* Your assessment is very important for improving the workof artificial intelligence, which forms the content of this project
Download IN VITRO TRANSCRIPTION . TRANSLATION - UTH e
Non-coding DNA wikipedia , lookup
Bottromycin wikipedia , lookup
Gene regulatory network wikipedia , lookup
Ancestral sequence reconstruction wikipedia , lookup
Nucleic acid analogue wikipedia , lookup
Promoter (genetics) wikipedia , lookup
Protein moonlighting wikipedia , lookup
List of types of proteins wikipedia , lookup
Genetic code wikipedia , lookup
Western blot wikipedia , lookup
Protein adsorption wikipedia , lookup
Homology modeling wikipedia , lookup
Intrinsically disordered proteins wikipedia , lookup
RNA polymerase II holoenzyme wikipedia , lookup
Protein–protein interaction wikipedia , lookup
Polyadenylation wikipedia , lookup
Eukaryotic transcription wikipedia , lookup
Artificial gene synthesis wikipedia , lookup
Proteolysis wikipedia , lookup
Deoxyribozyme wikipedia , lookup
Non-coding RNA wikipedia , lookup
Silencer (genetics) wikipedia , lookup
Transcriptional regulation wikipedia , lookup
Messenger RNA wikipedia , lookup
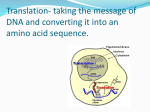
In Vitro Translation: The Basics The in vitro synthesis of proteins in cell-free extracts is an important tool for molecular biologists and has a variety of applications, including the rapid identification of gene products (e.g., proteomics), localization of mutations through synthesis of truncated gene products, protein folding studies, and incorporation of modified or unnatural amino acids for functional studies. In principle, it should be possible to prepare a cell-free extract for in vitro translation of mRNAs from any type of cells. In practice, only a few cell-free systems have been developed for in vitro protein synthesis. In general, these systems are derived from cells engaged in a high rate of protein synthesis. Cell-Free Expression Systems The most frequently used cell-free translation systems consist of extracts from rabbit reticulocytes and Escherichia coli. All are prepared as crude extracts containing all the macromolecular components (70S or 80S ribosomes, tRNAs, aminoacyl-tRNA synthetases, initiation, elongation and termination factors, etc.) required for translation of exogenous RNA. To ensure efficient translation, each extract must be supplemented with amino acids, energy sources (ATP, GTP), energy regenerating systems (creatine phosphate and creatine phosphokinase for eukaryotic systems, and phosphoenol pyruvate and pyruvate kinase for the E. coli lysate), and other co-factors (Mg2+, K+, etc.). There are two approaches to in vitro protein synthesis based on the starting genetic material: RNA or DNA. Standard translation systems, such as reticulocyte lysates and wheat germ extracts, use RNA as a template; whereas "coupled" and "linked" systems start with DNA templates, which are transcribed into RNA then translated. Rabbit Reticulocyte Lysate Rabbit reticulocyte lysate is a highly efficient in vitro eukaryotic protein synthesis system used for translation of exogenous RNAs (either natural or generated in vitro). In vivo, reticulocytes are highly specialized cells primarily responsible for the synthesis of hemoglobin, which represents more than 90% of the protein made in the reticulocyte. These immature red cells have already lost their nuclei, but contain adequate mRNA, as well as complete translation machinery, for extensive globin synthesis. The endogenous globin mRNA can be eliminated by incubation with Ca2+dependent micrococcal nuclease, which is later inactivated by chelation of the Ca2+ by EGTA. The Kit offers a nuclease-treated reticulocyte lysate. This type of lysate is the most widely used RNA-dependent cell-free system because of its low background and its efficient utilization of exogenous RNAs even at low concentrations. Exogenous proteins are synthesized at a rate close to that observed in intact reticulocyte cells. Standard in Vitro Translation Procedure Using Rabbit Reticulocyte Lysate Untreated reticulocyte lysate translates endogenous globin mRNA, exogenous RNAs, or both. This type of lysate is typically used for studying the translation machinery, e.g. studying the effects of inhibitors on globin translation. Both the untreated and treated rabbit reticulocyte lysates have low nuclease activity and are capable of synthesizing a large amount of full-length product. Both lysates are appropriate for the synthesis of larger proteins from either capped or uncapped RNAs (eukaryotic or viral). E. coli Cell-Free System E. coli cell-free systems consist of a crude extract that is rich in endogenous mRNA. The extract is incubated during preparation so that this endogenous mRNA is translated and subsequently degraded. Because the levels of endogenous mRNA in the prepared lysate is low, the exogenous product is easily identified. In comparison to eukaryotic systems, the E.coli extract has a relatively simple translational apparatus with less complicated control at the initiation level, allowing this system to be very efficient in protein synthesis. Bacterial extracts are often unsuitable for translation of RNA, because exogenous RNA is rapidly degraded by endogenous nucleases. There are some viral mRNAs that translate efficiently, because they are somewhat resistant to nuclease activity and contain stable secondary structure. However, E.coli extracts are ideal for coupled transcription:translation from DNA templates. "Linked" And "Coupled" Transcription:Translation Systems In standard translation reactions, purified RNA is used as a template for translation. "Linked" and "coupled" systems, on the other hand, use DNA as a template. RNA is transcribed from the DNA and subsequently translated without any purification. Such systems typically combine a prokaryotic phage RNA polymerase and promoter (T7, T3, or SP6) with eukaryotic or prokaryotic extracts to synthesize proteins from exogenous DNA templates. DNA templates for transcription:translation reactions may be cloned into plasmid vectors or generated by PCR Primer Sequences for PCR-generated Translation Templates DNA templates for translation using "coupled" or "linked" transcription:translation systems can be easily generated by PCR. Below are the upstream (5')primer sequences to produce PCR products for T7-driven transcription and subsequent translation in a retic lysate and E.coli extract, respectively. Note, translation systems that use T3 or SP6 polymerases are also available . To generate PCR templates for other polymerases, simply change the T7 Promoter Sequence Phage Polymerase Promoters: Minimal Sequence Requirements The bacteriophage promoters, T7, T3, and SP6, consist of 23 basepairs numbered -17 to +6, where +1 indicates the first base of the coded transcript. An important observation is that, of the +1 through +6 bases, only the base composition of +1 and +2 are critical and must be a G and purine, respectively, to yield an efficient transcription template. In addition, synthetic oligonucleotide templates only need to be double-stranded in the -17 to -1 region of the promoter, and the coding region can be all single-stranded. This can reduce the cost of synthetic templates, since the coding region (i.e., from +1 on) can be left single-stranded, and the short oligonucleotides required to render the promoter region double-stranded can be used with multiple templates. Consensus Promoter Sequences. The +1 base (in bold) is the first base incorporated into RNA during transcription. The underline indicates the minimum sequence required for efficient transcription. Linked Transcription:Translation The "linked" system is a two-step reaction, based on transcription with a bacteriophage polymerase followed by translation in the rabbit reticulocyte lysate or wheat germ lysate. Because the transcription and translation reactions are separate, each can be optimized to ensure that both are functioning at their full potential. Conversely, many commercially available eukaryotic coupled transcription:translation systems have compromised one or both reactions so that they can occur in a single tube. Thus, yield is sacrificed for convenience. Linked in Vitro Transcription and Translation Procedure Using Rabbit Reticulocyte Lysate Human In Vitro Translation Human In Vitro Protein Expression System is a method for expressing proteins from DNA or mRNA templates in a cell-free solution containing essential components of the cellular translational machinery. Extracts of an immortalized human cell line provide the ribosomes, initiation and elongation factors, tRNAs and other basic components required for protein synthesis. When supplemented with proprietary accessory proteins, ATP, and an energy regenerating system, these extracts sustain the synthesis of target proteins from DNA templates for up to 6 hours without the need to remove inhibitory byproducts. Protein synthesis begins with either a DNA or RNA template. When starting with DNA, mRNA is transcribed in one hour at 32°C using the included reaction components. The mRNA are then added to the in vitro translation reaction and incubated for 90 minutes at 30°C for general protein expression. Alternatively, the reaction is incubated for 90 minutes at 28°C for glycoprotein expression. Glycoprotein Expression Kits The major advantage of using the Human In Vitro Expression System is the ability to generate protein modifications just as they occur in vivo. The Glycoprotein Expression Kits are optimized for generating glycosylated protein. The yield of glycoprotein produced in the system out-performs traditional methods (i.e., those based on rabbit reticulocyte lysates combined with microsomal membranes) and have higher fidelity than insect expression systems, which lack N-linked oligosaccharide side chains Human choriogonadotropin hormone (hCG) β subunit was expressed in insect cell extract or the Pierce System according to the manufacturer's instructions. Insect lysates yielded no glycosylated protein when 1µg of hCGβ mRNA was used and multiple glycoforms when 12µg of mRNA was used. Note that the hCGβ was cloned into vectors that were recommended by the manufacturer of the insect expression system Traditional in vitro translation systems have limitations in the types of proteins that can be generated. The Human in vitro Translation System uses human HeLa or hybridoma lysates that endogenously contain the enzymes required for protein synthesis and proper post-translational modifications. The types of proteins that can be expressed using the Human in vitro Translation System, include: Proteins large and small Phosphoproteins Glycoproteins Membrane proteins Successful protein expression is influenced by protein stability, folding and solubility. High molecular weight proteins are difficult to express in vitro because of the greater chance of premature termination and the greater complexity of the tertiary structure compared to smaller proteins, which increases the chance of improper folding . Additionally, proteins with greater size can have large hydrophobic regions that promote the formation of inclusion bodies when expressed in E. coli extracts. The Human in vitro Translation System can be used to produce a broad range of proteins of different sizes. Size distribution of proteins generated with the Human in vitro Translation System. One hundred proteins ranging from 8kDa to greater than 250kDa were individually expressed using the Pierce Human in vitro Translation System with a 95% success rate. Coupled Transcription:Translation Unlike eukaryotic systems where transcription and translation occur sequentially, in E. coli, transcription and translation occur simultaneously within the cell. In vitro E. coli translation systems are thus performed the same way, coupled, in the same tube under the same reaction conditions (one-step reaction). During transcription, the 5' end of the RNA becomes available for ribosomal binding and undergoes translation while its 3' end is still being transcribed. This early binding of ribosomes to the RNA maintains transcript stability and promotes efficient translation. This bacterial translation system gives efficient expression of either prokaryotic or eukaryotic gene products in a short amount of time. For the highest protein yield and the best initiation fidelity, make sure the DNA template has a ShineDalgarno ribosome binding site upstream of the initiator codon. Capping of eukaryotic RNA is not required. Coupled in Vitro Transcription:Translation Procedure Using E. coli Extract. Important Elements For Translation There are some significant differences between prokaryotic and eukaryotic mRNA transcripts. Typically, eukaryotic mRNAs are characterized by two post-transcriptional modifications: a 5'-7 methyl-GTP cap and a 3' poly(A) tail. Both modifications contribute to the stability of the mRNA by preventing degradation. Additionally, the 5' cap structure enhances the translation of mRNA by helping to bind the eukaryotic ribosome and assuring recognition of the proper AUG initiator codon. This function may vary with the translation system and with the specific mRNA being synthesized. The consensus sequence 5'-GCCACCAUGG-3', also known as the "Kozak" sequence, is considered to be the strongest ribosomal binding signal in eukaryotic mRNA. For efficient translation initiation, the key elements are the G residue at the +1 position and the A residue at the -3 position. An mRNA that lacks the Kozak consensus sequence may be translated efficiently in eukaryotic cell-free systems if it possesses a moderately long 5'-untranslated region (UTR) that lacks stable secondary structure. In bacteria, the ribosome is guided to the AUG initiation site by a purinerich region called the Shine-Dalgarno (SD) sequence. This sequence is complementary to the 3' end of the 16s rRNA in the 30S ribosomal subunit. Upstream from the initiation AUG codon, the SD region has the consensus sequence 5'-UAAGGAGGUGA-3'. Specific mRNAs vary considerably in the number of nucleotides that complement the anti-Shine-Dalgarno sequence of 16S rRNA, ranging from as few as two to nine or more. The position of the ribosome binding site (RBS) in relation to the AUG initiator is very important for efficiency of translation (usually from -6 to -10 relative to the A of the initiation site). Ribosomal Binding Site Sequence Requirements Protein synthesis is regulated by the sequence and structure of the 5' untranslated region (UTR) of the mRNA transcript. In prokaryotes, the ribosome binding site (RBS), which promotes efficient and accurate translation of mRNA, is called the Shine-Dalgarno sequence. This purine-rich sequence of 5' UTR is complementary to the UCCU core sequence of the 3'-end of 16S rRNA (located within the 30S small ribosomal subunit). Various Shine-Dalgarno sequences have been found in prokaryotic mRNAs. These sequences lie about 10 nucleotides upstream from the AUG start codon. Activity of a RBS can be influenced by the length and nucleotide composition of the spacer separating the RBS and the initiator AUG. In eukaryotes, the Kozak sequence A/GCCACCAUGG, which lies within a short 5' untranslated region, directs translation of mRNA. An mRNA lacking the Kozak consensus sequence may be translated efficiently in vitro systems if it possesses a moderately long 5' UTR that lacks stable secondary structure. In contrast to the E. coli ribosome, which preferentially recognizes the ShineDalgarno sequence, eukaryotic ribosomes (such as those found in retic lysate) can efficiently use either the Shine-Dalgarno or the Kozak ribosomal binding sites. . The +1 A is the first base of the AUG initiator codon (shaded) responsible for binding of fMettRNAfMet. The underline indicates the ribosomal binding site sequence, which is required for efficient translation. Comparison of in vitro (cell-free) protein expression systems. Advantages and disadvantages of existing extract-based systems for human recombinant protein synthesis. Selection of a cell-free expression system should consider the biological nature of the protein, application, and the template used for protein expression. System Advantages Disadvantages •Very high protein yield •Relatively tolerant of additives •Many eukaryotic proteins insoluble upon expression •Eukaryotic co- and post-translational modifications not possible •Codon usage is different from eukaryotes •Mammalian system •Cap independent translation •Sensitive to additives •Protein glycosylation not possible •Co-expression of off-target proteins •Translation of large proteins possible •Devoid of off-target endogenous mammalian proteins •High protein yield •Mammalian co- and post-translational modifications are not possible •Premature termination of products •Translation of large proteins possible •No endogenous mammalian proteins •Certain forms of protein glycosylation possible •Non-mammalian •Human system •Co- and post-translational modifications are possible •Synthesis of functional proteins •Possible to make VPLs (virus-like particles) •Sensitive to additives •Lower yields than E. coli •New system E. coli Rabbit Reticulocyte (RRL) Wheat germ Insect Human