* Your assessment is very important for improving the work of artificial intelligence, which forms the content of this project
Download Bio1A - Lec 9 slides File
Ultrasensitivity wikipedia , lookup
Epitranscriptome wikipedia , lookup
Catalytic triad wikipedia , lookup
Basal metabolic rate wikipedia , lookup
Photosynthesis wikipedia , lookup
Photosynthetic reaction centre wikipedia , lookup
Mitochondrion wikipedia , lookup
Fatty acid synthesis wikipedia , lookup
Butyric acid wikipedia , lookup
Light-dependent reactions wikipedia , lookup
Biosynthesis wikipedia , lookup
Metalloprotein wikipedia , lookup
Amino acid synthesis wikipedia , lookup
Fatty acid metabolism wikipedia , lookup
Glyceroneogenesis wikipedia , lookup
Lactate dehydrogenase wikipedia , lookup
Electron transport chain wikipedia , lookup
Phosphorylation wikipedia , lookup
Enzyme inhibitor wikipedia , lookup
Microbial metabolism wikipedia , lookup
Evolution of metal ions in biological systems wikipedia , lookup
NADH:ubiquinone oxidoreductase (H+-translocating) wikipedia , lookup
Biochemistry wikipedia , lookup
Adenosine triphosphate wikipedia , lookup
Nicotinamide adenine dinucleotide wikipedia , lookup
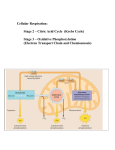
Metabolism Thermodynamics • Free Energy • Activation Energy Enzymes ATP coupling Factors on Enzymatic activity • Optimal activity graphs • Michealis Mentin Kinetics Regulation Reading Ch 8: Energy Homework Ch 9 Prequiz Cell Respiration prequiz Check your clicker grades Web registrations not working Extra Credit Science Seminar Enzyme requirements – Cofactors Low MW compounds at active site Terms are not strictly held – usage not fixed Cofactors are nonprotein enzyme helpers - inorganic (such as a metal in ionic form) or organic - Cofactors usually refers to inorganic • Coenzyme - loosely bound organic cofactor - Name stresses affect on enzyme - include vitamins • Prosthetic Group = covalently bound organic cofactor - Name stresses the fact that it is tightly bound - Function is partially to affect enzyme shape and partially for interacting with substrates Examples of cofactors Only Cofactor Carbonic anhydrase Zn2+ surrounded by 3 x His Also Covalently bound So also a Prosthetic group Cofactors Transition metals For redox reactions Fe3+ + e- Fe2+ Cu2+ + e- Cu+ or other … Generally when people say cofactor they mean a metal ion Organic Prosthetic groups coenzymes Derivative used as prosthetic groups Riboflavin flavin adenine dinucleotide (FAD+) Vit B-1 Thiamine Thiamine pyrophosphate (THPP) Vit B-3 Niacin Nicotinamide adenine dinucleotide (NAD+) Vit B-6 Pyridoxal Pyridoxal phosphate Vit B-12 Cobalmin adenosylcobalamine & methylcobalamin Vit B-2 Biotin (vit B-7) Covalently bound to protein Protein is modified O C Used in carboxylases Substrate + CO2 Protein Examples of cofactors Heme Not a hydrogen bond Coenzyme & Cofactor Covalently bound, so just call it a Prosthetic group Enzyme Inhibitors / Activators • Competitive inhibitors bind to the active site of an enzyme, competing with the substrate • Noncompetitive inhibitors bind to another part of an enzyme, causing the enzyme to change shape and making the active site less effective Allosteric Affect – Examples of inhibitors include toxins, poisons, pesticides, and antibiotics • Activators – opposite of a noncompetitve inhibitor Allosteric Regulation of Enzymes • Allosteric regulation may either inhibit or stimulate an enzyme’s activity • Allosteric regulation occurs when a regulatory molecule binds to a protein at one site (away from active site) and affects the protein’s function at another site (active site) Allosteric activators and inhibitors Fig. 8-19 Affects availability of substrate Alters Km Substrate Active site Competitive inhibitor Enzyme Noncompetitive inhibitor Normal binding Allosteric effect Affects Enzyme activity Alters Vmax Allosteric activators and inhibitors Allosteric enzyme with four subunits Regulatory site (one of four) Active site (one of four) Activator Active form Stabilized active form Oscillation Non- functional active site Inhibitor Stabilized inactive Inactive form form Affects of competitive inhibitor normal Rate Vmax + inhibitor ½ Vmax Km Km +inhibitor Km increases = “graph shifts right” No change in Vmax Substrate conc. Less productive There is no opposite of a competitive inhibitor No competitive “activator” But if you remove a competitive inhibitor… Vmax original ½ Vmax Km -inhibitor Km Km decreases = “graph shifts left” More productive Substrate Enzyme Competitive Inhibitor Substrate Rate is 5 / 5s =1 reaction / sec normal Same conc. But slower Rate is 2 / 5 sec Rate has decreased even though substrate concentration remains unchanged Some competitive inhibitors are better than other (don’t let go), but others can be “pushed out” or competed out. The more substrate around, the more chances to try to bump out inhibitor or to get in there before the inhibitor has a chance to bind Why does it have the same Vmax then? At V the ratio would be but evenof more There high enough be some concentrations affect, substrate maxwill extreme. So Inhibitor little to no you it willessentially be negligible. end upwould with this: interference. How non-competitve inhibitors, Allosteric activators Cofactors, Coenzymes, Prosthetic groups work These all work by affecting the enzyme itself Cofactors, Coenzymes & Prosthetic groups are REQUIRED portions of the enzyme. • often required for proper protein folding • typically required at the active site -For bonding -Supplies electrons or functional groups for the reaction -Temporarily for E-S transient complexes Non-competitve inhibitors and allosteric activators, both change the protein structure to make them better or worse. Typically away from the active site enzyme & substrate Enzymes not requiring a cofactor are only referred to as enzymes s or different enzyme s Cofactors Coenzyme prosthetic group •Holoenzyme – an enzyme when combined with cofactor etc. •Apoenzyme – enzyme that requires a cofactor but does not currently have one bound (NON-Functional) No cofactor = 0 or negligible activity Non-competitive inhibitors and activators work by altering the protein shape. Non-competitive inhibitor impairs enzyme activity. Unlike removing a cofactor, does not necessarily completely inactivate enzyme. s Activator makes the enzyme better. Improves the active site. Unlike cofactors, without activators enzymes still have some activity. Affects of non-competitive inhibitor or removal of a cofactor, coenzyme, prosthetic group or allosteric activator original Vmax Vmax +inhibitor + inhibitor (non-competitive) ½ Vmax ½ Vmax +inhibitor Km Vmax decreases = “graph shifts down” Less productive No change in Km Affects of allosteric activator or addition of a cofactor, coenzyme, prosthetic group or removal of a non-competitive inhibitor Vmax +activator + activator original Vmax ½ Vmax +inhibitor ½ Vmax Km Vmax increases = “graph shifts up” No change in Km More productive Substrate Allosteric Affector Non-competitve inhibitor Enzyme Notice how substrate can not displace a non-competiitve inhibitor Substrate Rate is 5 / 5s =1 reaction / sec Rate is 5 / 1 min =0.08 reactions / sec or effectively nothing So why doesn’t Km change? As with Vmax for competitive inhibitors, Km will change, but it should be a negligible amount. This is less true than for competitive inhibition, but for our understanding we will be using the following assumption. 1 reaction / sec Effectively or comparitively 0 reactions / sec So why isn’t the curve flat? Because you probably have a mixture Inhibitor No inhibitor Conc .1 M Reactions per sec .1 M This tube has “x” number of enzymes. All are active. Represented by 2 enzymes. Reactions per sec This tube has the same number of enzymes. But some portion are inactivated by inhibitor while others remain inactive. The inactive ones make essentially no contribution. Represented by one functional and one nonfunctional enzyme Let’s use some made up numbers. Substrate conc on left and rate below Because you probably have a mixture Inhibitor No inhibitor Conc .0 M .1 M .2 M .3 M .4 M .5 M .6 M .7 M .8 M .9 M 1.0 M Reactions per sec 0 15 30 42 50 54 57 59 60 60 60 0 15 30 42 50 54 57 59 60 60 60 Active enzymes should behave the same at the same conc Reactions per sec 0 15 30 42 50 54 57 59 60 60 60 0 0 0 0 0 0 0 0 0 0 0 Because you probably have a mixture Inhibitor No inhibitor Conc .0 M .1 M .2 M .3 M .4 M .5 M .6 M .7 M .8 M .9 M 1.0 M Reactions per sec 0 15 30 42 50 54 57 59 60 60 60 0 15 30 42 50 54 57 59 60 60 60 Reactions per sec Total 0 30 60 84 100 108 114 118 120 120 120 Total 0 15 30 42 50 54 57 59 60 60 60 0 15 30 42 50 54 57 59 60 60 60 0 0 0 0 0 0 0 0 0 0 0 Because you probably have a mixture Inhibitor No inhibitor Conc .0 M .1 M .2 M .3 M .4 M .5 M .6 M .7 M .8 M .9 M 1.0 M Reactions per sec Total 0 0 0 30 15 15 60 30 30 84 42 42 100 50 50 108 54 54 114 57 57 118 59 59 120 60 60 120 60 60 120 60 60 Reactions per sec Total 0 0 15 15 Km (0.2M) 30 30 at ½ Vmax 42 42 is the same 50 50 54 54 57 57 59 59 60 60 Vmax is 60 60 lower 60 60 0 0 0 0 0 0 0 0 0 0 0 Affects of non-competitive inhibitor or removal of a cofactor, coenzyme, prosthetic group or allosteric activator original Vmax Vmax +inhibitor + inhibitor (non-competitive) ½ Vmax ½ Vmax +inhibitor Km Vmax decreases = “graph shifts down” Less productive No change in Km Affects of allosteric activator or addition of a cofactor, coenzyme, prosthetic group or removal of a non-competitive inhibitor Vmax +activator + activator original Vmax ½ Vmax +inhibitor ½ Vmax Km Vmax increases = “graph shifts up” No change in Km More productive If an enzyme lacks a necessary cofactor, how will this affect the enzyme kinetics? a)Vmax up b)Vmax down c)Km up d)Km down How will a competitive inhibitor affect an enzyme’s kinetics? a)Vmax down b)Km up c)Km down d)Curve Shifts left How will a non-competitive inhibitor affect an enzyme’s kinetics? a)Vmax down b)Km up c)Km down d)Curve Shifts left Non-Michaelis-Menten Kinetics: Cooperativity One example of non-Michaelis-Menten Kinetics There are others, but this is the most significant Michaelis-Menten Single active site Cooperativity substrate Multiple active sites & Substrate is an activator Cooperativity • form of allosteric regulation amplifies enzyme activity • binding of substrate to one active site stabilizes favorable conformational changes at all other subunits Substrate = activator S-Curve Enzyme exhibiting cooperativity •Difference is in beginning representing initial low substrate binding •As substrate binds, activates enzyme, more easily binds substrate •Remainder of curve matches normal kinetics Same affects from inhibitors, activators, etc as in Michaelis Menten Kinetics: competitive Vmax unchanged Removing competitive inhibitor original inhibitors adding competitive inhibitor ½ Vmax Km decreases = “shift left” More productive Km Km Removing competitive inhibitor Km + competitive inhibitor Km increases =“shift right” Less productive •Non-competitive inhibitors •activators, cofactors, coenzymes, prosthetic groups Vmax -inhibitor Vmax “shift up” productive Removing non-competitive inhibitor or adding activator (allosteric) or adding cofactor / coenzyme / prosthetic group Vmax original ½ Vmax - inhibitor Vmax +inhibitor Vmax “shift down” productive ½ Vmax Adding non-competitive inhibitor or removing activator (allosteric) or removing cofactor etc ½ Vmax + inhibitor Km unchanged Feedback Inhibition • In feedback inhibition, the end product of a metabolic pathway shuts down the pathway • Feedback inhibition prevents a cell from wasting chemical resources by synthesizing more product than is needed Maintains a stable level of product Need Ile Too little? Enzymes produce Ile Later when enough Ile Need to turn off / down enzymes otherwise overproduction Post-Translational Modifications Can affect enzyme activity Example: Proteolysis = protein cleavage (by proteases) Ex: Zymogens = inactive precursor Inactive protease active Post-Translational Modifications Phosphorylation - adding phosphate • addition or removal can activate or repress • Kinases (generally to add Pi) • Phosphatases (generally to remove Pi) •Reversible & Instantaneous • added to Serine, Threonine, or Tyrosine - All have alcohol group (-OH) Chapter 9: Cellular Respiration Harvesting Chemical Energy / making ATP Broad overview of cell respiration With emphasis on enzymes & metabolic pathways Catabolic Pathways and Production of ATP • The breakdown of organic molecules is exergonic • Aerobic respiration consumes organic molecules and O2 and yields ATP • Fermentation is a partial degradation of sugars that occurs without O2 • Anaerobic respiration - similar, but consumes compounds other than O2 Cellular respiration • includes both aerobic and anaerobic respiration but is often used to refer to aerobic respiration • Although carbohydrates, fats, and proteins are all consumed as fuel, it is helpful to trace cellular respiration with the sugar glucose: C6H12O6 + 6 O2 6 CO2 + 6 H2O + Energy (ATP + heat) The NET reaction Stages of Cellular Respiration Cellular respiration has 3(4) stages: 1. Glycolysis 2. Pyruvate oxidation 3. The citric acid cycle Stepwise oxidation of glucose Releasing energy captured in the form of: NADH 4. Oxidative phosphorylation Each Phase is multi-step Redox Reactions: Oxidation & Reduction Chemical reactions that transfer electrons Oxidation - substance loses electrons (is oxidized) Reduction - substance gains electrons, (is reduced) (the amount of positive charge is reduced) •The electron donor is called the reducing agent •The electron receptor is called the oxidizing agent The transfer of electrons releases energy stored in organic molecules Fig. 9-UN1 becomes oxidized (loses electron) becomes reduced Reducing agent Causes Cl to be Oxidizing (gains electron) reduced agent • Some redox reactions do not transfer electrons but change the electron sharing in covalent bonds • An example is the reaction between methane and O2 Because O is more electronegative than carbon there is a release of energy Fig. 9-3 Oxidation – so where are the electrons? R R C R H R R H C O R O R Where do they go? What is reduced? H H 2H+ & 2e- R Electron shuttling by: Nicotinamide adenine dinucleotide (NAD+) NAD+ NADH + H+ NADP+ NADPH + H+ FAD FADH2 oxidized reduced ΔG° = 53 kcal/mole 2 e-’s and 2 H+ from substrates P NADP+ Dehydrogenases Enzymes that redox using NAD+/NADH & 2nd substrate Take electrons and add to NAD+ Oxidize substrate 1 & Reduce NAD+ Or vice versa Dehydrogenase HCOOH Formic Acid Formic Acid Dehydrogenase Cell Respiration Oxidation of C and Reduction of O Reduction C6H12O6 + 6 O2 Oxidation 6 CO2 + 6 H2O + Energy (ATP + heat) ΔG° = - 686 kcal/mole But broken down in a more controlled release Stepwise oxidation Each step releases significant energy Nothing 100% efficient Fig. 9-5 Electron shuttling by: H2 + 1/2 O2 1/ O 2 2 2H (from food via NADH) 2 H+ + 2 e– Controlled release of energy for synthesis of ATP Explosive release of heat and light energy Flour Mill explosions (a) Uncontrolled reaction 1/ O 2 2 (b) Cellular respiration Fig. 9-6-3 The Stages of Cellular Respiration Electrons carried via NADH and FADH2 Electrons carried via NADH Glycolysis Pyruvate Glucose Citric acid cycle Pyruvate oxidation Oxidative phosphorylation: electron transport & chemiosmosis Mitochondrion Cytosol ATP ATP ATP Substrate-level phosphorylation Substrate-level phosphorylation Oxidative phosphorylation Glycolysis • • • • Cytosol Simplest metabolic pathway Most cells do it Considered most primitive biochem process mitochondria not needed • 10 steps 10 enzymes Glycolysis : Splitting of sugar 2 phases Investment Phase 4 ADP & 2 NAD+ 2 ATP Glucose Payoff phase 2 G3P (C3) (C6) 2 ADP BPG 2Pyruvate (C3) 4 ATP 2 NADH + H+ Investment Phase Coupling C D ΔG° = 5 kcal/mol ATP ADP + Pi ΔG° = -7.3kcal/mol C + ATP D + ADP + Pi ΔG° = -2.3kcal/mol E F ΔG° = 14 kcal/mol Cannot be coupled to ATP, but maybe another reaction G H ΔG° = -3 kcal/mol Can be coupled but probably isn’t because it is already spontaneous Which of the following is a possible ΔG? a)-4 b)1 c)2 d)3 Which of the following is a possible ΔG for ONLY: Pi + Glu G6P Remember you coupled this to ATP hydrolysis a)-5 b)3 c)8 d)11 ATP ADP + Pi ΔG°= -7.3 kcal/mole ATP + Glu G6P + ADP ΔG° < 0 Fig. 9-8 Glucose CO2 Glycolysis Kreb’s ΔG° -686 kcal/mol Oxidative Phosphorylation 4 ADP & 2 NAD+ 2 ATP Glucose 2 G3P 2 ADP 2Pyruvate 4 ATP 2 NADH + H+ Glucose Pyruvate ΔG° -140 kcal/mol Glycolysis : Energy investment phase Glucose + 2ATP 2 G3P + 2 ADP Use up 2 ATP because: Glucose + Pi 2ATP 2 G3P ΔG is positive 2 ADP + 2 Pi •Also “priming” G6P allow more glucose into the cell & Blocks glucose from leaking out •2nd ATP Hydrolysis step is essentially irreversible committing molecule to the rest of glycolysis How many total ATP are produced during glycolysis? Payoff phase Investment Phase 4 ADP & 2 NAD+ 2 ATP Glucose a)-2 b)4 c)10 d)32 2 G3P 2 ADP 2Pyruvate 4 ATP 2 NADH + H+ How many NET ATP are produced during glycolysis? a)-2 b)2 c)10 d)32 Payoff phase 1st oxidation step oxidized •Dehydrogenase NAD+ involved Oxidation of C Coupled to reduction of NAD+ Glyceraldehyde3-phosphate 2 x 2 NAD+ 2 NADH aka G3P GAP PGAL 6 Triose phosphate dehydrogenase 2Pi + 2 H+ •Large energy release 2 x 1, 3-Bisphosphoglycerate BPG For G3P +Pi BPG? What is a feasible G? Remember: NAD+ NADH + H+ ΔG° = 53 kcal/mole Glyceraldehyde3-phosphate 2 x 2 NAD+ a)-61 2 NADH 6 Triose phosphate dehydrogenase 2Pi + 2 H+ b)-8 c)-2 d)+40 2 x 1, 3-Bisphosphoglycerate G3P +Pi BPG ΔG° = ? NAD+ NADH + H+ ΔG° = 53 kcal/mole G3P + NAD+ BPG + NADH + H+ a)-61 b)-8 c)-2 d)+40 oxidized R-CHO R-COOH NAD+ NADH ΔG° -10.3 kcal/mol Only that it is neg is important Glyceraldehyde3-phosphate 2 x 2 NAD+ 2 NADH 6 Triose phosphate dehydrogenase 2Pi + 2 H+ 2 x 1, 3-Bisphosphoglycerate BPG Fig. 9-9-6 2 NAD+ 2 NADH + 2 H+ 6 Triose phosphate dehydrogenase 2 Pi 2 1, 3-Bisphosphoglycerate 2 ADP 7 Phosphoglycerokinase 2 ATP 2 1, 3-Bisphosphoglycerate 2 ADP 2 3-Phosphoglycerate ΔG° 7.3 2 ATP 2 ΔG° -7.3 kcal/mol 7 Phosphoglycerokinase 3-Phosphoglycerate Overall ΔG° -0.1 kcal/mol Substrate level phosphorylation Transfer of a phosphate from substrate to ADP to generate ATP R-P + ADP R + ATP • A smaller amount of ATP is formed in glycolysis and the citric acid cycle by substrate-level phosphorylation • Oxidative phosphorylation accounts for almost 90% of the ATP generated by cellular respiration Pi + ADP ATP Fig. 9-9-9 2 NAD+ Substrate Phosphorylation 6 Triose phosphate dehydrogenase 2 Pi 2 NADH + 2 H+ 2 1, 3-Bisphosphoglycerate 2 ADP 7 Phosphoglycerokinase 2 ATP 2 Phosphoenolpyruvate ΔG° -15 kcal/mol 2 ADP 2 3-Phosphoglycerate 8 Phosphoglyceromutase 2 ATP 2 10 Pyruvate kinase 2-Phosphoglycerate 9 2 H2O Enolase 2 Phosphoenolpyruvate 2 ADP 10 Pyruvate kinase 2 ATP 2 2 Pyruvate Pyruvate 2 Phosphoenolpyruvate 2 ADP 10 Pyruvate kinase 2 ATP 2 Pyruvate What is a possible free energy change (ΔG) of phosphoenolpyruvate to pyruvate? PEP pyruvate + Pi a)+5 kcal/mole b)0 c) -5 d)-12 e)None of the above What is the primary mechanism of ATP generation in glycolysis? a)ATP b)NADP c) substrate level phosphorylation d)pyruvate Which of the following is NOT a product of glycolysis? a)ATP b)NADP c)Lactic Acid d)Pyruvate Lactic acid fermentation 2 ADP + 2 P i Glucose 2 ATP Glycolysis 2 NAD+ 2 NADH + 2 H+ 2 Pyruvate 2 Lactate Lactic acid fermentation • In lactic acid fermentation, pyruvate is reduced to NADH, forming lactate as an end product, with no release of CO2 • Lactic acid fermentation by some fungi and bacteria is used to make cheese and yogurt • Human muscle cells use lactic acid fermentation to generate ATP when O2 is scarce Why does a cell use fermentation? a)To produce ATP b) To produce lactic acid c)To produce NAD+ d)To produce NADH Fig. 9-8 How equilibrium affects Respiration Normally (under aerobic conditions): •Glucose resupplied •Pyruvate & NADH used in next step NADH + H+ NAD+ •ATP used for work Anaerobic conditions (NO O2): •Pyruvate & NADH not used (O2 is required) • “Run out” of NAD+ Net Glucose 2 ADP + 2 Pi 2 NAD+ + 4 e– + 4 H+ 2 Pyruvate + 2 H2O 2 ATP 2 NADH + 2 H+ Alcohol fermentation 2 ADP + 2 Glucose P i 2 ATP Glycolysis 2 Pyruvate 2 NAD+ 2 Ethanol 2 NADH + 2 H+ 2 CO2 2 Acetaldehyde Many other forms including production of methane and hydrogen gas What is the enzyme for alcohol fermentation? a)Kinase b)Phosphotase c)Dehydrogenase d)protease ? Pyruvate decarboxylation & citric acid (or kreb’s) cycle completes the energy-yielding oxidation of organic molecules • In the presence of O2, pyruvate enters the mitochondrial Matrix • Before the citric acid cycle can begin, pyruvate must be converted to acetyl CoA, which links the cycle to glycolysis Pyruvate oxidation Step 1: pyruvate decarboxylation CYTOSOL MITOCHONDRION NAD+ NADH + H+ 2 1 Pyruvate 3 CO2 Coenzyme A Acetyl CoA Transport protein / pump What is the enzyme? Pyruvate Dehydrogenase Fig. 9-10 Pyruvate AcetylCoA is what kind of reaction? a)Substrate phosphorylation b)Oxidative phosphorylation c)condensation d)Redox Pyruvate dehydrogenase is likely inhibited by: Consider homeostasis Consider feedback inhibition O2 NADH Glucose NADH a)pyruvate b)ADP c)ATP d)O2 pyruvate ATP CO2 ATP is a competitive inhibitor, what will happen to the enzyme kinetics of pyruvate dehyrogenase as ATP builds up? a)Vmax will go up b)Vmax will go down c)Km will shift right d)Km will shift left Pyruvate oxidation Step 1: pyruvate decarboxylation CYTOSOL MITOCHONDRION NAD+ NADH + H+ 2 1 Pyruvate 3 CO2 Coenzyme A Acetyl CoA Transport protein Oxidation produces NADH CoA carries 2 carbon unit to citric acid cycle CO2 released Fig. 9-10 AcetylCoA is starting material for Krebs cycle • The citric acid cycle, also called the Krebs cycle, takes place within the mitochondrial matrix (except succinate dehydrogenase which is loosely associated with inside membrane) • The cycle oxidizes organic fuel derived from pyruvate, generating 1 ATP, 3 NADH, and 1 FADH2 per turn • Complete oxidation of glucose CO2 Fig. 9-12-8 CoA for next pyruvate Acetyl CoA CoA—SH NADH +H+ H2O 1 NAD+ 8 Oxaloacetate 2 Malate Citrate Isocitrate NAD+ Citric acid cycle 7 H2O NADH + H+ 3 CO2 Fumarate CoA—SH 6 -Ketoglutarate 4 CoA—SH 5 FADH2 NAD+ FAD Succinate GTP GDP ADP ATP Pi Succinyl CoA NADH + H+ CO2 Pyruvate CO2 NAD+ NADH + H+ Glycolysis 1 glucose 2 Pyruvates 2 NADH & 2ATP CoA Acetyl CoA CoA Pyruvate Decarboxylation 2 pyruvate 2 CO2 2 Acetyl CoA 2 NADH Kreb’s CoA Citric acid cycle FADH2 2 CO2 3 NAD+ 3 NADH + 3 H+ FAD ADP + P i ATP 2 Acetyl 2 turns 1 turn •2 CO2 •3 NADH + H+ •1FADH2 •ATP Total 4 CO2 6 NADH 2 FADH2 2 ATP Before the krebs cycle, most of the free energy from glucose was in: a)Pyruvate b)ATP c)NADH d)FADH After the krebs cycle, most of the free energy from glucose was in: a)CO2 b)ATP c)NADH d)FADH Fig. 9-8 Glucose CO2 Glycolysis Kreb’s ΔG° -686 kcal/mol Oxidative Phosphorylation 4 ADP & 2 NAD+ 2 ATP Glucose 2 G3P 2 ADP 2Pyruvate 4 ATP 2 NADH + H+ Glucose Pyruvate ΔG° -140 kcal/mol Lactic acid fermentation 2 ADP + 2 P i Glucose 2 ATP Glycolysis 2 NAD+ 2 NADH + 2 H+ 2 Pyruvate 2 Lactate Lactic acid fermentation • In lactic acid fermentation, pyruvate is reduced to NADH, forming lactate as an end product, with no release of CO2 • Lactic acid fermentation by some fungi and bacteria is used to make cheese and yogurt • Human muscle cells use lactic acid fermentation to generate ATP when O2 is scarce Why does a cell use fermentation? a)To produce ATP b) To produce lactic acid c)To produce NAD+ d)To produce NADH Fig. 9-8 How equilibrium affects Respiration Normally (under aerobic conditions): •Glucose resupplied •Pyruvate & NADH used in next step NADH + H+ NAD+ •ATP used for work Anaerobic conditions (NO O2): •Pyruvate & NADH not used (O2 is required) • “Run out” of NAD+ Net Glucose 2 ADP + 2 Pi 2 NAD+ + 4 e– + 4 H+ 2 Pyruvate + 2 H2O 2 ATP 2 NADH + 2 H+ Alcohol fermentation 2 ADP + 2 Glucose P i 2 ATP Glycolysis 2 Pyruvate 2 NAD+ 2 Ethanol 2 NADH + 2 H+ 2 CO2 2 Acetaldehyde Many other forms including production of methane and hydrogen gas What is the enzyme for alcohol fermentation? a)Kinase b)Phosphotase c)Dehydrogenase d)protease ? Pyruvate decarboxylation & citric acid (or kreb’s) cycle completes the energy-yielding oxidation of organic molecules • In the presence of O2, pyruvate enters the mitochondrial Matrix • Before the citric acid cycle can begin, pyruvate must be converted to acetyl CoA, which links the cycle to glycolysis Pyruvate oxidation Step 1: pyruvate decarboxylation CYTOSOL MITOCHONDRION NAD+ NADH + H+ 2 1 Pyruvate 3 CO2 Coenzyme A Acetyl CoA Transport protein / pump What is the enzyme? Pyruvate Dehydrogenase Fig. 9-10 Coenzyme A Carries acetyl groups From vit B5 Pyruvate AcetylCoA is what kind of reaction? a)Substrate phosphorylation b)Oxidative phosphorylation c)condensation d)Redox Pyruvate dehydrogenase is likely inhibited by: Consider homeostasis Consider feedback inhibition O2 NADH Glucose NADH a)pyruvate b)ADP c)ATP d)O2 pyruvate ATP CO2 ATP is a competitive inhibitor, what will happen to the enzyme kinetics of pyruvate dehyrogenase as ATP builds up? a)Vmax will go up b)Vmax will go down c)Km will shift right d)Km will shift left Pyruvate oxidation Step 1: pyruvate decarboxylation CYTOSOL MITOCHONDRION NAD+ NADH + H+ 2 1 Pyruvate 3 CO2 Coenzyme A Acetyl CoA Transport protein Oxidation produces NADH CoA carries 2 carbon unit to citric acid cycle CO2 released Fig. 9-10 AcetylCoA is starting material for Krebs cycle • The citric acid cycle, also called the Krebs cycle, takes place within the mitochondrial matrix (except succinate dehydrogenase which is loosely associated with inside membrane) • The cycle oxidizes organic fuel derived from pyruvate, generating 1 ATP, 3 NADH, and 1 FADH2 per turn • Complete oxidation of glucose CO2 Fig. 9-12-8 CoA for next pyruvate Acetyl CoA CoA—SH NADH +H+ H2O 1 NAD+ 8 Oxaloacetate 2 Malate Citrate Isocitrate NAD+ Citric acid cycle 7 H2O NADH + H+ 3 CO2 Fumarate CoA—SH 6 -Ketoglutarate 4 CoA—SH 5 FADH2 NAD+ FAD Succinate GTP GDP ADP ATP Pi Succinyl CoA NADH + H+ CO2 Pyruvate CO2 NAD+ NADH + H+ Glycolysis 1 glucose 2 Pyruvates 2 NADH & 2ATP CoA Acetyl CoA CoA Pyruvate Decarboxylation 2 pyruvate 2 CO2 2 Acetyl CoA 2 NADH Kreb’s CoA Citric acid cycle FADH2 2 CO2 3 NAD+ 3 NADH + 3 H+ FAD ADP + P i ATP 2 Acetyl 2 turns 1 turn •2 CO2 •3 NADH + H+ •1FADH2 •ATP Total 4 CO2 6 NADH 2 FADH2 2 ATP Before the krebs cycle, most of the free energy from glucose was in: a)Pyruvate b)ATP c)NADH d)FADH After the krebs cycle, most of the free energy from glucose was in: a)CO2 b)ATP c)NADH d)FADH