* Your assessment is very important for improving the work of artificial intelligence, which forms the content of this project
Download Student notes in ppt
Photosynthesis wikipedia , lookup
Electron transport chain wikipedia , lookup
Nicotinamide adenine dinucleotide wikipedia , lookup
NADH:ubiquinone oxidoreductase (H+-translocating) wikipedia , lookup
Light-dependent reactions wikipedia , lookup
Lipid signaling wikipedia , lookup
Photosynthetic reaction centre wikipedia , lookup
Mitochondrion wikipedia , lookup
Metalloprotein wikipedia , lookup
Microbial metabolism wikipedia , lookup
Adenosine triphosphate wikipedia , lookup
Evolution of metal ions in biological systems wikipedia , lookup
Basal metabolic rate wikipedia , lookup
Amino acid synthesis wikipedia , lookup
Specialized pro-resolving mediators wikipedia , lookup
Oxidative phosphorylation wikipedia , lookup
Butyric acid wikipedia , lookup
Biosynthesis wikipedia , lookup
Citric acid cycle wikipedia , lookup
Biochemistry wikipedia , lookup
Glyceroneogenesis wikipedia , lookup
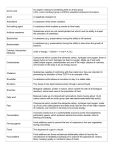
Lipid Metabolism 1: Overview of lipid transport in animals, fatty acid oxidation, ketogenesis in liver mitochondria Bioc 460 Spring 2008 - Lecture 35 (Miesfeld) Prime rib contains large amounts of saturated fats in the form of triacylglycerols Adipose tissue is the primary triacylglycerol storage depot in animals, fats are an excellent form of redox energy Stored fat comes from the conversion of carbohydrates into fatty acids in the liver Key Concepts in Lipid Metabolism • Stored lipids is the primary source of energy in most organisms. Lipids, such as triacylglycerols, are much more reduced than carbohydrates and are hydrophobic, which makes them ideal storage forms of high energy compounds. • The three sources of triacylglycerols in animals are dietary lipids, stored triacylglycerols in adipose tissue, and the conversion of carbon from either carbohydrate or protein into fatty acids in the liver. -oxidation is the mitochondrial process by which fatty acids are oxidized to yield NADH, FADH2, and acetyl-CoA. These metabolites are oxidized by the citrate cycle and electron transport system to yield large amounts of ATP. • Ketogenesis takes place in liver mitochondria when acetyl-CoA levels are high and oxaloacetate levels are low. Acetoacetate and D--hydroxybutyrate are exported are exported and converted back into acetyl-CoA by peripheral tissues. Overview of Lipid Transport in Animals There are three basic sources of fatty acids in animals that can be used for energy conversion processes: 1) fatty acids present in triacylglycerols obtained from the diet, 2) fatty acids stored as triacylglycerols in adipose tissue that are released by hydrolysis following hormone stimulation (glucagon or epinephrine signaling) 3) fatty acids synthesized in the liver from excess carbohydrates and exported as triacylglycerols. Fat is stored in fat cells (adipocytes). Obesity, especially childhood obesity, can be due to both more fat storage per cell, and to a larger number of adipocytes. In contrast, in normal healthy adults, the onset of old age and reduced metabolic rates leads to weight gain resulting primarily from storing more fat per cell (although adults can also add more fat cells if they become obese). Review of lipid structures: Fatty acids are stored as triacylglycerols Glycerol Fatty acid #2 Fatty acid #1 Fatty acid #3 Glycerol esterification of fatty acids protects cell membranes from the amphipathic nature of fatty acids. Soap is made out of fatty acids and works well to remove oils from hands and clothes by forming micelles that trap the lipids in a water soluble particle. Lipid metabolism is critical to animals who depend on lipids as a major energy source. Plants only use seeds as a major lipid storage depot. Conversion of carbohydrates to fatty acids is thought to be a major contributing factor to obesity and diabetes in developed countries over the last 30 years. The primary source of these carbohydrates are soft drinks, and processed foods (snack foods) that have been prepared with refined sugar and flour. Another dietary demon contributing to obesity has been transesterified fats. Pathway Questions 1. What purpose does fatty acid metabolism serve in animals? – Fatty acid oxidation in mitochondria is responsible for providing energy to cells when glucose levels are low. Triacylglycerols stored in adipose tissue of most humans can supply energy to the body for ~3 months during starvation. – Fatty acid synthesis reactions in the cytosol of liver and adipose cells convert excess acetyl CoA that builds up in the mitochondrial matrix when glucose levels are high into fatty acids that can be stored or exported as triacylglycerols. Pathway Questions 2. What are the net reactions of fatty acid degradation and synthesis for the C16 fatty acid palmitate? Fatty acid oxidation: Palmitate + 7 NAD+ + 7 FAD + 8 CoA + 7 H2O + ATP → 8 acetyl CoA + 7 NADH + 7 FADH2 + AMP + 2 Pi + 7 H+ Fatty acid synthesis: 8 Acetyl CoA + 7 ATP + 14 NADPH + 14 H+ → Palmitate + 8 CoA + 7 ADP + 7 Pi + 14 NADP+ + 6 H2O Pathway Questions 3. What are the key enzymes in fatty acid metabolism? Fatty acyl CoA synthetase – enzyme catalyzing the "priming" reaction in fatty acid metabolism which converts free fatty acids in the cytosol into fatty acyl-CoA using the energy available from ATP and PPi hydrolysis. Carnitine acyltransferase I - catalyzes the commitment step in fatty acid oxidation which links fatty acyl-CoA molecules to the hydroxyl group of carnitine. The activity of carnitine acyltransferase I is inhibited by malonylCoA, the product of the acetyl-CoA carboxylase reaction, which signals that glucose levels are high and fatty acid synthesis is favored. Pathway Questions 3. What are the key enzymes in fatty acid metabolism? Acetyl CoA carboxylase - catalyzes the commitment step in fatty acid synthesis using a biotin-mediated reaction mechanism that carboxylates acetyl-CoA to form the C3 compound malonyl-CoA. The activity of acetyl CoA carboxylase is regulated by both reversible phosphorylation (the active conformation is dephosphorylated) and allosteric mechanisms (citrate binding stimulates activity, palmitoyl-CoA inhibits activity). Fatty acid synthase - this large multi-functional enzyme is responsible for catalyzing a series of reactions that sequentially adds C2 units to a growing fatty acid chain covalently attached to the enzyme complex. The mechanism involves the linking malonyl-CoA to an acyl carrier protein, followed by a decarboxylation and condensation reaction that extends the hydrocarbon chain. Pathway Questions 4. What are examples of fatty acid metabolism in real life? A variety of foods are prominently advertised as "non-fat," even though they can contain a high calorie count coming from carbohydrates. Eating too much of these high calorie non-fat foods (e.g., non-fat bagels) activates the fatty acid synthesis pathway resulting in the conversion of acetyl-CoA to fatty acids, which are stored as triacylglycerols. Transport and storage of fatty acids and triacylglycerols Much of the triacylglycerol stored in adipose tissue originates from dietary lipids. Fats that enter the small intestine from the stomach are insoluble and must be emulsified by bile acids such as glycocholate which are secreted by the bile duct and function as detergents to promote the formation of micelles. Lipases are water soluble enzymes in the small intestine that hydrolyze the acyl ester bonds in triacylglycerols to liberate free fatty acids which then pass through the membrane on the lumenal side of intestinal epithelial cells. Pancreatic lipase cleaves the ester bond at the C-1 and C-3 carbons to release two free fatty acids and monoacylglyclerol. Transport and storage of fatty acids and triacylglycerols Transport and storage of fatty acids and triacylglycerols Chylomicrons transport the triacylglycerols to adipose tissue for storage, and to muscle cells for energy conversion processes. Apolipoprotein C-II on the surface of chylomicrons binds to and activates lipoprotein lipase on endothelial cells which leads to the release of fatty acids and glycerol. Fatty acids diffuse into the endothelial cells and then enter nearby adipose and muscle cells where they are stored or used for energy conversion pathways. The glycerol produced by lipoprotein lipase returns to the liver where it is converted to dihydroxyacetone phosphate. Transport and storage of fatty acids and triacylglycerols Fatty acids are synthesized in the liver from carbohydrates Dietary lipids are not the only source of triacylglycerols stored in adipocytes. The liver synthesizes triacylglycerols from fatty acids when glucose levels are high and the amount of acetyl CoA produced exceeds the energy requirements of the cell. Glucose provides the necessary substrates for triacylglycerol synthesis (acetyl CoA for fatty acid synthesis and glycerol) using reactions in the glycolytic pathway and the citrate cycle. The fatty acid oxidation pathway in mitochondria Fatty acids must first be activated by a two step reaction catalyzed by medium chain fatty acyl CoA synthetase. In the first step, the carboxylate ion of the fatty acid attacks a phosphate in ATP to form an acyl-adenylate intermediate and release pyrophosphate (PPi) which is quickly hydrolyzed by the enzyme inorganic pyrophosphatase to form 2 Pi. In the second step of the fatty acyl CoA synthetase reaction, the palmitoyl-adenylate intermediate is attacked by the thiol group of CoA to form the thioester palmitoyl-CoA product and release AMP. Fatty acid are transported into mitochondria by carnitine The fatty acyl-CoA products of the fatty acyl CoA synthetase reaction have two fates. If the energy charge of the cell is low, then they will be imported into the mitochondrial matrix by the carnitine transport cycle and degraded by the fatty acid oxidation reactions to yield acetyl CoA, FADH2 and NADH. However, if the energy charge is high, and fatty acid synthesis is favored, then mitochondrial import of fatty acyl-CoA is inhibited and the fatty acyl-CoA molecule is used instead for triacylglycerol or membrane lipid synthesis in the cytosol. Carnitine acyltransferase I is located in the outer mitochondrial membrane and replaces CoA with carnitine to form fatty acyl carnitine which is translocated across the inner mitochondrial membrane. The carnitine translocating protein is an antiporter that exchanges a fatty acyl carnitine molecule for a carnitine. Once inside the mitochondrial matrix, fatty acyl carnitine is converted back to fatty acyl CoA in a reaction catalyzed by carnitine acyltransferase II releasing the carnitine so that it can be shuttled back across the membrane. Fatty acid are transported into mitochondria by carnitine -oxidation yields large amounts of ATP Once the electron-rich carbons of fatty acids are moved into the mitochondrial matrix, their high energy redox potential is traded in for a substantial payout of ATP This energy conversion process of fatty acid --> ATP involves oxidation of fatty acids by sequential degradation of C2 units leading to the generation FADH2, NADH, and acetyl CoA. The subsequent oxidation of these reaction products by the citrate cycle and oxidative phosphorylation generates large amounts of ATP. -oxidation reactions The -oxidation pathway occurs at the carbon of the fatty acid, thereby releasing the C-1 carboxyl carbon and carbon as the acetate component of acetyl CoA. In the first of four reactions, the enzyme acyl CoA dehydrogenase catalyzes a dehydrogenation reaction (oxidation) that introduces a trans C=C bond between the and carbons of the fatty acyl-CoA molecule using a mechanism that reduces an enzyme bound FAD to form FADH2. Mitochondria contain three isozymes of acyl CoA dehydrogenase which differ in their specificity for hydrocarbon chains of different lengths, long chain (C12 to C18), medium chain (C4 to C14) and short chain (C4 to C8 ) acyl CoA dehydrogenases. -oxidation reactions The second reaction in the oxidation pathway is a hydration step catalyzed by the enzyme enoyl CoA hydratase that adds H2O across the C=C bond to convert trans-2-enoyl-CoA to 3-L-hydroxyacyl-CoA. The third reaction is another dehydrogenation (oxidation) step in which the enzyme -hydroxyacyl-CoA dehydrogenase removes an electron pair from the substrate and donates it to NAD+ to form NADH. Finally, coenzyme A is used in thiolysis reaction catalyzed by the enzyme acyl CoA acetyltransferase (also called thiolase) that releases a molecule of acetyl CoA and in the process, results in the formation of an fatty acyl CoA product that is two carbons shorter than the starting substrate. -oxidation reactions The complete oxidation of palmitoyl-CoA (C16) requires seven rounds of the oxidation pathway to convert one molecule of palmitoyl CoA into eight molecules of acetyl CoA in a net reaction that can be written as: Palmitoyl-CoA + 7 CoA + 7 FAD + 7 NAD+ + 7 H2O --> 8 acetyl CoA + 7 FADH2 + 7 NADH + 7 H+ After seven rounds of oxidation, palmitoyl-CoA yields 8 acetyl CoA, 7 NADH and 7 FADH2. The oxidation of acetyl CoA by the citrate cycle then generates 24 NADH, 8 FADH2 and 8 GTP (ATP). -oxidation reactions The combined reactions of the electron transport system and oxidative phosphorylation converts these 31 NADH (31 x ~2.5 ATP) = ~77.5 ATP 15 FADH2 (15 x ~1.5 ATP) = ~22.5 ATP For a grand total = 100 ATP After subtracting the 2 ATP required for fatty acyl CoA activation (AMP --> PPi) And adding the 8 ATP obtained from eight turns of the citrate cycle; The total payout for the complete oxidation of palmitate is 106 ATP -oxidation is a chemical source of water for desert animals Besides the payout of ATP that comes from fatty acid oxidation, another benefit is the generation of H2O that occurs when O2 is reduced by the final reaction in the electron transport system, as well as, the formation of H2O in the ATP synthesis reaction of oxidative phosphorylation as shown in the three reactions below: 2 NADH + 2 H+ + O2 --> 2 H2O 2 FADH2 + O2 --> 2 H2O ADP + PO42- --> ATP + H2O The water production that accompanies fatty oxidation benefits animals that live in dry climates where liquid water is scarce, for example, the desert kangaroo rat and Arabian camel. Large animals that hibernate over the winter, like the Alaskan brown bear, also take advantage of fatty acid oxidation in order to replace H2O that is lost by respiration. Ketogenesis Acetyl-CoA derived from fatty acid oxidation enters the Citrate Cycle only if carbohydrate metabolism is properly balanced. When fatty acid oxidation produces more acetylCoA than can be combined with OAA to form citrate, then the "extra" acetyl-CoA is converted to acetoacetyl-CoA and ketone bodies, including acetone. Ketogenesis (synthesis of ketone bodies) takes place primarily in the liver. Ketogenesis Three mitochondrial reactions are required to convert two acetyl CoA molecules into acetoacetate which is then reduced to form D--hydroxybutyrate. Acyl-CoA acetyltransferase (thiolase) is the same enzyme that releases one molecule of acetyl CoA in reaction 4 of the oxidation pathway, however in this case, the reaction is driven toward condensation by the high concentration of acetyl CoA in the mitochondria under ketogenic conditions. In the next step, the enzyme HMG-CoA synthase fadds another acetyl CoA group to form the intermediate -hydroxy-methylglutaryl-CoA, abbreviated as HMGCoA, and then the enzyme HMG-CoA lyase removes one of the original acetyl CoA groups to yield acetoacetate. Ketones are an energy source for tissues Acetoacetate and D-hydroxybutyrate are exported from the liver and used by other tissues such as skeletal and heart muscle to generate acetyl CoA for energy conversion reactions. Even the brain which prefers glucose as an energy source, can adapt to using ketone bodies as chemical energy during times of extreme starvation. Ketogenesis occurs when glycogen stores are depleted such as during fasting and in undiagnosed diabetics Diabetes is a metabolic form of carbohydrate "starvation," and characterized by elevated concentrations of acetoacetate and D-hydroxybutyrate in the blood and urine. Diabetics can have high levels of acetone in their blood which can be detected on their breath as a fruity odor. Acetone is a spontaneous breakdown product of acetoacetate (decarboxylation), or is formed by enzymatic cleavage of acetoacetate by the enzyme acetoacetate decarboxylase