* Your assessment is very important for improving the work of artificial intelligence, which forms the content of this project
Download (TCA) cycle
Basal metabolic rate wikipedia , lookup
Mitochondrion wikipedia , lookup
Biosynthesis wikipedia , lookup
Photosynthesis wikipedia , lookup
Lactate dehydrogenase wikipedia , lookup
Light-dependent reactions wikipedia , lookup
Adenosine triphosphate wikipedia , lookup
Photosynthetic reaction centre wikipedia , lookup
Fatty acid synthesis wikipedia , lookup
Fatty acid metabolism wikipedia , lookup
Evolution of metal ions in biological systems wikipedia , lookup
NADH:ubiquinone oxidoreductase (H+-translocating) wikipedia , lookup
Electron transport chain wikipedia , lookup
Nicotinamide adenine dinucleotide wikipedia , lookup
Amino acid synthesis wikipedia , lookup
Biochemistry wikipedia , lookup
Glyceroneogenesis wikipedia , lookup
Microbial metabolism wikipedia , lookup
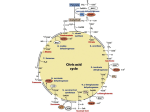
발효화학 (Fermentation Chemistry), Bacterial Physiology and Metabolism Chapter 4. Tricarboxylic acid (TCA) cycle, Electron transport and Oxidative phosphorylation Yong-Cheol Park (http://park.openwetware.org) Department of Advanced Fermentation Fusion Science & Technology F L &B T 1/30 5.1 Oxidative decarboxylation of pyruvate Pyruvate is oxidized by the pyruvate dehydrogenase complex to acetyl-CoA and CO2 reducing NAD+ under aerobic conditions The pyruvate dehydrogenase complex consists of (1) 24 molecules of pyruvate dehydrogenase containing thiamin pyrophosphate (TPP) (2) 24 molecules of dihyrolipoate acetyltransferase containing dihydrolipoate (3) 12 molecules of dihydrolipoate dehydrogenase containing flavin adenine dinucleotide (FAD) In addition, NAD+ and coenzyme A participate in the reaction. F L &B T 5.1 Oxidative decarboxylation of pyruvate This reactions is irreversible, and takes place in the mitochondria in eukaryotic cells. In summarized reaction, Pyruvate dehydrogenase is activated by pyruvate and AMP and repressed by acetyl-CoA, NADH and ATP. F L &B T 5.2 Tricarboxylic acid (TCA) cycle Discovered by Krebs and his colleagues in animal cell. Tricarboxylic acid (TCA) cycle = Krebs cycle = citric acid cycle A main role is the full oxidation of acetyl-CoA to CO2, reducing electron carriers such as NAD+, NADP+ and FAD. These reduced electron carriers (NADH and FADH2) are oxidized through the processes of electron transport and oxidative phosphorylation to form the proton motive force and to synthesize ATP. The TCA cycle provides not only reducing equivalents for ATP synthesis but also precursors for biosynthesis. F L &B T 5.2 Tricarboxylic acid (TCA) cycle F L &B T 5.2 Tricarboxylic acid (TCA) cycle 5.2.1 Citrate synthesis and the TCA cycle Citrate synthase (1) oxaloacetate + acetyl-CoA citrate + CoA-SH (2) Irreversible and exergonic reaction (∆Go = -32.2 kJ/mol acetyl-CoA) (3) Its reverse reaction in the reductive TCA cycle is catalyzed by ATP:citrate lyase (Section 5.4.2) Aconitase : citrate isocitrate Isocitrate dehydrogenase : (1) isocitrate + NAD(P)+ 2-ketoglutarate + CO2 + NAD(P)H + H+ (1) In most bacteria, it is NADP+ dependent. (2) Two separate enzymes are found in eukaryotes using NADP+ and NAD+. 2-Ketoglutarate dehydrogenase complex (1) 2-ketoglutarate + CoA-SH + NAD+ succinyl-CoA + CO2 + NADH + H+ (2) catalyzes an irreversible reaction and another oxidative decarboxylation. (3) The reverse reaction is catalyzed by 2-ketoglutarate synthase in the reductive TCA cycle to fix CO2. (Section 5.4.2) F L &B T 5.2 Tricarboxylic acid (TCA) cycle 5.2.1 Citrate synthesis and the TCA cycle (continued) Succinyl-CoA thiokinase (succinyl-CoA synthetase) (1) succinyl-CoA + ADP + Pi succinate + CoA-SH + ATP (2) An example of substrate-level phosphorylation (3) Guanine triphosphate (GTP) is synthesized in the mitochondrion in eukaryotic cells. Succinate dehydrogenase (1) succinate + FAD fumarate + FADH2 (2) Since the redox potential of fumarate/succinate (-0.03V) is remarkable higher than NAD+/NADH (-0.32V), NAD(P)+ cannot be reduced in this reaction. (3) FAD, the prosthetic group of succinate dehydrogenase, is reduced. Fumarase (fumarate hydratase) (1) fumarate + H2O malate Malate dehydrogenase (1) malate + NAD+ oxaloacetate + NADH + H+ F L &B T 5.2 Tricarboxylic acid (TCA) cycle 5.2.1 Citrate synthesis and the TCA cycle (continued) In summary, The reduced electron carriers (NADH, FADH2) channel electrons to the electron transport chain to synthesize ATP through the proton motive force (Section 5.8). 5.2.2 Regulation of the TCA cycle It is regulated by the energy status of the cell and the availability of biosynthetic precursors. In addition, oxygen regulates the TCA cycle since the reduced electron carriers are recycled, consuming oxygen as the electron acceptor. Oxygen controls the expression of genes for TCA cycle enzymes (1) Facultative anaerobes do not synthesize 2-ketoglutarate dehydrogenase under anaerobic conditions without alternative electron acceptors such as nitrate. (2) In G(-) bacteria (Escherichia coli), the regulatory proteins FNR and Arc regulate the transcription of many genes for aerobic and anaerobic metabolism. F L &B T 5.2 Tricarboxylic acid (TCA) cycle 5.2.2 Regulation of the TCA cycle (continued) Citrate synthase is regulated to control the TCA cycle. (1) Repressed with the accumulation of NADH and ATP or 2-ketoglutarate. (2) G(-) bacteria have two different citrate synthase enzymes, one repressed by NADH and the other unaffected. (3) G(+) bacteria have only one enzyme which is not repressed by NADH but by ATP. (4) In some bacteria, AMP activates the citrate synthase and NADH inhibits it. F L &B T 5.3 Replenishment of TCA cycle intermediates For efficient operation of the TCA metabolism, the intermediates used for biosynthesis should be replenished. Otherwise the concentration of oxaloacetate would be too low to start the TCA cycle. Oxaloacetate is replenished through a process called the anaplerotic sequence (Figure 5.3). F L &B T 5.3 Replenishment of TCA cycle intermediates 5.3.1 Anaplerotic sequence Pyruvate carboxylase in many organisms, from bacteria to mammals (1) This enzyme requires biotin. (2) Activated by acetyl-CoA in many bacteria, as in animals. (3) But some bacteria such as Pseudomonas aerugenosa have a pyruvate carboxylase that is not activated by acetyl-CoA. PEP carboxylase in many bacteria including Escherichia coli, Bacillus anthractis, Thiobacillus novellus, Acetobacter xylinum and Azotobacter vinelandii, (1) A PEP carboxylase mutant of Escherichia coli is unable to grow in a glucosemineral salts medium, but can grow when supplemented with TCA cycle intermediates. (2) E. coli has PEP carboxylase as the anaplerotic sequence,, not pyruvate carboxylase. F L &B T 5.3 Replenishment of TCA cycle intermediates 5.3.2 Glyoxylate cycle Bacteria growing on carbon sources that are not metabolized through pyruvate or PEP cannot replenish TCA cycle intermediates through the anaplerotic sequence And they need more oxaloacetate to produced PEP for gluconeogenesis.. The glyoxylate cycle : metabolism for acetate utilization through acetyl-CoA F L &B T 5.3 Replenishment of TCA cycle intermediates 5.3.2 Glyoxylate cycle (continued) By isocitrate lyase and malate synthase in conjunction with TCA cycle enzymes, 2 molecules of acetyl-CoA are converted to q molecule of malate. A molecule of acetyl-CoA is converted to isocitrate through the TCA cycle . Isocitrate lyase: Succinate is oxidized to oxaloacetate through the TCA cycle. Malate synthase: In summary, F L &B T 5.3 Replenishment of TCA cycle intermediates 5.3.2.1 Regulation of the glyoxylate cycle The TCA cycle supplies energy while the glyoxylate cycle supplied precursors for biosynthesis when an organism is growing on carbon sources that are not metabolized through pyruvate or PEP. Accumulation of acetyl-CoA induces the transcription of the genes coding for isocitrate lyase and malate synthase. The activity of isocitrate lyase is inhibited by PEP, succinate and pyruvate. Isocitrate is a branch point of the TCA cycle and glyoxylate cycle. Activities of isocitrate dehydrogenase and isocitrate lyase should be regulated to control the flux. The bacteria solve this problem through differences in the substrate affinity and by controlling the activity of the enzyme with the higher activity. (1) The dehydrogenase has a much higher affinity (Km = 1~2 μM) for the substrate than that of the lyase (Km = 3 mM). (2) When TCA cycle is needed to generate energy, isocitrate dehydrogenase is activated. But this enzyme is inactivated when precursors for biosynthesis should be synthesized through the glyoxylate cycle. F L &B T 5.3 Replenishment of TCA cycle intermediates 5.3.2.1 Regulation of the glyoxylate cycle (continued) An enzyme with kinase-phosphatase activity controls the activity of isocitrate dehydrogenase. F L &B T 5.3 Replenishment of TCA cycle intermediates 5.3.2.1 Regulation of the glyoxylate cycle (continued) Dephosphorylation active form of isocitrate dehydrogenase Phosphorylation inactive form of isocitrate dehydrogenase Dephosphorylation to induce flux through the TCA cycle (1) When metabolic intermediates such as isocitrate, PEP, OAA, 2-KG and 3-PG are in high concentration (2) When more ATP is needed with the accumulation of AMP and ADP Phosphorylation to induce flux through the glyoxylate cycle (1) When NADPH accumulates, isocitrate is directed toward the glyoxylate cycle. The TCA cycle is controlled by citrate synthase activity. Isocitrate dehydrogenase activity is regulated to control the glyoxylate cycle. F L &B T