effects of model parameter variability. Further modifications to the recording model
could also be considered such as: inclusion of an explicit representation of the electrodeelectrolyte interface, incorporation of capacitive effects of the electrode and biological
media, and the development of a more detailed model of the encapsulation domain.
Possible in vivo recording experiments should also be considered as a means to validate
the model results and to examine the effect of contact size on tissue parameters in the
impedance model.
For this study, we investigated the effect of recording contact size on the
recording SNR and the effects of thermal and biological noise. However, the modeling
infrastructure originally developed by Moffitt and McIntyre (2005) and expanded in this
study, can be used to investigate numerous factors affecting neural recording. For
example, this infrastructure provides an excellent tool for testing the accuracy of various
spike sorting techniques because the location and exact firing patterns of the individual
neuron are known. Although electrode contact size was the only design parameter
considered in this study, this recording model allows any arbitrary electrode design to be
considered. This model can also be used to study numerous experimental conditions that
affect the shape of the recorded extracellular action potential, such as the detailed neuron
66
geometry and relative location of the neuron to the recording site, as well as signal
filtering from the brain tissue and recording electronics.
3.5
Conclusion
This study utilized detailed computer models of cortical recording with silicon-
substrate microelectrodes and experimental measurements of electrode impedance to
investigate the effects of recording contact size and thermal and biological noise on
recording quality. This type of analysis would be very difficult, if not impossible, to
perform experimentally. The model analysis resulted in recording amplitude, noise, and
SNR that resembled the experimental values reported in the literature. While noise levels
decreased for large contacts so did the recording amplitude of the action potentials. These
trends resulted in the smallest contact size (i.e. 177 µm2) producing the highest SNR by
providing increased signal amplitude in the recordings with only a small increase in the
recording noise. Model analysis also showed theoretical levels of thermal and biological
noise that can be expected in chronic intracortical microelectrode recordings and
potential confounding factors (e.g. signal filtering, variable electrode-tissue interface
impedance, neuron density and firing rates) that can significantly alter recording quality.
The variables examined in this study need to be considered when analyzing cortical
recording data and when optimizing microelectrode design so that recording systems can
be designed with the long-term performance necessary for brain machine interface
applications.
67
4
CHAPTER 4: IMPEDANCE OF DEEP BRAIN STIMULATION
ELECTRODES
Lempka SF, Miocinovic S, Johnson MD, Vitek JL, and McIntyre CC. (2009) In vivo
impedance spectroscopy of deep brain stimulation electrodes. J. Neural Eng.
6(4):046001.l
4.1
Introduction
Deep brain stimulation (DBS) is an established therapy for the treatment of
movement disorders (Limousin and Martinez-Torres, 2008; Ostrem and Starr, 2008), and
shows promise for the treatment of several neuropsychiatric disorders (Ackermans et al.,
2008; Lujan et al., 2008). Commercial DBS systems apply high-frequency (~100-185
Hz) voltage-controlled (~1-3 V) stimulus pulses (~60-90 µs) to the brain to achieve their
therapeutic effect. The use of voltage-controlled stimulation results in voltage
distributions generated in the brain that depend upon the impedance of the electrodetissue interface (Gimsa et al., 2005; Wei and Grill, 2005; Butson et al., 2006; Miocinovic
et al., 2009). In turn, DBS electrode impedance can affect the actual stimulus applied to
the tissue medium and the corresponding volume of tissue activated by the stimulation
(Butson et al., 2006; Miocinovic et al., 2009). The goal of this study was to examine the
in vivo changes in DBS electrode impedance that occur after electrode implantation and
during clinically-relevant stimulation.
In the days and weeks after surgical implantation of an electrode into peripheral
or central nervous system tissue, electrode impedances typically increase (Grill and
68
Mortimer, 1994; Johnson et al., 2005; Williams et al., 2007). These changes have been
attributed to the nervous system’s foreign body reaction, which involves the attachment
of proteins and cells directly to the electrode and the development of an encapsulation
layer around the implanted device (Xu et al., 1997; Haberler et al., 2000; Szarowski et
al., 2003; Moss et al., 2004; Biran et al., 2005). After several weeks, the foreign body
reaction and the electrode-tissue impedance typically stabilize (Grill and Mortimer,
1994), but this stability can be perturbed with electrical stimulation (Johnson et al., 2005;
Otto et al., 2006). Clinical measurements have also shown reversible decreases in DBS
electrode impedance following electrical stimulation (Hemm et al., 2004). However, the
electrode-tissue interface has a substantial frequency dependence that can be more
completely monitored with electrode impedance spectroscopy (EIS) (Buitenweg et al.,
1998; Johnson et al., 2005; Otto et al., 2006; Frampton et al., 2007; Williams et al.,
2007).
Changes in DBS electrode impedance observed under clinically-relevant
conditions represent an opportunity to characterize the electrode-brain interface.
Moreover, DBS impedance fluctuations may be responsible for some clinical
observations relevant to patient programming. For example, most clinical centers find it
is necessary to wait 3-4 weeks after electrode implantation before beginning the process
of therapeutic stimulation parameter selection (Deuschl et al., 2006). Another common
clinical observation is the appearance of unwanted side effects (e.g. muscle contractions,
dyskinesias, and paresthesias) within the first few hours of stimulation at settings that
were initially therapeutic (Volkmann et al., 2002). Understanding how and why these
69
effects occur could assist in the definition of new or alternative programming paradigms
to improve the DBS patient experience.
The purpose of this study was to provide a detailed description of the impedance
of chronically-implanted DBS electrodes and develop a theoretical understanding of the
factors that influence these observed impedances. DBS leads with four electrode contacts
were implanted in the subthalamic nucleus and thalamus of a rhesus macaque and used to
study the in vivo impedance spectra of the electrode-tissue interface over time. Equivalent
circuit models were then fit to the measured impedance data to characterize the factors
affecting the electrode impedance. Preliminary portions of this work have been presented
in abstract form (Lempka et al., 2007; Lempka et al., 2008).
4.2
4.2.1
Materials and methods
Electrode impedance spectroscopy
Electrode impedance spectroscopy (EIS) was used to provide a comprehensive
description of the DBS electrode impedance. Impedance measurements were performed
with a two-electrode cell configuration using an Autolab potentiostat (PG-STAT12, Eco
Chemie, Utrecht, The Netherlands) with a built-in frequency response analyzer (FRA2,
Brinkmann Instruments, Westbury, NY). A 25 mV (rms) sine wave was applied to
measure impedances over a frequency range from 0.5 Hz to 10 kHz. The 25 mV signal
was chosen to provide increased signal strength while still operating in a linear
measurement range. The impedance was measured using either a single-sine or a multisine technique. In the single-sine technique, a 25 mV sinusoid was applied at a specific
70
frequency and the resulting impedance measured. For this technique, the frequency range
examined was 1 Hz to 10 kHz with 41 frequency points spaced logarithmically. For more
rapid impedance measurements, a multi-sine technique was often utilized in which 15
sine waves were applied simultaneously at 15 different frequencies. A desired base
frequency was selected and multiples of this frequency were calculated (frequencies =
base frequency * (1, 3, 5, 7, 9, 13, 19, 25, 33, 41, 51, 61, 73, 87, 99)). The results
presented in this study used base frequencies of 0.5, 1, 10, and 100 Hz. Each sinusoid had
an amplitude of 25 mV and random phase to minimize the overall excursion of the
applied voltage signal. The electrode impedance at a specific frequency was calculated in
the frequency domain by dividing the applied voltage signal by the measured current
output.
4.2.1.1 In vitro impedance measurements
In vitro EIS measurements were performed with an individual DBS electrode
contact as the working electrode and the counter electrode was a coiled Ag|AgCl wire.
The DBS electrodes were scaled-down versions of clinical electrodes suitable for
implantation in the monkey brain and were fabricated by the Advanced Bionics
Corporation (now Boston Scientific Neuromodulation, Valencia, CA). The electrodes had
a 45 mm polyurethane shaft with four cylindrical platinum/iridium contacts located near
the distal end of the lead shaft. Each electrode was 0.75 mm in diameter and 0.5 mm
height with 0.5 mm insulation separating the individual contacts. In vitro measurements
were performed in a 250 mL beaker containing 0.9% NaCl that was placed inside a
copper Faraday cage to help minimize noise. In vitro EIS measurements showed the
71
standard constant phase element (CPE) behavior of solid metal electrodes in which the
impedance data followed a straight line on the impedance plot with a phase angle less
then unity (data not shown) (McAdams et al., 1995). In vitro measurements were also
used to estimate the shunt capacitance of the DBS electrode and coupling capacitances
between the individual contacts. The shunt capacitance was estimated by submerging the
shaft of the DBS lead in saline while keeping the contacts exposed to air. The coupling
capacitance between pairs of DBS contacts was estimated by measuring the impedance
between the lead wires. The total stray capacitance of the electrode was then calculated
by adding these parallel capacitances (i.e. Cstray = Cshunt + Ccoupling). The total stray
capacitance was typically 20 pF and this value was used in the model analysis described
below.
In vitro EIS measurements were also used to examine the effect of protein
adsorption on possible changes to the DBS electrode impedance. For this particular
experiment, a DBS electrode was submerged in 0.1 M phosphate buffered saline
(pH=7.4) containing 0.2 mg/mL bovine serum albumin (Sigma-Aldrich, St. Louis, MO,
Product No. A-7906), equal to the albumin concentration in cerebrospinal fluid. EIS
measurements were performed periodically for two weeks using the two-electrode cell
configuration described above.
4.2.1.2 In vivo impedance measurements
For this study, in vivo impedance measurements were performed on chronically
implanted electrode leads in the brain of a rhesus macaque monkey (Macaca mulatta; 8
years old; weighing 6 kg) (Fig. 4.1). The first electrode lead was implanted in the ventral
72
thalamus of the left hemisphere and a second electrode lead was implanted in the
subthalamic nucleus (STN) of the right hemisphere using previously described implant
procedures (Elder et al., 2005; Miocinovic et al., 2007b; Miocinovic et al., 2009). All
surgical and recording protocols were approved by the Cleveland Clinic Institutional
Animal Care and Use Committee and complied with United States Public Health Service
policy on the humane care and use of laboratory animals.
73
Figure 4.1. Surgical planning and electrode implantation.
A) Cicerone software was used to plan the locations of the access chambers on the skull
and the DBS electrodes to be implanted in the thalamus (left hemisphere) and the STN
(right hemisphere). B) Sagittal X-ray images of the two DBS electrodes after
implantation. C, E) The post-DBS-implantation CT was co-registered to the pre-operative
MRI scan. The white contours were defined from the CT and represent the implanted
DBS electrodes. D, F) The approximate location of the implanted DBS electrodes with
respect to specific brain nuclei was defined with an atlas scaled to match the
neuroanatomy of the animal.
74
In vivo EIS measurements were performed with a single DBS electrode contact as
the working electrode and the counter electrode was a Ag|AgCl coiled wire placed in the
contralateral access chamber and submerged in 0.9% NaCl (Fig. 4.2). The surface area of
the Ag|AgCl counter electrode was much larger than the individual DBS contacts and
was removed and cleaned at the end of each experiment to ensure the EIS measurements
were dominated by the DBS electrode-tissue interface. The animal’s chair was grounded
to help minimize the effects of surrounding noise. During the experimental procedures,
the animal was lightly sedated with acepromazine (1 mg/kg) to help minimize movement
and pressure exerted on the head implant. The animal also received 4 mg of prednisolone
7 days a week to treat an endogenous intestinal disorder.
Figure 4.2. EIS of the implanted DBS electrodes.
A) A two-cell electrode configuration was used to perform the EIS measurements. The
working electrode was the DBS contact of interest and the counter electrode consisted of
a Ag|AgCl electrode placed in the contralateral chamber. B) The typical impedance
spectrum was linear in the low frequency range, which followed the constant phase
element (CPE) behavior at electrode-electrolyte interfaces. In the higher frequency range,
a semicircular arc represented the tissue component surrounding the electrode contact.
75
EIS measurements were performed before electrode implantation, immediately
following implantation, and periodically after implantation. These measurements
provided a means to detect the temporal profile of proteins and cells adhering to the
electrode, which appeared as a semi-circular arc in the high frequency range of the
impedance spectra (e.g. 0.1-10 kHz) (Buitenweg et al., 1998; Williams et al., 2007).
Changes in the properties of the electrode-electrolyte interface could also be observed as
changes in the magnitude and phase of the low frequency range of the impedance spectra
(e.g. 0.5-100 Hz).
Stimulation applied through a DBS contact has been shown to alter the electrode
impedance (Hemm et al., 2004). To examine the specific effects of stimulation on
electrode impedance, voltage-controlled stimulation was applied through a DBS contact
using an Itrel II implantable pulse generator (Medtronic, Minneapolis, MN) with the
contralateral titanium access chamber as the return electrode. A clinically-relevant
stimulus train of -1.0 V amplitude 90 µs pulses delivered at a frequency of 135 Hz was
used for this study. In vivo EIS measurements were performed before and after
stimulation to examine the effect of DBS on the electrode impedance.
4.2.2
Equivalent circuit models
Equivalent circuit models were used to quantify the factors influencing the DBS
electrode impedance. The circuit models were fit to measured impedance data to examine
changes that occur after electrode implantation and during stimulation. Multiple
impedance models were examined to identify the model components that were important
for reproducing the observed impedance data. Overall, each individual model had one
76
section representing the impedance of the electrode-electrolyte interface and a second
section representing the impedance of the tissue layer near the electrode resulting from
the adhesion or accumulation of cells and proteins (Fig. 4.3A-D). In the first model
(model A), the impedance of the electrode-electrolyte interface was represented as an
ideal capacitor (Fig. 4.3A). The tissue layer impedance consisted of the classical
Lapicque model with a resistance in series with a parallel combination of a resistor and
capacitor (McAdams and Jossinet, 1995; Foster and Schwan, 1996). In the remaining
three models (models B-D), the electrode-electrolyte interface was represented by a
constant phase element (CPE) according the following equation:
Z CPE =
K
( jω )α
(4.1)
where K was a magnitude scaling factor and α was a phase factor defined for 0<α<1. The
CPE accounted for the non-ideal capacitive behavior of the electrode-electrolyte interface
for solid metal electrodes that is believed to arise from specific adsorption and surface
roughness effects (McAdams et al., 1995). To represent the impedance of the tissue layer
around the electrode, model B had the same Lapicque model as described above for
model A (Fig. 4.3B). In model C, this impedance was represented by an encapsulation
resistance in series with cellular and extracellular compartments (Fig. 4.3C). The cellular
compartment contained a specific membrane conductance (gm=0.3 mS/cm2) and
membrane capacitance (cm=1 µF/cm2) multiplied by a cell membrane area scaling term
(Am). This cellular compartment represented the membrane conductance and capacitance
of glial cells and macrophages that typically accumulate around the electrode over time.
The extracellular compartment was composed of a single resistor representing the
resistance to ionic current flow through the extracellular space. This model has
77
previously been used to understand the composition of the electrode-tissue interface in
chronic microelectrode recording applications (Johnson et al., 2005; Otto et al., 2006;
Williams et al., 2007). In the fourth impedance model (model D), the tissue layer
impedance was represented by a modified Lapicque model of a resistance (R∞) in series
with a parallel combination of a resistance (∆R) and a CPE (Fig. 4.3D) (McAdams and
Jossinet, 1995). R∞ represents the tissue layer resistance measured at an infinite
stimulation frequency while ∆R represents the difference between the tissue layer
resistance measured at DC (R0) and R∞ (i.e. ∆R = R0-R∞). Each of the four models used
in this study contained a capacitor representing the stray capacitance of the electrode.
This capacitance was connected in parallel to the electrode and tissue components of each
model. This stray capacitance represented a combination of the shunt and coupling
capacitances of the DBS electrode and was set to a fixed value of 20 pF. This capacitor is
not shown in the circuit diagrams.
78
Figure 4.3. Equivalent circuit models.
Each model consisted of an electrode component that represented the impedance of the electrodeelectrolyte interface and a tissue component that represented the impedance of the tissue layer
around the electrode. Each model had a capacitor connected in parallel to account for the stray
capacitance of the electrode (capacitor not shown). A) Classical model with the electrodeelectrolyte interface impedance represented by a capacitor and the tissue layer impedance
represented with a Lapicque equivalent circuit model. B) The electrode-electrolyte interface
impedance is replaced by a constant phase element (CPE) to account for non-ideal capacitive
properties of solid metal electrodes. C) A model used in previous studies to understand the
impedance of cortical microelectrodes in which the tissue component has cellular and
extracellular compartments. D) The electrode-electrolyte interface impedance is represented by a
CPE and the tissue layer impedance is represented by a modified Lapicque equivalent circuit
model in which the tissue capacitance is modeled using a second CPE. E) An example in which
each of the four models was fit to the same impedance data. F) Average error for each model
when fitting the impedance data measured 15 days and 16 days after implantation for the DBS
leads implanted in the thalamus and STN, respectively.
79
4.2.3
Parameter estimation
Parameter estimates were calculated with a direct-search method using the
PATTERNSEARCH function available in Matlab (Mathworks, Natick, MA). Model fits
were performed by minimizing the following objective function:
(
⎛ ' '
⎜ Zj −Zj
J = ∑⎜
2
j =1 ⎜
Z 'j
⎝
N
( )
) + (Z
2
''
j
− Z ''j
( )
Z ''j
2
)
2
⎞
⎟
⎟
⎟
⎠
(4.2)
where j indicates a particular frequency at which the impedance was measured, N
represents the total number of frequencies, Zj’ represents the real part of the measured
impedance at the specific frequency and Żj’ the corresponding model estimate, and Zj’’
represents the imaginary part of the measured impedance at the specific frequency and
Żj’’ the corresponding model estimate. According to (4.2), function weighting was used to
estimate the model parameters. Function weighting was selected over other possible
weighting methods, such as modulus weighting, because function weighting is less likely
to lead to biased parameter estimates (Macdonald, 1992). For each data set, five sets of
initial parameter values were randomly chosen throughout the entire parameter space and
the set of optimized parameter values that produced the minimum error was selected. The
use of a direct-search method and multiple initial starting points helped improve the
probability of finding the global minimum.
Model analysis was applied to the measured impedance data to identify
significant changes in the model parameters following electrode implantation and during
stimulation. Statistically-significant changes in the model parameters were detected with
the Wilcoxon matched-pairs signed-ranks test, a nonparametric procedure applied to
80
hypothesis testing in which there are two dependent samples (e.g. parameter values
before stimulation and after stimulation). Significance was set to p<0.05.
4.3
4.3.1
Results
Model analysis
The model complexity necessary to accurately reproduce the measured impedance
spectra was examined by comparing four impedance models (Fig. 4.3). Each model had
two major sections; an electrode component representing the electrode-electrolyte
interface and a tissue component representing the tissue layer around the electrode
formed by the adhesion and accumulation of proteins and cells. Each model was fit to
experimentally recorded impedance data and the model error was calculated using (4.2)
(Fig. 4.3E-F). On average, model D returned the lowest total model error (Fig. 4.3F) by
providing the lowest model error in both the low frequency range that described the
electrode-electrode interface and the high frequency range that described the surrounding
tissue layer (data not shown). Therefore, model D was used for the remaining analysis in
this paper.
4.3.2
Changes in electrode impedance after implantation
DBS electrode impedance was examined as a function of time after implantation
by performing EIS measurements periodically after implantation. Fig. 4.4 displays a
typical data set where substantial changes in the impedance spectra can be seen in the
days following implantation. Changes occurred in both the magnitude and phase of the
81
electrode-electrolyte interface, as well as development of a semicircular arc in the high
frequency range of the impedance measurements (i.e. 0.1-10 kHz). This semicircular arc,
referred to here as the tissue component, became apparent within the first five days and
began to stabilize within the first few weeks after implantation (Fig. 4.4).
82
Figure 4.4. Evolution of DBS electrode impedance after implantation.
A) Nyquist plots of the complex impedance during the first nine days after implantation.
Immediately after implantation in the brain (day 0), the DBS electrode exhibited CPE behavior.
Within the first five days, there was a significant change in the DBS electrode impedance due to
the foreign body reaction and a prominent semicircular arc developed in the high frequency
range. The 1, 10, and 100 Hz frequency points are indicated for the impedance measurements
performed on day 0 and day 9. B) The resistive and reactive components of the measured
impedance as a function of frequency during the first nine days after implantation. C) The 1 kHz
impedance during the first nine days after implantation for the same electrode shown in (A) and
(B). The inset shows stabilization of the 1 kHz electrode impedance over the first 100 days after
implantation.
83
We compared the impedance data measured 24 hours and approximately two
weeks after implantation using model D (Fig. 4.5). Parameter values were determined for
impedances measured from the four contacts of the DBS electrode implanted in the
thalamus and the four contacts of the DBS electrode implanted in the STN (n=8). All of
the model parameters showed a statistically-significant change except for R∞ (Fig. 4.5B).
Figure 4.5. Model parameters after electrode implantation.
A) Model D was used to analyze the changes in DBS electrode impedance that occurred
following implantation. B) Impedance data measured 24 hours (gray bars) and more than
2 weeks (black bars) after implantation was used to examine potential changes in model
parameters. The data presented in (B) represents the mean parameter values for the
impedance measured at 8 DBS electrode contacts. The error bars represent the standard
deviations. Model analysis was performed for impedance data measured 1 day and 15
days after implantation for the thalamic DBS lead and 1 day and 16 days after
implantation for the STN lead. All of the model parameters, except R∞, showed
statistically-significant changes (* for p=0.023 and ** for p=0.008).
84
The model analysis showed a decrease in the magnitude and phase of the
electrode-electrolyte interface impedance two weeks after implantation (p=0.008 for Ke
and p=0.023 for αe). However, the most significant changes in the model parameters
were encountered in the impedance of the tissue layer surrounding the electrode in which
Kt, αt, and ∆R all increased (p=0.008). On average, the magnitude of the tissue layer
capacitance (Kt) increased more than 67 times its initial value while ∆R increased more
than 11 times its initial value two weeks after implantation.
4.3.3
Stimulation-induced changes in electrode impedance
The effect of clinically-relevant stimulation on DBS electrode impedance was
examined using a train of charge-balanced biphasic stimulus waveforms. The stimuli
were generated using a Medtronic pulse generator (Itrel II, Minneapolis, MN) and had the
following parameters: -1.0 V amplitude, 90 µs pulse width, and 135 Hz frequency.
Stimulation was applied for a total of 60 minutes and EIS measurements were performed
before and after stimulation (Fig. 4.6A).
Model analysis showed stimulation caused statistically-significant changes in both
the electrode and tissue components of the impedance model (Fig. 4.6B). The electrodeelectrolyte interface impedance exhibited an increase in both magnitude and phase (Ke
and αe, p=0.031). However, stimulation caused the most substantial changes in the
impedance of the tissue layer surrounding the electrode. These impedance changes were
dominated by a large decrease in both the capacitive (Kt and αt) and resistive (∆R)
components of the tissue parameters (p=0.031) that appeared as a large decrease in the
size of the semicircular arc in the high frequency range of the impedance spectra (Fig.
85
4.6A). Specifically, Kt exhibited an 81% decrease while ∆R showed a 70% decrease in
magnitude.
Although stimulation caused a large decrease in the electrode impedance, these
changes were reversible and the electrode impedance began increasing immediately after
stimulation was stopped (data not shown). Typically, small changes were observed
minutes after the end of stimulation and a complete recovery to pre-stimulation
impedances often occurred within days. These rapid and reversible changes in the
electrode impedance demonstrate the dynamic nature of the foreign body reaction.
86
Figure 4.6. Effect of clinically-relevant stimulation on DBS electrode impedance.
A) An example of typical impedance spectra measured before stimulation and after 60
minutes of stimulation. The corresponding model fits are also shown. B) The resistive
and reactive components of the impedance data shown in (A) as a function of frequency.
C) Averages of the model parameters estimated for six separate experiments performed
on contacts from both the DBS electrode implanted in the thalamus and the DBS
electrode implanted in the STN (n=6). The error bars represent the standard deviation. All
of the model parameters, except R∞, showed a statistically-significant change (* for
p=0.031).
87
4.4
Discussion
The goal of this study was to investigate changes in DBS electrode impedance
that occur after electrode implantation and clinically-relevant stimulation. We utilized
EIS as a non-invasive and rapid technique to characterize impedance changes over a wide
range of frequencies to better understand the electrode-tissue interface. EIS provides
more information than the standard impedance measurement at 1kHz and has been used
extensively to study the composition of the electrode-tissue interface in various
microelectrode applications (Buitenweg et al., 1998; Keese et al., 2004; Johnson et al.,
2005; Otto et al., 2006; Frampton et al., 2007; Williams et al., 2007). Our results show
that: 1) DBS electrode impedance significantly increased during the first few weeks after
electrode implantation, and 2) application of clinically-relevant stimulation significantly
and reversibly decreased the electrode impedance. These results have important clinical
implications for the use of voltage-controlled stimulators since the current delivered to
the tissue is inversely proportional to the electrode impedance.
4.4.1
Characterizing DBS electrode impedance
Equivalent circuit models were used to interpret the impedance spectra measured
from the implanted DBS electrodes. Multiple impedance models were investigated to
assess the model complexity necessary to accurately reproduce the experimental
measurements. Each of the four models examined in this study had two major sections:
one representing the impedance of the electrode-electrolyte interface and the other
representing the impedance of the tissue layer formed around the electrode due to the
88
adhesion and accumulation of proteins and cells (Fig. 4.3A-D). Comparison of the four
models, showed model D produced the lowest model error (Fig. 4.3F).
Model A relied on an ideal capacitor to represent the electrode-electrolyte
interface and produced the highest errors. The error in the other three models was
substantially reduced by incorporating a constant phase element (CPE) to represent the
impedance of the electrode-electrolyte interface. A CPE is an empirically-derived model
of the electrode-electrolyte interface that accounts for the non-ideal capacitive properties
of solid metal electrodes (McAdams et al., 1995).
Models B and C differed in their respective tissue components. Model B consisted
of a classical Lapicque model composed of a resistance (R∞) in series with a parallel
combination of a capacitance and resistance (∆R) (McAdams and Jossinet, 1995). R∞
represented the tissue resistance at an infinite stimulus frequency and ∆R represented the
difference between the tissue resistance at DC and R∞. The tissue component of model C
had an encapsulation resistance in series with cellular and extracellular components. The
physical interpretation of the encapsulation or sealing resistance is protein adsorption
onto the electrode contact and/or an adjacent layer of connective tissue (Johnson et al.,
2005), while the cellular component represents the membrane conductance and
capacitance of cells near the electrode (Otto et al., 2006; Williams et al., 2007). A
specific membrane conductance (gm) and membrane capacitance (cm) are multiplied by a
scaling term, Am. Thus Am provides an estimate of the total area of cellular membrane
that affects the electrode impedance. This cellular component is in parallel to an
extracellular resistance that represents pathways for ions to travel in the extracellular
space. Models B and C often exhibited fitting errors that were similar; however, one
89
advantage of model C is the implied physical meaning of its parameters. This type of
model may be more advantageous for microelectrode applications where the dimensions
of the neural and glial cells are of a similar order of magnitude as the electrode contact.
However, physical interpretation of these parameters is limited by the assumptions
required in formulating this model such as the use of a purely resistive encapsulation
layer, the membrane capacitance of cells near the electrode are the only capacitive
component of the tissue (i.e. the extracellular pathway is purely resistive), and the
conductivity and capacitance of the cell membranes are constant.
We used model D for the statistical analyses done in this study. Model D is not
dependent upon the assumptions described above for model C, but physical interpretation
of the model results is not as clear. Model D relied on a CPE to model the impedance of
the electrode-electrolyte interface and a second CPE to represent the tissue capacitance.
Thus the tissue component of model D represented a modified Lapicque model that was
first proposed by Cole (Cole, 1940; McAdams and Jossinet, 1995). Incorporation of a
CPE representing the capacitive behavior of the tissue allowed for model D to produce
increased accuracy in fitting the experimentally measured impedance data (Fig. 4.3F).
We used the model analysis to quantify the effect of the foreign body reaction on
the implanted electrode impedance. All of the model parameters (except R∞) underwent a
statistically-significant change during the first two weeks after implantation (Fig. 4.5B)
that included a decrease in both the impedance magnitude and phase of the electrode
component (Ke and αe). These changes show potential modification to the behavior of the
electrode-electrolyte interface caused by the adsorption of various ions, proteins, and
cells to the electrode (Contu et al., 2002; Frampton et al., 2007). These changes in the
90
electrode-electrolyte interface impedance could also be due to changes in the ionic
composition of the cerebrospinal fluid near the electrode during vasogenic and cellular
edema (Unterberg et al., 2004). The largest changes were the increases in the model
parameters representing the impedance of the tissue layer surrounding the electrode (Kt,
αt, and ∆R). The drastic increase in both the magnitude of the tissue layer capacitance,
Kt, and the resistance, ∆R, suggests the migration of various glial cells to the electrode
site as a part of the foreign body reaction. This conclusion was further supported by the
time course of these impedance changes that closely paralleled the time course for
establishing the chronic foreign body reaction that is characterized by the formation of
foreign body giant cells and granulation tissue around the implanted device (Anderson et
al., 2008). Direct adhesion of cells and the development of an encapsulation layer around
electrodes implanted in the central nervous system has been documented for both cortical
microelectrode (Szarowski et al., 2003; Biran et al., 2005) and DBS applications
(Haberler et al., 2000; Moss et al., 2004; Nielsen et al., 2007).
Before cells can adhere to the electrode, proteins need to adsorb to the electrode
surface. Therefore, it is possible the impedance changes measured in vivo could largely
be attributed to the adsorption of proteins to the electrode surface and not the
accumulation of cells near the electrode (Gimsa et al., 2005). In order to examine this
possibility, a DBS lead was submerged in 0.2 mg/mL albumin and EIS measurements
were performed periodically during the following two weeks. Impedance measurements
of all four contacts showed only minor changes during this time and no development of a
semicircular arc in the high frequency range (data not shown). These results suggest the
91
semicircular arc in the EIS measurements of a chronically-implanted DBS electrode was
due to the accumulation of cells around the electrode contact.
Electrical stimulation caused a significant decrease in the tissue component (i.e.
high frequency range) of the impedance spectra and small changes in the magnitude and
phase of the electrode-electrolyte interface impedance (Fig. 4.6). The largest changes
were in the magnitude of the tissue capacitance (Kt) and ∆R. While it is difficult to
interpret the exact physical basis for these results, one possible explanation is that
stimulation applied through the electrode polarizes the surface causing the attached
proteins and cells to desorb. This “cleaning” of the electrode surface could produce a
corresponding decrease in the electrode impedance. This phenomenon has been observed
for both in vitro and in vivo applications (Keese et al., 2004; Johnson et al., 2005). Keese
et al. (2004) observed electroporation of cultured cells adhered to the electrode after
application of high frequency (40 kHz) voltage pulses and a corresponding decrease in
the electrode impedance to levels similar to the bare electrode impedance. Johnson et al.
(2005) applied DC voltage signals to help improve the chronic recording capabilities of
cortical microelectrodes and showed a substantial decrease in both the electrode and
tissue impedance parameters. The described cleaning of the electrode surface is further
supported by the observation that stimulation applied through one contact did not cause
impedance changes to adjacent contacts (data not shown). These results suggest that the
measured impedance is dominated by the properties of the tissue within ~100 µm or less
of the electrode contact and not the properties of the bulk brain tissue.
The impedance spectra of the DBS electrodes did not show significant changes in
R∞ either after implantation or after electrical stimulation (Fig. 4.5B and 4.6C) that would
92
correspond to resistive shifts of the entire impedance spectra along the horizontal axis.
These small resistive shifts are in contrast to the impedance changes observed for
microelectrodes in which large increases in R∞ have been documented after implantation,
along with large decreases in R∞ after applying electrical stimulation (Johnson et al.,
2005; Otto et al., 2006). This lack of significant change in R∞ for the DBS electrodes
could be attributed to the relatively large contact size. The large DBS electrode size
provides an increased number of pathways for current to flow. In turn, DBS electrodes
may have a lower access resistance and a lower sensitivity to the adsorption of various
proteins directly on the electrode that could produce large changes in R∞. This conclusion
was further supported by the in vitro experiment described above in which the DBS
electrode impedance showed little resistive shifts when placed in a solution containing
0.2 mg/mL albumin (data not shown).
4.4.2
Model limitations
The equivalent circuit models used in this study provided accurate fits to the
experimentally measured impedance spectra in a non-human primate, however, these
models have notable limitations. The most significant limitation was the use of models
composed of linear circuit elements. EIS measurements were performed with a small
amplitude voltage sinusoid to ensure that the electrode was operating under linear
conditions and permitted the electrode impedance to be determined at multiple
frequencies using Ohm’s law. However, under stimulating conditions, the DBS electrode
likely exhibits nonlinear behavior that would make these linear circuit elements invalid.
Because of this limitation, the main purpose of the EIS measurements and model analysis
93
performed in this study was to study interval changes in the composition of the electrodetissue interface for individual electrodes under multiple experimental conditions. This
limitation is justified because of the extreme difficulties of attempting to examine these
changes for individual electrodes using histological techniques in which the animal
would need to be sacrificed and/or the electrode removed.
A second limitation was the use of spatially-lumped circuit elements to describe a
spatially-distributed environment. However, the advantage of using spatially-lumped
circuit elements was their ease to parameterize while still providing insight into the
physical significance of the measured electrode impedance. Another limitation was the
inability to guarantee parameter uniqueness in the model optimization. Even though
multiple initial parameter values were utilized to help improve the likelihood of locating
the global minimum, it was not possible to guarantee the parameter values generated
from the optimization methods were unique or represented the global minimum.
4.4.3
Experimental limitations
While the experimental conditions used in this study closely mimicked clinical
DBS, there are a number of potential issues that should be recognized. The first limitation
was the physical stability of the DBS electrode in the monkey chamber system. The
electrode was fixed to the access chamber attached to the skull and allowed for
micromotion between the skull and brain. Brain shift could therefore affect the stability
of the electrode-tissue interface and the corresponding electrode impedance. However,
clinical DBS electrodes are also fixed to the skull and thus generate a similar degree of
brain shift relative to the DBS electrode. A second limitation was the treatment of the
94
experimental animal with the steroid, prednisolone, which could influence the extent of
the foreign body reaction to the implanted electrodes. However, similar changes in DBS
electrode impedance after implantation and during stimulation were observed with other
animals not receiving this drug (data not shown).
4.4.4
Clinical relevance of results
The results of this study show major changes in DBS electrode impedance occur
after electrode implantation and during stimulation. These impedance changes could be
an important issue for DBS patients implanted with voltage-controlled stimulators.
Typically, patient programming is not started until 3-4 weeks after electrode implantation
to ensure that disease symptoms stabilize from any micro-lesioning effects induced in the
operating room and to allow time for the foreign-body reaction to stabilize (Deuschl et
al., 2006). If patient programming is started within the initial 3-4 weeks after
implantation, there is often a need to frequently adjust the parameter settings to maintain
therapeutic benefit while minimizing unwanted side effects. The electrode impedance
changes observed in this study help explain why this initial delay in patient programming
is necessary as fluctuating electrode impedance directly affects the voltage distributions
generated during voltage-controlled stimulation (Butson et al., 2006; Yousif et al., 2008;
Miocinovic et al., 2009).
Following the initial electrode stabilization period, stimulation parameters
(amplitude, pulse width, frequency) are selected by an experienced clinical programmer,
to be delivered through an electrode contact(s) that maximize therapeutic benefit while
minimizing any stimulation-induced side effects. However, after a couple hours of
95
stimulation unwanted side effects can appear (Volkmann et al., 2002). The appearance of
these unwanted side affects may be attributed to the changes in electrode impedance
induced by the stimulation (Fig. 4.6). The overall decrease in electrode impedance that
occurs due to the applied stimulation would result in a larger area of tissue being
stimulated for the same stimulation parameters (Butson et al., 2006). Thus, as the
impedance decreases over time, supra-threshold stimulation can reach regions of the
brain implicated in the appearance of various side effects (e.g. sustained muscle
contractions, dyskinesias, paresthesias, etc.).
While clinically-relevant stimulation caused changes to the properties of the
electrode-electrolyte interface of the DBS electrode, the most dramatic changes occurred
in the tissue component of the electrode impedance (Fig. 4.6). We observed a dramatic
decrease in the size of the semicircular arc in the high frequency range of the impedance
spectrum (Fig. 4.6A). After 1 hour of stimulation, the electrode impedance qualitatively
resembled the impedance of a freshly implanted electrode except for a small residual
tissue component in the high frequency range of the impedance spectrum (Fig. 4.6A).
Similar results have also been observed following stimulation through microelectrodes
(Johnson et al., 2005; Otto et al., 2006). This remaining semicircular arc could be
attributed to several factors. It is possible that stimulation fails to clean off all of the
adhered proteins and cells. The remaining tissue component observed during stimulation
could be also be representative of the encapsulation sheath surrounding the electrode that
may be unaffected by the stimulation. This hypothesis is supported by histological studies
that have been unable to detect any measurable differences in the encapsulation sheath
around stimulated and non-stimulated DBS electrode contacts or the polyurethane tubing
96
(Haberler et al., 2000; Nielsen et al., 2007). This remaining tissue component shows
stimulation does not reverse all of the impedance changes due to the foreign body
reaction that may directly affect the voltage distribution generated during stimulation
(Butson et al., 2006; Yousif et al., 2008; Miocinovic et al., 2009).
4.5
Conclusion
This study utilized electrode impedance spectroscopy to characterize deep brain
stimulation electrodes implanted in the brain of a rhesus macaque monkey. These
electrodes were permanently implanted in regions of the brain that are common targets
for deep brain stimulation applications (i.e. thalamus and subthalamic nucleus). Model
analysis was used to understand the changes that occur in electrode impedance after
implantation and following prolonged stimulation. We observed a major increase in the
electrode impedance during the first two weeks after implantation, presumably caused by
the foreign body reaction to the implanted electrode. This increase in electrode
impedance was composed of changes to both the electrode and tissue components of the
overall impedance. The most substantial change occurred in the tissue component of the
impedance and was characterized by the development of a semicircular arc in the high
frequency range of the impedance measurements. Electrical stimulation caused a
decrease in the impedance within the first hour of stimulation that was characterized by a
major decrease in the tissue component of the electrode impedance. Because clinical
DBS systems rely on voltage-controlled stimulation, these impedance changes could play
an important role during patient programming (e.g. stabilization periods, assessment
times, and parameter adjustments).
97
5
CHAPTER 5: CURRENT-CONTROLLED DEEP BRAIN STIMULATION
REDUCES IN VIVO VOLTAGE FLUCTUATIONS OBSERVED DURING
VOLTAGE-CONTROLLED STIMULATION
Lempka SF, Johnson MD, Miocinovic S, Vitek JL, and McIntyre CC. (2010) Currentcontrolled deep brain stimulation reduces in vivo voltage fluctuations observed during
voltage-controlled stimulation. Clin. Neurophysiol. Submitted.
5.1
Introduction
Deep brain stimulation (DBS) is an established therapy for the treatment of
movement disorders and shows promise for the treatment of several neuropsychiatric
disorders (Perlmutter and Mink, 2006). Traditionally, clinical DBS systems have relied
on voltage-controlled pulse generators; however, the recent introduction of currentcontrolled pulse generators has expanded the clinical options for DBS therapy. The use of
voltage-controlled stimulation results in voltage distributions in the target neural tissues
that depend upon the impedance of the electrode-tissue interface (Butson et al., 2006;
Miocinovic et al., 2009). The impedance of the DBS electrode-tissue interface has been
shown to fluctuate both after implantation and during stimulation (Lempka et al., 2009).
These varying impedance conditions are suspected to produce instability in the voltages
produced in the target neural tissues during voltage-controlled DBS, and may be at least
partially responsible for the frequent need to adjust stimulation parameters during the
initial patient programming process.
98
Unlike voltage-controlled DBS, current-controlled DBS regulates the current
through the electrode-tissue interface. In theory, the voltages generated in the target brain
tissues by current-controlled DBS should be fairly independent of the electrode
impedance. This increased stability in the extracellular voltages produced from
stimulation could help stabilize the therapeutic efficacy of stimulation parameters
selected during patient programming. Therefore, we attempted to experimentally verify
the theoretical advantage of current-controlled DBS relative to voltage-controlled DBS.
DBS was applied through leads implanted in rhesus macaque monkeys and the
voltages in the surrounding neural tissue were monitored with microelectrode recordings.
We first examined the temporal voltage fluctuations during voltage-controlled DBS, both
after electrode implantation and during stimulation. Then we compared the magnitude of
the voltage fluctuations that occur during voltage-controlled DBS relative to currentcontrolled DBS. Our results show that substantial voltage fluctuations occur within the
first hour after activating a DBS electrode contact with voltage-controlled stimulation,
but these fluctuations were minimized with current-controlled DBS. These results have
important implications for clinical research analyses of the time-dependent wash-in
behavioral effects of DBS, as well as for standard clinical DBS device programming.
5.2
5.2.1
Materials and Methods
Stimulation and recording protocols
The DBS electrodes used in this study were scaled-down versions of clinical DBS
electrodes suitable for implantation in the brain of rhesus macaque monkeys (Macaca
99
mulatta). The DBS leads were fabricated by Advanced Bionics Corporation (now Boston
Scientific Neuromodulation, Valencia, CA) and each lead had a 45 mm polyurethane
shaft with four cylindrical platinum/iridium contacts located near the distal end of the
lead shaft. Each electrode contact was 0.75 mm in diameter and 0.5 mm height with 0.5
mm insulation separating individual contacts.
Both voltage-controlled and current-controlled stimulation were examined in this
study. A voltage-controlled stimulus train (-1.0V cathodic amplitude and 90 µs pulses
delivered at a frequency of 135Hz) was applied through a DBS contact using a clinical
pulse generator (IPG; Itrel II model, Medtronic Inc., Minneapolis, MN) with the
contralateral titanium access chamber as the return electrode (Fig. 5.1B). Currentcontrolled stimulation (200 µA amplitude and 90 µs pulses delivered at a frequency of
135 Hz) was applied with an external pulse generator (S88; Grass Instruments, Quincy,
MA) and two photoelectric constant-current stimulus isolation units (PSIU6, Grass
Instruments) (Fig. 5.1B). The amplitude of the current-controlled stimulus train was
determined using current measurements during one of the aforementioned experiments
utilizing voltage-controlled stimulation. The measured current typically varied between
100-300 µA in these experiments, and this variance was dependent on the DBS electrode
impedance (data not shown). A 200 µA amplitude was thus selected for the currentcontrolled experiments.
The voltages generated in the brain during DBS were measured with differential
recordings using an acutely implanted microelectrode. A guide tube was used to puncture
the dura so that the recording microelectrode could be inserted with ~2 mm separation
from the chronically-implanted DBS electrode using a microdrive (MO-95-Ip, Narishige
100
Scientific Instruments, Tokyo, Japan) (Fig. 5.1A). The stainless steel guide tube was
placed a few millimeters below the dura and served as the reference electrode.
Recordings were performed with both single-channel and multi-channel microelectrodes.
Single-channel
recordings
were
performed
with
epoxylite-coated
tungsten
microelectrodes with tip lengths of approximately 50 µm (FHC, Bowdoinham, ME). The
recorded signal was amplified (50x) and band-pass filtered between 0.1 Hz and 20 kHz
using a differential amplifier connected to a high-impedance headstage (model 3000, AM Systems, Sequim, WA). The recorded signal was digitized at a sampling rate of 100
kHz and stored for offline analysis (Power 1401 and Spike2 software, Cambridge
Electronic Design, Cambridge, UK). To increase the number of voltage recording
locations, multi-channel recordings were performed in a subset of experiments using a
linear array of 8 microelectrodes (NeuroNexus, Ann Arbor, MI). Recordings were
sampled at 50 kHz through an Alpha-Lab system (Alpha Omega, Nazareth, Israel) and
band-pass filtered between 1 Hz and 10 kHz. For both single-channel and multi-channel
microelectrode recordings, the peak cathodic voltage at each recording location was
calculated off-line by averaging peak voltages for 1 sec of recording data (i.e. 135
waveforms).
Because the impedance of the recording microelectrode can also influence the
voltages measured in the brain, both DBS electrode and microelectrode impedances were
monitored during the experiments. While DBS electrode impedances were measured at
multiple time points during each experiment, the microelectrode impedance was
monitored at the beginning and end of each experiment in order to ensure the
microelectrode impedance remained stable throughout the duration of the experiment.
101
Microelectrode impedances at 1 kHz were typically 0.5-1 MΩ. For both the DBS
electrodes and recording microelectrodes, impedance measurements were performed at 1
kHz using an two-electrode configuration with an Autolab potentiostat (PGSTAT-12,
Eco Chemie, Utrecht, The Netherlands) by applying a 25 mV (rms) sine wave between
the working electrode (i.e. DBS contact or recording microelectrode) and a large surface
area Ag|AgCl wire placed in the saline-filled contralateral access chamber. The current
output was measured and the impedance was calculated in the frequency domain using
Ohm’s law. The animal’s chair was grounded to help minimize the effects of surrounding
noise.
102
Figure 5.1. In vivo microelectrode recordings examining the temporal evolution of the voltages
generated in the brain during DBS.
A) X-ray image showing a chronically-implanted DBS electrode and an acutely-inserted
recording microelectrode used to monitor the voltages generated in the brain during DBS. The
recording electrode was inserted approximately 2 mm away from the DBS lead. B) Recordings of
the voltage-controlled and current-controlled stimulus waveforms examined in this study and
examples of the voltage waveforms recorded in the brain tissue. The example of in vivo voltages
recorded during voltage-controlled stimulation is from one of the recording locations shown in
Fig. 5.2A and shows the changes that occur during the first week after DBS electrode
implantation. The example recordings for current-controlled stimulation show the small changes
that occur during one hour of stimulation.
103
5.2.2
Surgical procedure and DBS electrode implantation
The data presented in this study were acquired from three DBS electrodes
chronically implanted in the brains of two rhesus macaque monkeys (8-10 years old;
weighing 5-6 kg) following previously described surgical implant procedures (Elder et
al., 2005; Miocinovic et al., 2007a). The electrodes were implanted in regions of the
brain that are common targets for treating movement disorders with DBS (i.e. thalamus,
subthalamic nucleus (STN), and globus pallidus (GP)). All surgical and recording
protocols were approved by the Cleveland Clinic Institutional Animal Care and Use
Committee and complied with the United States Public Health Service policy on the
humane care and use of laboratory animals.
5.2.3
Voltage changes after DBS electrode implantation
The effect of the foreign body reaction on the voltages generated in the brain
during DBS was investigated by performing microelectrode recordings periodically after
implantation. Microelectrode recordings during voltage-controlled DBS were acquired at
multiple insertion locations parallel to the DBS electrode lead, and the peak recorded
voltage was compared at one day and seven days after implantation. Two DBS electrodes
implanted in the STN of one animal and the GP of a second animal were used in this
analysis (n = 6, 3 contacts from each DBS lead) and the average peak voltages were
determined from 10 recording locations centered around each DBS contact. The relative
position of the DBS electrode and recording microelectrode was monitored with X-ray
images during each experimental session (Fig. 5.1A).
104
5.2.4
Voltage changes during voltage-controlled DBS
Temporal fluctuations in the voltages generated in the brain during clinically-
relevant, voltage-controlled DBS were examined with microelectrode recordings. DBS
was applied for a total of 60 minutes and voltage recordings were obtained using a linear
array of 8 microelectrodes. Multiple experiments (n = 4) were performed when applying
stimulation through multiple contacts of a single DBS lead implanted in the thalamus and
the peak voltages at each recording location of the microelectrode array were averaged.
5.2.5
Voltage-controlled v. current-controlled DBS
A separate set of experiments was also performed to examine differences in the
temporal voltage fluctuations generated during voltage-controlled and current-controlled
DBS. At the beginning of the experiment, a short duration (~one second) voltagecontrolled stimulation train was applied through a DBS contact and the voltage generated
in the brain was recorded with a single-channel microelectrode. After this brief voltagecontrolled stimulation train, 60 minutes of current-controlled stimulation was applied
through the same DBS contact, and the voltage measured at the adjacent microelectrode
was again recorded. At the end of the 60 minutes of current-controlled DBS, an
additional one second voltage-controlled stimulation train was applied and the voltage
was measured at the same location. This experimental design provided a means to
compare the effects of stimulation-induced changes at the electrode-tissue interface on
the voltage distributions generated during voltage-controlled and current-controlled DBS.
Three separate experiments were performed on multiple contacts from a single DBS lead
implanted in the thalamus (n=3).
105
For all of the experimental situations described above, the Spearman’s rank-order
correlation coefficient (rs) was calculated in order to examine possible correlations
between changes in the DBS electrode impedance and the voltages recorded in the brain.
Significance was set to p < 0.05.
5.3
Results
When comparing microelectrode voltage recordings of short periods of acute
stimulation on day 1 to day 7 after chronic implantation of the DBS electrode, we
observed an average decrease of 38.6 ± 10.6% in the peak cathodic voltage during
voltage-controlled stimulation (n = 6) (Fig. 5.2). This voltage decrease was accompanied
by a corresponding average increase in the 1 kHz DBS electrode impedance of 298 ±
136%. The decrease in the peak cathodic voltage between day 1 and day 7 exhibited a
strong negative correlation with the increase in the DBS electrode impedance (rs = -0.804,
p = 9.65e-29).
106
Figure 5.2. Effect of the foreign-body reaction on the voltages generated in the brain during
voltage-controlled DBS.
A) Microelectrode recordings were performed 1 day and 7 days after implantation of the DBS
electrode at the locations indicated by the linear array of black dots parallel to the electrode. The
plotted values display an example of the average peak cathodic voltages measured during
voltage-controlled stimulation 1 day and 7 days after implantation for the DBS contact shown in
black. B) Plots of the DBS electrode impedance versus the peak cathodic voltages recorded 1 day
and 7 days after implantation for the DBS lead implanted in the STN. Each marker type (i.e.
circle, triangle, cross) correspond to an individual DBS contact. Between 1 day and 7 days after
implantation, there was a 38.3 ± 10.5% decrease in the peak cathodic voltage and a corresponding
average increase in the 1 kHz DBS electrode impedance of 298 ± 136% (n=6). The decrease in
the peak cathodic voltage exhibited a strong negative correlation with the increase in the DBS
electrode impedance (rs = -0.803, p = 2.61e-28). Ten recording locations centered around each
DBS contact were used in this analysis (see ‘5.2’), corresponding to ten peak voltage
measurements for each DBS electrode impedance.
107
During experiments in which we applied continuous high frequency (135 Hz)
DBS, there was a rapid decrease in the DBS electrode impedance during the first 10-15
minutes of stimulation that began to stabilize within the first hour of stimulation (Fig.
5.3A). During voltage-controlled DBS, voltage recordings from the tissue medium
showed an increase in the peak cathodic amplitude that followed a similar time scale
(Fig. 5.3A). After 60 minutes of stimulation, there was an average decrease in the 1 kHz
DBS electrode impedance of 47.9 ± 16.7% that coincided with an average increase in the
peak recorded cathodic amplitude of 19.3 ± 6.2% (n = 4) (Fig. 5.3B). This increase in the
peak voltage amplitude was negatively correlated with the measured decrease in 1kHz
DBS electrode impedance (rs = -0.590, p = 4.42e-7).
108
Figure 5.3. Temporal voltage fluctuations observed in the brain during voltage-controlled
DBS.
A) Double y-axis plot showing an example of the temporal changes in both the 1 kHz
impedance of the DBS electrode and the peak cathodic voltages during one hour of
voltage-controlled DBS. The black line and circle markers indicate the 1 kHz impedance
of the DBS electrode and the gray dashed line and triangle markers indicate the peak
cathodic voltages. B) Plot of the DBS electrode impedance versus the peak cathodic
voltages recorded after 0 and 60 minutes of stimulation. Each marker type (e.g. circle,
asterisk) corresponds to an individual experiment. After 60 minutes of stimulation, there
was an average decrease in the 1 kHz DBS electrode impedance of 47.9 ± 16.7% that
coincided with an average increase in the peak recorded cathodic amplitude of 19.3 ±
6.2% (n = 4). This increase in the peak voltage amplitude was negatively correlated with
the measured decrease in 1kHz DBS electrode impedance (rs = -0.590, p = 4.42e-7).
Voltages for this set of experiments were recorded with an eight-contact microelectrode
array (see ‘5.2’), corresponding to eight peak voltage measurements for each DBS
electrode impedance.
109
In a separate set of experiments, current-controlled DBS showed an average
increase of only 6.5 ± 1.1 % in the peak recorded cathodic amplitude after 1 hour of
stimulation while voltage-controlled stimulation exhibited a larger and much more
variable average change of 54.8 ± 54.5% (n=3) (Fig. 5.4). The average decrease in DBS
electrode impedance for this set of experiments was 48.9 ± 30.1 %. The increase in the
peak cathodic voltage recorded during voltage-controlled DBS was highly correlated with
the decrease in DBS electrode impedance (rs = -0.927, p = 0.017) while the voltage
changes recorded during current-controlled DBS were not significantly correlated with
the decrease in DBS electrode impedance (rs = 0.657, p = 0.175).
110
Figure 5.4. Temporal voltage changes observed during current-controlled and voltage-controlled
DBS.
A) Microelectrode recordings of the voltages generated in the brain during current-controlled and
voltage-controlled DBS. The black line represents the stimulus waveform recorded at the
beginning of stimulation and the gray-dashed line represents the stimulus waveform recorded
after one hour of stimulation. B) Plot of the DBS electrode impedance versus the peak cathodic
voltages recorded during current-controlled (gray open markers) and voltage-controlled (black
markers) stimulation. Each marker type corresponds to an individual experiment (i.e. triangle,
square, and circle). Current-controlled DBS showed an average increase of only 6.5 ± 1.1 % in
the peak recorded cathodic amplitude after 1 hour of stimulation while voltage-controlled
stimulation exhibited a larger and much more variable average change of 54.8 ± 54.5% (n=3).
The average decrease in DBS electrode impedance for this set of experiments was 48.9 ± 30.1 %.
The increase in the peak cathodic voltage recorded during voltage-controlled DBS was highly
correlated with the decrease in DBS electrode impedance (rs = -0.927, p = 0.017) while the
voltage changes recorded during current-controlled DBS were not significantly correlated with
the decrease in DBS electrode impedance (rs = 0.657, p = 0.175).
111
5.4
Discussion
The goal of this study was to examine temporal fluctuations in the voltages
generated in the brain during DBS and investigate potential advantages of currentcontrolled over voltage-controlled DBS. Our results show that (1) changes in the
composition of the electrode-tissue interface after implantation produce an increase in
DBS electrode impedance and a decrease in the voltage magnitudes generated in the
brain by voltage-controlled DBS, (2) stimulation produces a decrease in DBS electrode
impedance and a corresponding increase in the voltage magnitudes, and (3) the observed
temporal voltage changes are reduced during current-controlled stimulation relative to
voltage-controlled stimulation.
After an electrode is implanted into the nervous system, there is a foreign body
reaction to the implanted device that results in the attachment of proteins and cells
directly to the electrode contact and accumulation of extracellular matrix proteins and
glia surrounding the device (Szarowski et al., 2003; Polikov et al., 2005; Anderson et al.,
2008). These changes at the electrode-tissue interface appear as an increase in the
electrode impedance (Williams et al., 2007; Lempka et al., 2009). Stimulation through a
DBS electrode contact reverses some of these impedance changes induced by the foreign
body reaction (Hemm et al., 2004; Lempka et al., 2009), which is noteworthy because
these impedance changes may directly affect the voltage distributions generated in the
brain.
The fundamental purpose of DBS is to modulate neural activity with electric
fields, and as such, understanding the factors that affect the voltage distribution in the
tissue medium have relevance to the clinical application of DBS, and in particular, the
112
clinical programming of DBS systems. After a DBS system is surgically implanted, there
is typically a period of time (~3-4 weeks) in which initial device programming is delayed
(Deuschl et al., 2006). This delay provides an opportunity for microlesioning effects and
local edematous changes to subside and the foreign-body reaction to stabilize. Fig. 5.2
shows that the changing electrode-tissue interface during this delay period can
substantially alter the voltage magnitudes generated in the brain tissue during voltagecontrolled DBS. As a result, stimulation parameters may require frequent adjustment if
programming is performed during the first few weeks after DBS electrode implantation.
Voltage-controlled stimulation may also contribute to additional difficulties
related to the identification of therapeutic stimulation parameter settings. Soon after
clinical identification of an initial therapeutic stimulation setting, unwanted side effects
(e.g. muscle contractions, dyskinesias, and parasthesias) can gradually appear over ~1-2
hours of continuous stimulation. The results presented in this study suggest the
appearance of these unwanted side effects are due in part to the decrease in the electrode
impedance during stimulation and the corresponding increase in the voltage magnitudes
generated in the neural tissue (Fig. 5.3). Such alterations likely cause the stimulation to
activate a larger volume of tissue that may include brain regions responsible for the
appearance of such side effects (Butson et al., 2006).
The utilization of current-controlled DBS should minimize voltage fluctuations
generated by impedance changes and may help reduce the amount of time required for
patient programming. Our results show that voltage distributions generated during
current-controlled stimulation are minimally affected by varying DBS electrode
impedance conditions (Fig. 5.4). Therefore, changes in therapeutic outcome observed
113
during patient programming with current-controlled DBS would not be contaminated by
stimulation induced changes at the electrode-tissue interface that may occur over time. As
an alternative, it should also be noted that one could minimize the consequences of using
voltage-controlled DBS systems by first applying DBS at the contact of interest for an
initial period (~30 minutes) to induce the major component of the decrease in the
electrode impedance (Fig. 5.3A). Clinical programming performed immediately after this
initial stimulation phase would only be subjected to relatively small changes in the
electrode impedance.
Clinical and/or electrophysiological research studies characterizing the wash-in or
wash-out effects of DBS with voltage-controlled stimulation also need to be cognizant of
the electrode impedance and voltage fluctuations that occur following device activation.
For example, recent interest has focused on studying the modulation of beta-band activity
in the STN immediately following DBS by recording local field potential activity through
the DBS electrode (e.g. Kuhn et al., 2008; Bronte-Stewart et al., 2009). Electrode
impedance fluctuations need to be considered in these types of experiments because the
stimulation induced impedance changes at the active electrode contact(s) could affect the
frequency content of the recorded neural signals through those contacts. These effects
would be time dependent because the electrode impedance would rapidly decrease during
stimulation and immediately begin to increase once stimulation is turned off. For the
DBS electrodes examined in this study, electrode impedance began to rebound
immediately after stimulation was turned off and often returned to pre-stimulation
baseline levels within 1-2 days (data not shown). Other clinical experiments in which
voltage-controlled DBS is turned off for many hours before the start of the experiment
114
and then turned on to address clinical efficacy at a given parameter setting (e.g. Lopiano
et al., 2003), should be aware that the first ~1 hour of data collection would be
contaminated by the decreasing electrode impedance, increasing voltage distribution, and
the subsequent non-stationary volume of stimulation. These impedance fluctuations are
also important to consider during animal studies examining the therapeutic mechanisms
of DBS in which stimulation is typically not chronic but only performed during the
experiment and a variety of voltage-controlled stimulation parameter settings are
examined within a short period of time (e.g. Hashimoto et al., 2003; Johnson et al.,
2009).
While we believe the results of this study to be highly relevant to the field of DBS
and neurostimulation in general, this study was subject to a number of limitations. For
example, although the results presented in this study suggest potential clinical advantages
with current-controlled stimulation, these experiments were performed in an animal
model and can only provide a hypothetical framework for future studies in human DBS
patients. Further, generalization of the results presented in this study may also be limited
because of the small number of animals used in the experiments.
Another potential limitation of this study was modifications to the voltage
waveform profiles recorded in the tissue from various extraneous factors. One of these
factors was the band-pass filtering of the recordings that produced significant distortions
in both the low and high frequency components of the recorded waveforms. In spite of
these distortions produced from filtering, the shapes of the recorded waveforms were
indicative of the system elements that were significantly contributing to the overall
electrode impedance. For voltage-controlled stimulation, experimental situations were
115
encountered in which the shape of the voltage waveform produced in the brain tissue
during stimulation was dominated by the DBS electrode impedance and other instances in
which the waveform shape was dominated by the tissue capacitance. For high electrode
impedances (>10 kΩ), the waveform shape was often dominated by the electrode
capacitance and exhibited a peak at the beginning of the cathodic pulse and an
exponential decay over the duration of the cathodic pulse (gray-dashed line at day 7 in
Fig. 5.1B and the black line at 0 min in Fig. 5.4A). For low electrode impedances (<10
kΩ), the waveform shape was often dominated by the tissue capacitance and exhibited an
exponential increase in the cathodic voltage throughout the duration of the cathodic pulse
(black line at day 1 in Fig. 5.1B and the gray-dashed line in Fig. 5.4A). For currentcontrolled stimulation, the shape of the voltage waveform generated in the brain was
independent of the DBS electrode impedance and showed an exponential increase in the
voltage due to tissue capacitance (Fig. 5.1B and Fig. 5.4A).
The shape of the voltage waveforms generated in the brain may also have been
altered by the non-ideal behavior of the Medtronic IPG used to apply voltage-controlled
stimulation in the described experiments. The IPG stimulation waveforms were not truly
voltage-controlled, but applied stimulation via an output capacitor that was charged
through charge pump circuitry. Because the IPG was not an ideal voltage source,
variations in the DBS load impedance likely produced differences in the amplitude and/or
time course of the stimulus waveform generated at the IPG output. To investigate these
potential changes, the IPG output was measured for a range of experimentally-relevant
load impedances (i.e. 1-30 kΩ). Differences in the amplitude and time course of the IPG
output were observed for this range of impedances, however, these differences were very
116
small relative to the voltage changes recorded in the tissue during the described
experiments (data not shown).
In this study, the relationship between changes in DBS electrode impedance and
the corresponding voltage distribution generated in the brain was quantified using the
Spearman’s rank-order correlation coefficient. The results of this study suggest that the
DBS electrode impedance strongly affects the corresponding voltages generated in the
brain during voltage-controlled DBS. However, it is important to keep in mind that there
are possible extraneous variables (e.g. differences between animals, individual DBS
contacts, electrode location, and the relative distance between the DBS contact and
individual microelectrode recording locations) that were not accounted for in this
analysis.
This study utilized experimental techniques to monitor the temporal evolution of
the voltage distribution generated in the brains of non-human primates during DBS. Our
results show that substantial variability in extracellularly recorded voltages can occur
during voltage-controlled DBS due to variable DBS electrode impedance conditions.
Such changes can directly affect the volume of neural tissue activated during stimulation
(Butson et al., 2006). In contrast, current-controlled DBS produced minimal changes in
the voltage distribution generated in the brain even with large decreases in electrode
impedance. Therefore, current-controlled DBS should be considered as a way to
minimize variability in the spread of stimulation for a given set of stimulation parameter
settings. In turn, adoption of current-controlled DBS should provide three advantages
over voltage-controlled DBS: 1) enable more consistent comparison of parameter settings
within and across patients, 2) reduce confounding variables when researching the time-
117
dependent behavioral and/or electrophysiological effects related to the onset of DBS, and
3) provide a more consistent stimulation effect during the initial clinical programming
process.
118
6
CHAPTER 6: DISCUSSION AND CONCLUSION
6.1
Contributions of the research
The results of this project have generated a more detailed description of the
electrode-tissue interface (ETI) during chronic recording and stimulation in the central
nervous system (CNS). The results will help lead to improved neural stimulation and
recording systems for chronic use in human patients.
6.1.1
Neural recording – intracortical microelectrodes
In terms of neural recordings, the specific results of this project considered the
effects of contact size on recording quality during intracortical microelectrode recordings.
The results show a slight trend in an improved recording quality (i.e. signal-to-noise ratio
(SNR)) for small surface area recording sites. The small contact sizes actually had
increased recording noise due to higher electrode impedance that produced both
increased thermal and biological noise levels. Increased biological noise for small surface
area recording sites is in opposition to the standard doctrine in the field of neural
recording. Typically, it is believed that large recording sites experience more neural
“hash” because these large recording sites are able to detect currents from an increased
number of neurons. However, the lower impedance of large surface area recording sites
actually resulted in lower noise levels because a given amount of current produced a
lower voltage at the recording site. This voltage decrease meant an improvement in
recording noise, but it also corresponded to lower recording amplitudes of extracellular
action potentials. Therefore, small surface area recording sites produced an increased
119
SNR even with increased recording noise because of the large action potential recording
amplitude.
Additional variables affecting the recording SNR were also considered with the
described modeling infrastructure. For example, the standard band-pass filtering of neural
spike recordings significantly distorted the perceived shape and amplitude of the action
potential (compare Fig. 3.2C to Fig. 3.5A-C). The filter-induced distortions in the action
potential recordings were highly dependent on the selected recording bandwidth. The
recording bandwidth also had a significant effect on both thermal and biological noise
(i.e. a wide bandwidth resulted in increased thermal and biological noise). The estimated
biological noise was also dependent on the neuron density and firing behavior of the
neurons immediately surrounding the recording microelectrode (i.e. high neuron density
and high neural firing rates produced increased biological noise).
In general, the model infrastructure developed in this study provided a way to
analyze numerous factors influencing the quality of intracortical microelectrode
recordings that would be very difficult, if not impossible, to analyze experimentally.
6.1.2
Neurostimulation - deep brain stimulation
This study produced a detailed description of changes at the ETI of chronically-
implanted deep brain stimulation (DBS) electrodes. The project investigated impedance
changes that occurred after implantation and during prolonged stimulation. The project
involved electrode impedance spectroscopy (EIS) measurements, augmenting, standard 1
kHz impedance measurements. EIS measurements provided a detailed description of the
frequency-dependent characteristics of the ETI and allowed the different components of
120
the ETI (i.e. electrode and tissue components) to be studied through model analysis. The
rapid and non-invasive nature of EIS measurements allowed single electrode contacts to
be examined at multiple time points throughout the experiment. The ability to
characterize the ETI of an individual contact at multiple time points (e.g. days after
electrode implantation, total stimulation time) provided a major advantage over
histological techniques in which animals would need to be sacrificed and/or leads
explanted at each time point.
This project also characterized the significance of ETI composition and the
corresponding electrode impedance on the voltage distributions generated in the brain
during DBS. Typical clinical DBS systems apply voltage-controlled stimulation in which
the resulting voltages generated in the target neural tissue are a function of the electrode
impedance. This study produced direct measurements of the voltage distributions
generated in the brain under variable impedance conditions (e.g. days after implantation,
stimulation time). It is commonly assumed that DBS electrode impedance is correlated
with the corresponding voltage distributions generated in the brain during voltagecontrolled DBS; however, this correlation has never been directly investigated or
quantified. The results of this study show DBS electrode impedance can significantly
affect the voltages generated in the target neural tissue. This study also shows that this
dependence on electrode impedance can be minimized with current-controlled
stimulation.
121
6.2
6.2.1
Implications of the research and future directions
Neural recording – intracortical microelectrodes
The theoretical framework established in this study will help provide a method for
optimizing the design of cortical microelectrodes and recording techniques to help
advance the state of the art for brain-machine interface (BMI) technology. Improved BMI
systems are necessary if this technology is to achieve the long-term performance required
for human applications.
Although the specifics of this research project only considered the effects of
contact size during single-unit recording, the modeling framework designed in this study
would allow for optimization of any arbitrary microelectrode design. This analysis can
also be easily adapted to analyze the effect of electrode design on the recording of local
field potentials (LFP). Microelectrode contact size would likely have a more pronounced
effect on the SNR during LFP recordings because of the pass band at low frequencies
(e.g. 10-100 Hz) in the region where microelectrode impedance dominates the overall
impedance of the ETI. This modeling infrastructure would also provide a means to test
many experimental techniques used in analyzing or processing neural recordings so that
these recordings can provide a neural control signal for BMI technology. For example,
this modeling infrastructure would serve as an excellent platform for testing the accuracy
of various spike sorting methods and help improve their implementation.
This research generated an improved theoretical understanding of the factors that
affect chronic microelectrode recordings. The results show the effect of recording contact
size and the corresponding electrode impedance on the action potential recording
122
amplitude and recording noise. The modeling infrastructure also provided a more detailed
understanding of thermal and biological noise sources and the effects of signal filtering,
neuron density, and neural firing rates. It would be extremely difficult to study/isolate
these variables in an in vivo environment.
6.2.2
Neurostimulation – deep brain stimulation
The results of this study suggest possible alterations in DBS therapy that could
improve the patient experience. For example, this study suggests current-controlled DBS
would help improve the patient programming experience by minimizing the
consequences of the impedance fluctuations at the ETI. Current-controlled DBS would
likely increase the stability in the therapeutic outcome of a given set of stimulation
parameters and allow the parameters to be selected soon after electrode implantation.
Alternatively, during voltage-controlled DBS, patient programming could be modified by
first applying DBS at the contact of interest for an initial period (~30 minutes) to induce
the major component of the decrease in electrode impedance observed experimentally.
Clinical programming performed immediately after this stimulation phase would then
only be subjected to relatively small changes in the electrode impedance and the
corresponding voltage distributions generated in the brain.
Although the impedance and voltage measurements performed in this study
suggest clinical relevance, this study was performed in an animal model of DBS and only
suggests a hypothetical framework for clinical investigation. Therefore, the main future
direction of this research will be to perform relevant analyses in human DBS patients.
One possible experiment would be EIS measurements on DBS leads implanted in human
123
patients at different periods of time after lead implantation. These measurements could be
performed soon after implantation while the lead wires are still externalized and after
longer implantation times when the patient must undergo surgery to replace the IPG
battery. EIS measurements could also be performed during stimulation to see if the ETI
exhibits impedance changes similar to the changes observed in the scaled-down DBS
leads considered in the present study. In human DBS patients, it is not possible to
perform the detailed voltage recordings described in chapter 5, but if the human DBS
leads show similar impedance changes, this would suggest there are significant voltage
fluctuations in the brain during voltage-controlled DBS.
Additional clinical studies could also investigate the potential advantages of
current-controlled DBS. This analysis would involve comparing the patient programming
times required during voltage-controlled and current-controlled DBS. Theoretically,
patient programming performed with current-controlled stimulation would lead to
increased stability in the therapeutic efficacy of a given set of stimulation parameters.
This increased stability would decrease the occurrence of stimulation-induced side effects
and lead to a decrease in the total time required for patient programming. It may also be
possible to investigate the potential use of current-controlled DBS for defining
therapeutic stimulation parameters within a shorter delay period after lead implantation
(i.e. days instead of weeks).
124
6.3
6.3.1
Limitations
Neural recording – intracortical microelectrodes
Although this study showed small surface area recording sites provide a higher
recording SNR relative to large contact sizes, these differences were small and it is
difficult to determine if they were significant. Another limitation of this study was the
lack of direct validation through in vivo experiments. However, this inability to directly
validate the model results with in vivo experiments was the main driving force behind this
theoretical approach. It would be very difficult to perform this type of design
optimization in an experimental environment because of the inability to control potential
confounding factors. This study was also subject to a number of other limitations that is
discussed in detailed in chapter 3 (see section ‘3.4.4’).
In spite of the number of limitations of the model analysis performed in this
study, the modeling infrastructure provides a necessary step for the “smart” design of
recording electrodes and the details included in this model represent a major
advancement in model sophistication. Future iterations of this recording model will only
provide improvements in the accuracy of this theoretical analysis. This model will
provide valuable insight to other researchers in the area of neural recording and help
guide future model development, experimental design and analysis, and technological
developments in BMI and other neural recording applications.
125
6.3.2
Neurostimulation – deep brain stimulation
Although the results of this study suggest significant changes occur at the ETI of
chronically-implanted DBS electrodes and these impedance changes significantly affect
the voltage distributions generated in the brain during DBS, these experiments were
performed in an animal model of DBS with scaled-down DBS leads. Because these
experiments were performed in an animal model and with a small number of animals, the
results of this study only provide a theoretical framework for clinical analysis in human
DBS patients. This study was also subject to a number of other limitations that are
described in detail in chapters 4 and 5 (see sections ‘4.4.2’, ‘4.4.3’, and ‘5.4’).
The current study was performed in an animal model because it was not possible
to perform these detailed experiments in human patients. Hopefully, this limitation will
largely be overcome by performing the type of clinical studies described above (see
section ‘6.2.2’) and will allow the results from the animal study to be generalized to
human DBS patients.
126
6.4
Final conclusion
This study included a detailed investigation into the significance of the ETI during
chronic recording and stimulation applications in the CNS. The ETI consists of a number
of elements that create a complex environment around the implanted electrode. The
details of this complex environment are often oversimplified or neglected. However,
consideration of these details is necessary to understand the confounding factors that can
limit the success of recording and stimulation applications in the CNS so that these
therapies can be improved. This study provides a significant step towards improving the
technologies and therapies for BMI and DBS applications and the results of the study can
also be applied to the general fields of neural recording and stimulation.
127
REFERENCES
Ackermans L, Temel Y and Visser-Vandewalle V 2008 Deep brain stimulation in
Tourette's Syndrome Neurotherapeutics 5 339-44
Anderson J M, Rodriguez A and Chang D T 2008 Foreign body reaction to biomaterials
Seminars in immunology 20 86-100
Barsoukov E and Macdonald J R 2005 Impedance spectroscopy: theory, experiment, and
applications John Wiley & Sons, Inc.: Hoboken
Bedard C, Kroger H and Destexhe A 2004 Modeling extracellular field potentials and the
frequency-filtering properties of extracellular space Biophysical journal 86 182942
Biran R, Martin D C and Tresco P A 2005 Neuronal cell loss accompanies the brain
tissue response to chronically implanted silicon microelectrode arrays
Experimental neurology 195 115-26
Bronte-Stewart H, Barberini C, Koop M M, Hill B C, Henderson J M and Wingeier B
2009 The STN beta-band profile in Parkinson's disease is stationary and shows
prolonged attenuation after deep brain stimulation Experimental neurology 215
20-8
Buitenweg J R, Rutten W L C, Willems W P A and van Nieuwkasteele J W 1998
Measurement of sealing resistance of cell-electrode interfaces in neuronal cultures
using impedance spectroscopy Medical & Biological Engineering & Computing
36 630-7
Butson C R, Maks C B and McIntyre C C 2006 Sources and effects of electrode
impedance during deep brain stimulation Clin Neurophysiol 117 447-54
Cole K S 1940 Permeability and impermeability of cell membranes for ions Spring
Harbor Symp Quant Biol 8 110-22
Contu F, Elsener B and Bohni H 2002 Characterization of implant materials in fetal
bovine serum and sodium sulfate by electrochemical impedance spectroscopy. I.
Mechanically polished samples Journal of Biomedical Materials Research 62
412-21
Cragg B G 1967 The density of synapses and neurones in the motor and visual areas of
the cerebral cortex Journal of anatomy 101 639-54
Deuschl G, Herzog J, Kleiner-Fisman G, Kubu C, Lozano A M, Lyons K E, RodriguezOroz M C, Tamma F, Troster A I, Vitek J L, Volkmann J and Voon V 2006 Deep
brain stimulation: postoperative issues Mov Disord 21 Suppl 14 S219-37
Dymond A M 1976 Characteristics of the metal-tissue interface of stimulation electrodes
IEEE transactions on bio-medical engineering 23 274-80
Elder C M, Hashimoto T, Zhang J Y and Vitek J L 2005 Chronic implantation of deep
brain stimulation leads in animal models of neurological disorders Journal of
Neuroscience Methods 142 11-6
Evarts E V 1964 Temporal Patterns of Discharge of Pyramidal Tract Neurons During
Sleep and Waking in the Monkey Journal of neurophysiology 27 152-71
Fetz E E 1969 Operant conditioning of cortical unit activity Science (New York, N.Y 163
955-8
128
Foster K R and Schwan H P 1996 Dielectric Properties of Tissues In Handbook of
Biological Effects of Electromagnetic Fields Polk C and Postow E eds CRC Press:
Boca Raton pp 25-102
Frampton J P, Hynd M R, Williams J C, Shuler M L and Shain W 2007 Threedimensional hydrogel cultures for modeling changes in tissue impedance around
microfabricated neural probes Journal of neural engineering 4 399-409
Friehs G M, Zerris V A, Ojakangas C L, Fellows M R and Donoghue J P 2004 Brainmachine and brain-computer interfaces Stroke; a journal of cerebral circulation
35 2702-5
Gabriels L, Cosyns P, Nuttin B, Demeulemeester H and Gybels J 2003 Deep brain
stimulation
for
treatment-refractory
obsessive-compulsive
disorder:
psychopathological and neuropsychological outcome in three cases Acta
psychiatrica Scandinavica 107 275-82
Geddes L A 1997 Historical evolution of circuit models for the electrode-electrolyte
interface Annals of biomedical engineering 25 1-14
Gimsa J, Habel B, Schreiber U, Rienen U, Strauss U and Gimsa U 2005 Choosing
electrodes for deep brain stimulation experiments-electrochemical considerations
Journal of neuroscience methods 142 251-65
Glees P 1955 Neuroglia, morphology and function Thomas: Springfield, Ill.,
Gold C, Henze D A, Koch C and Buzsaki G 2006 On the origin of the extracellular action
potential waveform: A modeling study Journal of neurophysiology 95 3113-28
Goldberg J A, Boraud T, Maraton S, Haber S N, Vaadia E and Bergman H 2002
Enhanced synchrony among primary motor cortex neurons in the 1-methyl-4phenyl-1,2,3,6-tetrahydropyridine primate model of Parkinson's disease J
Neurosci 22 4639-53
Grill W M and Mortimer J T 1994 Electrical-Properties of Implant Encapsulation Tissue
Annals of Biomedical Engineering 22 23-33
Gulie S 2007 A Shock to the System Wired 15
Haberler C, Alesch F, Mazal P R, Pilz P, Jellinger K, Pinter M M, Hainfellner J A and
Budka H 2000 No tissue damage by chronic deep brain stimulation in Parkinson's
disease Annals of Neurology 48 372-6
Hashimoto T, Elder C M, Okun M S, Patrick S K and Vitek J L 2003 Stimulation of the
subthalamic nucleus changes the firing pattern of pallidal neurons J Neurosci 23
1916-23
Haueisen J, Tuch D S, Ramon C, Schimpf P H, Wedeen V J, George J S and Belliveau J
W 2002 The influence of brain tissue anisotropy on human EEG and MEG
NeuroImage 15 159-66
Hemm S, Vayssiere N, Mennessier G, Cif L, Zanca M, Ravel P, Frerebeau P and Coubes
P 2004 Evolution of brain impedance in dystonic patients treated by GPi electrical
stimulation Neuromodulation 7 67-75
Henze D A, Borhegyi Z, Csicsvari J, Mamiya A, Harris K D and Buzsaki G 2000
Intracellular features predicted by extracellular recordings in the hippocampus in
vivo Journal of neurophysiology 84 390-400
Hines M L and Carnevale N T 1997 The NEURON simulation environment Neural
computation 9 1179-209
129
Hochberg L R, Serruya M D, Friehs G M, Mukand J A, Saleh M, Caplan A H, Branner
A, Chen D, Penn R D and Donoghue J P 2006 Neuronal ensemble control of
prosthetic devices by a human with tetraplegia Nature 442 164-71
Hodaie M, Wennberg R A, Dostrovsky J O and Lozano A M 2002 Chronic anterior
thalamus stimulation for intractable epilepsy Epilepsia 43 603-8
Holt G R and Koch C 1999 Electrical interactions via the extracellular potential near cell
bodies Journal of computational neuroscience 6 169-84
Johnson M D, Otto K J and Kipke D R 2005 Repeated voltage biasing improves unit
recordings by reducing resistive tissue impedances Ieee Transactions on Neural
Systems and Rehabilitation Engineering 13 160-5
Johnson M D, Vitek J L and McIntyre C C 2009 Pallidal stimulation that improves
parkinsonian motor symptoms also modulates neuronal firing patterns in primary
motor cortex in the MPTP-treated monkey Experimental neurology
Jones K E, Campbell P K and Normann R A 1992 A glass/silicon composite intracortical
electrode array Annals of biomedical engineering 20 423-37
Keese C R, Wegener J, Walker S R and Giaever I 2004 Electrical wound-healing assay
for cells in vitro Proceedings of the National Academy of Sciences of the United
States of America 101 1554-9
Kennedy P, Andreasen D, Ehirim P, King B, Kirby T, Mao H and Moore M 2004 Using
human extra-cortical local field potentials to control a switch Journal of neural
engineering 1 72-7
Kennedy P R and Bakay R A 1998 Restoration of neural output from a paralyzed patient
by a direct brain connection Neuroreport 9 1707-11
Kim Y T, Hitchcock R W, Bridge M J and Tresco P A 2004 Chronic response of adult rat
brain tissue to implants anchored to the skull Biomaterials 25 2229-37
Kissinger P T and Heineman W R 1996 Laboratory techniques in electroanalytical
chemistry Marcel Dekker: New York
Kuhn A A, Kempf F, Brucke C, Gaynor Doyle L, Martinez-Torres I, Pogosyan A,
Trottenberg T, Kupsch A, Schneider G H, Hariz M I, Vandenberghe W, Nuttin B
and Brown P 2008 High-frequency stimulation of the subthalamic nucleus
suppresses oscillatory beta activity in patients with Parkinson's disease in parallel
with improvement in motor performance J Neurosci 28 6165-73
Lapicque 1907 Rescherches quatitatives sur l'exitation electrique des nerf traitee comme
une polarisation J Physiol Pathol Gen 9 620-35
Lauer R T, Peckham P H and Kilgore K L 1999 EEG-based control of a hand grasp
neuroprosthesis Neuroreport 10 1767-71
Lempka S F, Johnson M D, Barnett D W, Moffitt M A, Otto K J, Kipke D R and
McIntyre C C 2006 Optimization of microelectrode design for cortical recording
based on thermal noise considerations Conf Proc IEEE Eng Med Biol Soc 1 33614
Lempka S F, Johnson M D, Miocinovic S, Vitek J L and McIntyre C C 2008 Timedependent effects of electrode impedance on the voltage distribution generated
during voltage-controlled or current-controlled deep brain stimulation Society for
Neuroscience 641.1
130
Lempka S F, Miocinovic S, Johnson M D, Vitek J L and McIntyre C C 2009 In vivo
impedance spectroscopy of deep brain stimulation electrodes Journal of neural
engineering 6 46001
Lempka S F, Miocinovic S, Russo G S, Zhang J, Vitek J L and McIntyre C C 2007 In
vivo deep brain stimulation electrode impedance spectroscopy Society for
Neuroscience 693.8
Lewicki M S 1998 A review of methods for spike sorting: the detection and classification
of neural action potentials Network (Bristol, England) 9 R53-78
Limousin P and Martinez-Torres I 2008 Deep brain stimulation for Parkinson's disease
Neurotherapeutics 5 309-19
Ling E 1981 The origin nature of microglia In Advances in cellular neurobiology
Federoff S ed Academic Press: Orlando pp 33-82
Liu X, McCreery D B, Carter R R, Bullara L A, Yuen T G and Agnew W F 1999
Stability of the interface between neural tissue and chronically implanted
intracortical microelectrodes IEEE Trans Rehabil Eng 7 315-26
Logothetis N K, Kayser C and Oeltermann A 2007 In vivo measurement of cortical
impedance spectrum in monkeys: implications for signal propagation Neuron 55
809-23
Lopiano L, Torre E, Benedetti F, Bergamasco B, Perozzo P, Pollo A, Rizzone M, Tavella
A and Lanotte M 2003 Temporal changes in movement time during the switch of
the stimulators in Parkinson's disease patients treated by subthalamic nucleus
stimulation European neurology 50 94-9
Ludwig K A, Uram J D, Yang J, Martin D C and Kipke D R 2006 Chronic neural
recordings using silicon microelectrode arrays electrochemically deposited with a
poly(3,4-ethylenedioxythiophene) (PEDOT) film Journal of neural engineering 3
59-70
Lujan J L, Chaturvedi A and McIntyre C C 2008 Tracking the mechanisms of deep brain
stimulation for neuropsychiatric disorders Front Biosci 13 5892-904
Lyons K E and Pahwa R 2008 Deep brain stimulation and tremor Neurotherapeutics 5
331-8
Macdonald J R 1992 Impedance spectroscopy Annals of biomedical engineering 20 289305
Mainen Z F, Joerges J, Huguenard J R and Sejnowski T J 1995 A model of spike
initiation in neocortical pyramidal neurons Neuron 15 1427-39
Mainen Z F and Sejnowski T J 1996 Influence of dendritic structure on firing pattern in
model neocortical neurons Nature 382 363-6
Mayberg H S, Lozano A M, Voon V, McNeely H E, Seminowicz D, Hamani C, Schwalb
J M and Kennedy S H 2005 Deep brain stimulation for treatment-resistant
depression Neuron 45 651-60
McAdams E T 1989a Effect of surface topography on the electrode-electrolyte interface
impedance, Part 1. The high frequency, small signal interface impedance. Surface
Topography 2 107-22
McAdams E T 1989b Effect of surface topography on the electrode-electrolyte interface
impedance, Part 2. The low frequency (f < 1 Hz), small signal interface
impedance. Surface Topography 2 223-32
131
McAdams E T 1990 Effect of surface topography on the electrode-electrolyte interface
impedance, Part 3. The fractal approach. Surface Topography 3 3-24
McAdams E T and Jossinet J 1995 Tissue Impedance - a Historical Overview
Physiological Measurement 16 A1-A13
McAdams E T, Lackermeier A, McLaughlin J A, Macken D and Jossinet J 1995 The
Linear and Nonlinear Electrical-Properties of the Electrode-Electrolyte Interface
Biosensors & Bioelectronics 10 67-74
McConnell G C, Butera R J and Bellamkonda R V 2009 Bioimpedance modeling to
monitor astrocytic response to chronically implanted electrodes Journal of neural
engineering 6 55005
Merrill D R and Tresco P A 2005 Impedance characterization of microarray recording
electrodes in vitro IEEE transactions on bio-medical engineering 52 1960-5
Meyer G 1987 Forms and spatial arrangement of neurons in the primary motor cortex of
man The Journal of comparative neurology 262 402-28
Miocinovic S, Lempka S F, Russo G S, Maks C B, Butson C R, Sakaie K E, Vitek J L
and McIntyre C C 2008 Experimental and theoretical characterization of the
voltage distribution generated by deep brain stimulation Experimental neurology
Miocinovic S, Lempka S F, Russo G S, Maks C B, Butson C R, Sakaie K E, Vitek J L
and McIntyre C C 2009 Experimental and theoretical characterization of the
voltage distribution generated by deep brain stimulation Experimental neurology
216 166-76
Miocinovic S, Parent M, Parent A, Butson C R, Russo G S, Vitek J L and McIntyre C C
2007a Activation of the subthalamic nucleus and lenticular fasciculus during
therapeutic deep brain stimulation Stereotactic and Functional Neurosurgery 85
32Miocinovic S, Zhang J Y, Xu W D, Russo G S, Vitek J L and McIntyre C C 2007b
Stereotactic neurosurgical planning, recording, and visualization for deep brain
stimulation in non-human primates Journal of Neuroscience Methods 162 32-41
Moffitt M A and McIntyre C C 2005 Model-based analysis of cortical recording with
silicon microelectrodes Clin Neurophysiol 116 2240-50
Moss J, Ryder T, Aziz T Z, Graeber M B and Bain P G 2004 Electron microscopy of
tissue adherent to explanted electrodes in dystonia and Parkinson's disease Brain
127 2755-63
Moulin C, Gliere A, Barbier D, Joucla S, Yvert B, Mailley P and Guillemaud R 2008 A
new 3-D finite-element model based on thin-film approximation for
microelectrode array recording of extracellular action potential IEEE transactions
on bio-medical engineering 55 683-92
Najafi K 1994 Solid-State Microsensors for Cortical Nerve Recordings Ieee Engineering
in Medicine and Biology Magazine 13 375-87
Nathaniel E J H and Nathaniel D R 1981 The reactive astrocyte In Advances in cellular
neurobiology Federoff S ed Academic Press: Orlando pp 249-301
Nielsen M S, Bjarkam C R, Sorensen J C, Bojsen-Moller M, Sunde N A and Ostergaard
K 2007 Chronic subthalamic high-frequency deep brain stimulation in Parkinson's
disease--a histopathological study Eur J Neurol 14 132-8
Nordhausen C T, Maynard E M and Normann R A 1996 Single unit recording
capabilities of a 100 microelectrode array Brain Res 726 129-40
132
Ostrem J L and Starr P A 2008 Treatment of dystonia with deep brain stimulation
Neurotherapeutics 5 320-30
Otto K J, Johnson M D and Kipke D R 2006 Voltage pulses change neural interface
properties and improve unit recordings with chronically implanted
microelectrodes IEEE transactions on bio-medical engineering 53 333-40
Pajkossy T 1994 Impedance of Rough Capacitive Electrodes Journal of Electroanalytical
Chemistry 364 111-25
Papoulis A 1984 Probability, random variables, and stochastic processes McGraw-Hill:
New York
Perlmutter J S and Mink J W 2006 Deep brain stimulation Annual review of neuroscience
29 229-57
Polikov V S, Tresco P A and Reichert W M 2005 Response of brain tissue to chronically
implanted neural electrodes Journal of neuroscience methods 148 1-18
Rall W 1962 Electrophysiology of a dendritic neuron model Biophysical journal 2 14567
Schwan H P 1968 Electrode polarization impedance and measurements in biological
materials Annals of the New York Academy of Sciences 148 191-209
Schwartz A B 2004 Cortical neural prosthetics Annual review of neuroscience 27 487507
Serruya M D, Hatsopoulos N G, Paninski L, Fellows M R and Donoghue J P 2002 Instant
neural control of a movement signal Nature 416 141-2
Sloper J J, Hiorns R W and Powell T P S 1979 Qualitative and Quantitative ElectronMicroscopic Study of the Neurons in the Primate Motor and Somatic Sensory
Cortices Philosophical Transactions of the Royal Society of London Series BBiological Sciences 285 141-&
Stensaas S S and Stensaas L J 1976 The reaction of the cerebral cortex to chronically
implanted plastic needles Acta neuropathologica 35 187-203
Stroncek J D and Reichert W M 2008 Overview of wound healing in different tissue
types In Indwelling neural implants: strategies for contending with the in vivo
environment Reichert W M ed CRC Press: Boca Raton pp 3-38
Suner S, Fellows M R, Vargas-Irwin C, Nakata G K and Donoghue J P 2005 Reliability
of signals from a chronically implanted, silicon-based electrode array in nonhuman primate primary motor cortex IEEE Trans Neural Syst Rehabil Eng 13
524-41
Szarowski D H, Andersen M D, Retterer S, Spence A J, Isaacson M, Craighead H G,
Turner J N and Shain W 2003 Brain responses to micro-machined silicon devices
Brain research 983 23-35
Taylor D M, Tillery S I and Schwartz A B 2002 Direct cortical control of 3D
neuroprosthetic devices Science (New York, N.Y 296 1829-32
Taylor D M, Tillery S I and Schwartz A B 2003 Information conveyed through braincontrol: cursor versus robot IEEE Trans Neural Syst Rehabil Eng 11 195-9
Turner J N, Shain W, Szarowski D H, Andersen M, Martins S, Isaacson M and Craighead
H 1999 Cerebral astrocyte response to micromachined silicon implants
Experimental neurology 156 33-49
Unterberg A W, Stover J, Kress B and Kiening K L 2004 Edema and brain trauma
Neuroscience 129 1021-9
133
Vaughan T M, McFarland D J, Schalk G, Sarnacki W A, Krusienski D J, Sellers E W and
Wolpaw J R 2006 The Wadsworth BCI Research and Development Program: at
home with BCI IEEE Trans Neural Syst Rehabil Eng 14 229-33
Vetter R J, Williams J C, Hetke J F, Nunamaker E A and Kipke D R 2004 Chronic neural
recording using silicon-substrate microelectrode arrays implanted in cerebral
cortex IEEE transactions on bio-medical engineering 51 896-904
Volkmann J, Herzog J, Kopper F and Deuschl G 2002 Introduction to the programming
of deep brain stimulators Movement Disorders 17 S181-S7
Ward M P, Rajdev P, Ellison C and Irazoqui P P 2009 Toward a comparison of
microelectrodes for acute and chronic recordings Brain Res 1282 183-200
Wei X F and Grill W M 2005 Current density distributions, field distributions and
impedance analysis of segmented deep brain stimulation electrodes Journal of
neural engineering 2 139-47
Wessberg J, Stambaugh C R, Kralik J D, Beck P D, Laubach M, Chapin J K, Kim J,
Biggs S J, Srinivasan M A and Nicolelis M A 2000 Real-time prediction of hand
trajectory by ensembles of cortical neurons in primates Nature 408 361-5
Williams J C, Hippensteel J A, Dilgen J, Shain W and Kipke D R 2007 Complex
impedance spectroscopy for monitoring tissue responses to inserted neural
implants Journal of neural engineering 4 410-23
Williams J C, Rennaker R L and Kipke D R 1999 Long-term neural recording
characteristics of wire microelectrode arrays implanted in cerebral cortex Brain
Res Brain Res Protoc 4 303-13
Wilson J A, Felton E A, Garell P C, Schalk G and Williams J C 2006 ECoG factors
underlying multimodal control of a brain-computer interface IEEE Trans Neural
Syst Rehabil Eng 14 246-50
Wise K D, Anderson D J, Hetke J F, Kipke D R and Najafi K 2004 Wireless implantable
microsystems: High-density electronic interfaces to the nervous system
Proceedings of the Ieee 92 76-97
Wolpaw J R, Birbaumer N, McFarland D J, Pfurtscheller G and Vaughan T M 2002
Brain-computer interfaces for communication and control Clin Neurophysiol 113
767-91
Wolpaw J R and McFarland D J 2004 Control of a two-dimensional movement signal by
a noninvasive brain-computer interface in humans Proceedings of the National
Academy of Sciences of the United States of America 101 17849-54
Xu J, Shepherd R K, Millard R E and Clark G M 1997 Chronic electrical stimulation of
the auditory nerve at high stimulus rates: A physiological and histopathological
study Hearing Research 105 1-29
Yousif N, Bayford R, Wang S and Liu X 2008 Quantifying the effects of the electrodebrain interface on the crossing electric currents in deep brain recording and
stimulation Neuroscience 152 683-91
Zhang J, Riehle A, Requin J and Kornblum S 1997 Dynamics of single neuron activity in
monkey primary motor cortex related to sensorimotor transformation J Neurosci
17 2227-46
134