* Your assessment is very important for improving the work of artificial intelligence, which forms the content of this project
Download Database Modeling in Bioinformatics
Transcriptional regulation wikipedia , lookup
Gene regulatory network wikipedia , lookup
Paracrine signalling wikipedia , lookup
Non-coding DNA wikipedia , lookup
Metalloprotein wikipedia , lookup
Magnesium transporter wikipedia , lookup
Biochemical cascade wikipedia , lookup
G protein–coupled receptor wikipedia , lookup
Point mutation wikipedia , lookup
Expression vector wikipedia , lookup
Silencer (genetics) wikipedia , lookup
Signal transduction wikipedia , lookup
Protein purification wikipedia , lookup
Gene expression wikipedia , lookup
Interactome wikipedia , lookup
Ancestral sequence reconstruction wikipedia , lookup
Artificial gene synthesis wikipedia , lookup
Endogenous retrovirus wikipedia , lookup
Protein structure prediction wikipedia , lookup
Nuclear magnetic resonance spectroscopy of proteins wikipedia , lookup
Western blot wikipedia , lookup
Proteolysis wikipedia , lookup
Homology modeling wikipedia , lookup
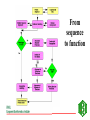
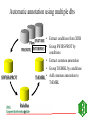
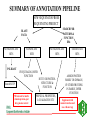
GENOME ANNOTATION AND FUNCTIONAL GENOMICS The protein sequence perspective GENOME ANNOTATION • Two main levels: – STRUCTURAL ANNOTATION – Finding genes and other biologically relevant sites thus building up a model of genome as objects with specific locations – FUNCTIONAL ANNOTATION – Objects are used in database searches (and expts) aim is attributing biologically relevant information to whole sequence and individual objects WHY PROTEIN RATHER THAN DNA? • • • • • • • • Larger alphabet -more sensitive comparisons Protein sequences lower signal to noise ratio Less redundancy and no frameshifts Each aa has different properties like size, charge etc Closer to biological function 3D structure of similar proteins may be known Evolutionary relationships more evident Availability of good, well annotated protein sequence and pattern databases Large-scale genome analysis projects • Rate-limiting step is annotation • Whole genome availability provides context information • Main goal is to bridge gap between genotype and phenotype Definitions of Annotation • Addition of as much reliable and up-to-date information as possible to describe a sequence • Identification, structural description, characterisation of putative protein products and other features in primary genomic sequence • Information attached to genomic coordinates with start and end point, can occur at different levels • Interpreting raw sequence data into useful biological information ANNOTATION/FUNCTION CAN BE MAPPED TO DIFFERENT LEVELS: ORGANISM -phenotypic function (morphology, physiology, behaviour, environemntal response), context NB CELLULAR -metabolic pathway, signal cascades, cellular localisation. Context dependent MOLECULAR -binding sites, catalytic activity, PTM, 3D structure DOMAIN SINGLE RESIDUE Annotation is the description of: • • • • • • • Function(s) of the protein Post-translational modification(s) Domains and sites Secondary structure Quaternary structure Similarities to other proteins Disease(s) associated with deficiencie(s) in the protein • Sequence conflicts, variants, etc. Additional information for proteins • • • • • • • • • ALTERNATIVE PRODUCTS CATALYTIC ACTIVITY COFACTOR DEVELOPMENTAL STAGE DISEASE DOMAIN ENZYME REGULATION FUNCTION INDUCTION • • • • • • PATHWAY PHARMACEUTICALS POLYMORPHISM PTM SIMILARITY SUBCELLULAR LOCATION • SUBUNIT • TISSUE SPECIFICITY Amino-acid sites are: • • • • • • • • Post-translational modification of a residue Covalent binding of a lipidic moiety Disulfide bond Thiolester bond Thioether bond Glycosylation site Binding site for a metal ion Binding site for any chemical group (co-enzyme, prosthetic group, etc.) Regions: • • • • • • SIGNAL SEQUENCE TRANSIT PEPTIDE PROPEPTIDE CHAIN PEPTIDE DOMAIN • • • • • ACTIVE SITE DNA BIND SITE METAL BIND SITE MOLECULE BIND SITE TRANSMEMBRANE Annotation sources: • publications that report new sequence data • review articles to periodically update the annotation of families or groups of proteins • external experts • protein sequence analysis Approaches to functional annotation: Automatic annotation (sequence homology, rules, transfer info from pdb) Automatic classification (pattern databases, clustering, structure) Automatic characterisation (functional databases) Context information (comparitive genome analysis, metabolic pathway databases) Experimental results (2D gels, microarrays) Full manual annotation (SWISS-PROT style) PROTEIN SEQUENCE ANALYSIS • Protein sequence can come from gene predictions, literature or peptide sequencing • Analysis on different levels: – molecular – cellular – organism • Simplest case- match for whole sequence in databasedetermination of structure and function • In between- partial matches across sequence to diverse or hypothetical proteins • Difficult case- no match, have to derive information from amino acid properties, pattern searches etc From sequence to function Predicting function from sequence similarity • Orthologues- arose from speciation, same gene in different organisms -can have <30% homology • Paralogues- from duplication within a genome, second copy may have new or changed function (difficult to distinguish between otho- and paralogues unless whole genome is available) • Equivalog- proteins with equivalent functions • Analog- proteins catalyzing same reaction but not structurally related • Some enzymes may have seq similarity simply because common catalytic site, substrate, pathway. TYPES OF HOMOLOGY Superfamily PROTEIN/DOMAIN Duplication within species Paralogues may have different functions A B Speciation Orthologues may have different functions, if same - Equivalogs B1 B2 Sequence homology in genomes When you do a whole genome BLAST search there is a general pattern of results: Maverick genes shared with some other species Common genes Incorrect predictions Maverick genes unique function Maverick genes tend to diverge more frequently than core genes Using homology information for automatic annotation- automatic annotation of TrEMBL as an example Requirements for automatic annotation • Well-annotated reference database (eg SWISS-PROT) • Highly reliable diagnostic protein family signature database with the means to assign proteins to groups (eg CDD, InterPro, IProClass) • A RuleBase to store and manage the annotation rules, their sources and their usage Direct Transfer XDB • Search target • Transfer annotation to target database • Example: Target FASTA against sequence database and transfer of DE line of best hit Multiple Sources • Usually more than one external database is used • Combine the different results XDB Target Conflicts • • • • Contradiction Inconsistencies Synonyms Redundancy Translation • Use a translator to map XDB language to target language XDB Target Translation Examples • ENZYME TrEMBL CA L-ALANINE=D-ALANINE CC -!- CATALYTIC ACTIVITY: L-ALANINE= CC D-ALANINE. • PROSITE TrEMBL /SITE=3,heme_iron FT METAL IRON • Pfam TrEMBL FT DOMAIN zf_C3HC4 FT ZN_FING C3HC4-TYPE • • • • • • Demands on a system for automated data analysis and annotation Correctness Scalability Updateable Low level of redundant information Completeness Standardized vocabulary What do we have? • • • • SWISS-PROT RuleBase TrEMBL PROSITE (and Pfam, PRINTS, ProDom, SMART, Blocks etc) • SWISS-PROT/TrEMBL/RuleBase in Oracle Standardized transfer of annotation from characterized proteins in SWISS-PROT to TrEMBL entries • TrEMBL entry is reliably recognized by a given method as a member of a certain group of proteins • corresponding group of proteins in SWISS-PROT shares certain annotation • common annotation is transferred to the TrEMBL entry and flagged as annotated by similarity Automatic annotation information flow • Get information necessary to assign proteins to groups eg using InterPro or other biological or family informationstore in RuleBase • Group proteins in SWISS-PROT by these conditions • Extract common annotation shared by all these proteinsstore in RuleBase • Group unannotated sequences by the conditions • Transfer common annotation flagged with evidence tags • Note: can add taxonomic constraints Extract Reference Entries • Use XDB to extract entries from standard database • Example: Pfam Pfam:PF00509 Hemagglutinin SWISS-PROT TrEMBL HEMA_IAVI7/P03435 HEMA_IANT6/P03436 HEMA_IAAIC/P03437 HEMA_IAX31/P03438 HEMA_IAME2/P03439 HEMA_IAEN7/P03440 HEMA_IABAN/P03441 HEMA_IADU3/P03442 HEMA_IADA1/P03443 HEMA_IADMA/P03444 HEMA_IADM1/P03445 HEMA_IADA2/P03446 HEMA_IASH5/P03447 Extract Common Annotation 132 131 125 6 131 130 130 125 125 75 31 131 102 1 130 107 102 entries read ID HEMA_XXXXX DE HEMAGGLUTININ PRECURSOR. DE HEMAGGLUTININ. GN HA CC -!- FUNCTION: HEMAGGLUTININ IS RESPONSIBLE FOR ATTACHING THE CC VIRUS TO CELL RECEPTORS AND FOR INITIATING INFECTION. CC -!- SUBUNIT: HOMOTRIMER. EACH OF THE MONOMER IS FORMED BY TWO CC CHAINS (HA1 AND HA2) LINKED BY A DISULFIDE BOND. DR HSSP; P03437; 1HGD. DR HSSP; P03437; 1DLH. KW HEMAGGLUTININ; GLYCOPROTEIN; ENVELOPE PROTEIN KW SIGNAL KW COAT PROTEIN; POLYPROTEIN; 3D-STRUCTURE FT CHAIN HA1 CHAIN. FT CHAIN HA2 CHAIN. FT SIGNAL Store Common Annotation • Store the used conditions and the extracted common annotation in a separate database XDB SWISS-PROT TrEMBL RuleBas e RULES • Rules describe: – the content of the annotation to be transferred (ACTIONS), – the CONDITIONS which the target TrEMBL entry must fulfill in order to allow transfer of the annotation. • Rules uniquely describe or delineate a set of SWISSPROT entries. – The common annotation in these entries is transferred to TrEMBL. // #RULE RU000482 #DATE 2001-01-11 #USER OPS$WFL #PACK PROSITE ?PSAC PS00449 ACTIONS ?EMOT PS00449 } CONDITIONS !ECNO 3.6.1.34 !SPDE ATP synthase A chain !CCFU KEY COMPONENT OF THE PROTON CHANNEL; IT MAY PLAY A DIRECT ROLE IN THE TRANSLOCATION OF PROTONS ACROSS THE MEMBRANE (BY SIMILARITY) !CCSU F-TYPE ATPASES HAVE 2 COMPONENTS, CF(1) - THE CATALYTIC CORE - AND CF(0) - THE MEMBRANE PROTON CHANNEL. CF(1) HAS FIVE SUBUNITS: ALPHA(3), BETA(3), GAMM A(1), DELTA(1), EPSILON(1). CF(0) HAS THREE MAIN SUBUNITS: A, B AND C (BY SIMILARITY) !CCLO INTEGRAL MEMBRANE PROTEIN (By Similarity) !CCSI TO THE ATPASE A CHAIN FAMILY !SPKW CF(0) !SPKW Hydrogen ion transport !SPKW Transmembrane // Add Annotation to Target • Use conditions to extract entries from TrEMBL • Add common annotation to the entries XDB SWISS-PROT TrEMBL RuleBas e Automatic annotation using multiple dbs • Extract conditions from XDB ENZYME Pfam • Group SWISS-PROT by INTERPRO PROSITE conditions • Extract common annotation • Group TrEMBL by conditions TrEMBL • Add common annotation to TrEMBL SWISS-PROT RuleBas e Using tree structure of InterPro RU000652 with additional condition connected by ‘AND’ // #RULE RU000652 #DATE 2001-01-11 #USER OPS$WFL #PACK PROSITE ?IPRO IPR002379 ?PSAC PS00605 Additional condition (parent signature) ?EMOT PS00605 !SPDE ATP synthase C chain (Lipid-binding protein) (Subunit C) !ECNO 3.6.1.34 !CCSU F-TYPE ATPASES HAVE 2 COMPONENTS, CF(1) - THE CATALYTIC CORE - AND CF(0) - THE MEMBRANE PROTON CHANNEL. CF(1) HAS FIVE SUBUNITS: ALPHA(3), BETA(3), GAMMA(1), DELTA(1), EPSILON(1). CF(0) HAS THREE MAIN SUBUNITS: A, B AND C (By Similarity) !CCSI TO THE ATPASE C CHAIN FAMILY !SPKW CF(0) !SPKW Hydrogen ion transport !SPKW Lipid-binding !SPKW Transmembrane // Condition types • Signature hits: - Prosite, Prints, Pfam, Prodom •Taxonomy: - Broad groups like: Archaea Bacteriophage Eukaryota Prokaryota Eukaryotic viruses - more specific such as species •Organelle •Conditions •Negated conditions Rule-building •Grouping and extraction of common annotation: - semi automated but involves manual data-mining assisted by perl/shell scripts. •Algorithmic data-mining: - fully automated. - fast. - exhaustive exploration of condition-set/annotation search-space . - non-biological, validity of rules being assessed by comparison with semi-manual approach. Advantages of this method • Uses reliable ref database, prevents propagation of incorrect annotation • Using common annotation of multiple entries, lower over-prediction than from best hit of BLAST • Can standardize annotation and nomenclature of target sequences, since reference is standardized • Can have different levels of common annotation from different levels of family hierarchy • Independent of multi-domain organisation • Evidence tags allow for easy tracking and updating Pitfalls of automatic functional analysis • Multifunctional proteins- genome projects often assign single function, info is lost in homology search • Hypothetical proteins (40% oRFs unknown), and poorly or even wrongly annotated proteins • No coverage of position-specific annotation eg active sites • Current methods provide only a phrase describing some properties of the unknown protein It is important to have evidence for all annotation added EVIDENCE TAGS Predicting function from non-homology • Look at position of genes relative to others, compare with other organisms • Can still build up rules from annotated sequences using information you have on other features like fold, physical properties etc. • Use physical properties and known attributes Protein functions from regions • Active sites- short, highly conserved regions • Loops- charged residues and variable sequence • Interior of protein- conservation of charged amino acids Protein functions from specific residues • C • • • • DE G H KR • P • SR • ST disulphide-rich, metallothionein, zinc fingers acidic proteins (unknown) collagens histidine-rich glycoprotein nuclear proteins, nuclear localisation collagen, filaments RNA binding motifs mucins • Polar (C,D,E,H,K,N,Q,R,S,T) - active sites • Aromatic (F,H,W,Y) - protein ligandbinding sites • Zn+-coord (C,D,E,H,N,Q) - active site, zinc finger • Ca2+-coord (D,E,N,Q) - ligand-binding site • Mg/Mn-coord (D,E,N,S,R,T) - Mg2+ or Mn2+ catalysis, ligand binding • Ph-bind (H,K,R,S,T) - phosphate and sulphate binding Supplement annotation with Xrefs to other databases • • • • DDBJ/EMBL/GenBank Nucleotide Sequence Database PDB Genomic databases (FlyBase, MGD, SGD) 2D-Gel databases (ECO2DBASE, SWISS-2DPAGE, Aarhus/Ghent, YEPD, Harefield), Gene expression data • Specialized collections (OMIM, InterPro, PROSITE, PRINTS, PFAM, ProDom, SMART, ENZYME, GPCRDB, Transfac, HSSP) Approaches to functional annotation: Automatic annotation (sequence homology, rules, transfer info from pdb) Automatic classification (pattern databases, clustering, structure) Automatic characterisation (functional databases) Context information (comparitive genome analysis, metabolic pathway databases) Experimental results (2D gels, microarrays) Full manual annotation (SWISS-PROT style) AUTOMATIC CLASSIFICATION Annotation can by using Clustering methods eg CluSTR (EBI), and pattern searches (InterPro etc)classification of proteins into different families AUTOMATIC CHARACTERIZATIONFUNCTIONAL ANNOTATION SCHEMES • • • • First attempt –Riley classification of E.coli Genome sequencing projects driving force Need standardised system and vocabulary Functional schemes normally hierarchies of different levels of generalisation Databases for Functional Information • KEGG -Kyoto encyclopedia of genes and genomes – (http://www.genome.ad.jp/kegg/) – Links genome information (GENES database) to high order functional information stored in PATHWAY database. – Also has LIGAND database for chemical compounds, molecules and reactions. • PEDANT -Protein Extraction, Description and Analysis Tool – (http://pedant.gsf.de/) – Annotation for complete and incomplete genomes eg. List of ORFs, EC numbers, functional categories, list seqs with homologs, gene clusters, domain hits, TM, structure links, search facility for sequences etc • WIT –What is there – ( http://www.cme.msu.edu/WIT) – Database of metabolic pathways, can text search for ORFs, pathways, enzymes Databases for Functional Information (2) • COG -Clusters of Orthologous Groups – – – – (http://www.ncbi.nlm.nih.gov/COG) Phylogenetic classification of proteins encoded in complete genomes. Contains 2791 COGs including 30 genomes. COGs thought to contain orthologous proteins, classified into broad functional categories (transciption, replication, cell division). – COGNITOR assigns proteins to COGs based on best-hit, divides multi-domain proteins – Can compare results with complete genomes, look for missing functions • GO –Gene Ontology – (http://www.geneontology.org) – Standard vocabulary first used for mouse, fly and yeast – Three ontologies: molecular function, biological process and cellular component Databases for Functional Information (3) • MIPS:MYGD FunCat –Functional catalogue (yeast) http://www.mips.biochem.mpg.de/proj/yeast • EcoCyc -Encyclopedia of E. coli Genes and Metabolism http://ecocyc.doubletwist.com/ecocyc/ecocyc.html • Enzyme database http://wwwexpasy.ch/sprot/enzyme.html • TIGR –Gene identification list http://www.tigr.org/tdb/mdb/mdb.html All schemes have different depths, breadths and resolutions Schemes need to be applicable to all organisms, standardized for comparisons and permit multiple assignments Assignment of function • Use a combination of databases, especially those with standardised functional information • Search function databases with sequences to find matches -assign function eg PENDANT, PIR superfamilies, COGs, GO (via InterPro) FUNCTIONAL CLASSIFICATION USING INTERPRO • InterPro classification with 3-4 letter codes • Mapping of InterPro entries to GO • GO- Gene Ontology (SGD, FB & MGD) universal ontology for – molecular function – biological process – cellular component Classification of IPRs CGD Cell cycle/growth/death -CGDc cell cycle/division -CGDg cell growth/development -CGDd cell death CYS Cytoskeletal/structural -CYSc cytoskeletal -CYSs structural -CYSv virus coat/capsid protein DPT Defense/pathogenesis/toxin DRG DNA/RNA-binding/regulation DRM DNA/RNA metabolism -DRMr DNA repair/recombination -DRMp DNA replication -DRMm DNA/RNA modification -DRMt transcription/translation -DRMb ribosomal protein MET Metabolism -METs substrate metabolism -METe electron transfer -METa amino acid metabolism -METn nucleic acid metabolism -METm metal binding proteins OTH Other functions -OTHm cell motility -OTHt transposition -OTHa cell adhesion -OTHg miscellaneous functions -OTHh hormones -OTHi immune-response proteins -OTHf multifunctional proteins -OTHo multifunctional domains PFD Protein folding & degradation -PFDc chaperone -PFDp protease/endopeptidase -PFDi protease inhibitor PRG Protein-binding/other regulation -PRGg GPCRs -PRGr other receptors -PRGo other regulation STD Signal transduction -STDk sig transduction -STDp sig transduction -STDr sig transduction -STDs sig transduction -STDc cell signalling kinases phosphatases response reg sensors TRS Transport and secretion -TRSt transport (subtrates) -TRSi transport (ions) -TRSs secretion -TRSr carrier proteins UNK Unknown function Pie charts of whole proteome analysis of 4 organisms Unknown Transport Signal transduction Protein folding/degradation Miscellaneous Structural Defense/Pathogenesis Cell cycle DNA/RNA metabolism Regulation Metabolism Distribution of protein functions 25 20 15 M. tuberculosis 10 E. coli B. subtilis S. cerevisae 5 0 S. cerevisae B. subtilis E. coli M. tuberculosis GENOME ANNOTATION TOOLS • Oakridge Genome Annotation Channel (http://compbio.ornl.gov/channel/) • ENSEMBL (http://ensembl.ebi.ac.uk) • Artemis (http://www.sanger.ac.uk/Software/Artemis) Sequence viewer and annotation tool • GeneQuiz (http//www.sander.ebi.ac.uk/genequiz/) System for automated annotation of sequences, web access required • Genome Annotation Assessment Project (GASP1) (http://www.fruitfly.org/GASP1) PEDANT SYSTEM Layer 1 bioinformatics tools PSI-BLAST IMPALA PREDATOR CLUSTALW TMAP SIGNALP SEG PROSEARCH COILS HMMER Databases for searching MIPS PROSITE BLOCKS PIR COGS Layer 2 database to store information -MySQL Layer 3 user interface to display results parser of results Manual annotation tool Programs written in Perl5 and some in C++ -portable. Processing of one sequence takes about 3 minutes Summary of protein sequence annotation • • • • • • Mask compositionally-biased and coiled-coil regions Identify transmembrane regions, signal peptides, GPI anchors Predict secondary structure Look for known domains from protein pattern databases Search sequence database for similar sequences If no or few results search with subsequences, do iterative searches • Functional annotation: consider function of each domain present, annotation from database homologs, function from hits with 3D structure SUMMARY OF ANNOTATION PIPELINE NEW SEQUENCES FROM SEQUENCING PROJECT SEARCH FOR PATTERNS & FUNCTION DBs BLAST/ FASTA NO SIGNIFICANT HITS PSI-BLAST SIGNIFICANT HITS IF EQUIVALOG, INFER FUNCTION Search SCOP NB look out for multidomain proteins, put into genome context NO SIGNIFICANT HITS HIT TO 3D PROTEINSTRUCTURE & FUNCTION PHYSICAL PROPERTIES, LOCALISATION ETC SIGNIFICANT HITS ASSIGN PROTEIN FAMILY OR DOMAIN, CF OTHER PROTEINS IN FAMILY, INFER FUNCTION Supplement with manual curation and use evidence tags LIMITS OF PROTEIN SEQUENCE ANALYSIS • Predicting function from sequence requires another sequence to be mapped to a function –many hypothetical proteins in db and UPFs • If sequence homologues are found, may not be functional homologues -qualitative rather than quantitative process - orthologues may have different functions -enzyme homologues may be inactive -equivalent functions may use different genes, not orthologue • Analogy can infer molecular function, but not necessarily cellular function LIMITS OF PROTEIN SEQUENCE ANALYSIS (2) • Databases are biased in sequence and aa composition and search is dependent on size • If no homology found- limited amount of information can be inferred • Incorrect annotation can be propagated when similarity is over part on sequence not used in annotation • No answers to tissue-specificity, binding of ligands, relationship between genotype and phenotype LIMITS OF PROTEIN SEQUENCE ANALYSIS (3) • Need additional information from experiments, eg can predict glycosylation sites, but not kind of sugar attached • Problem with multidomain proteins (assign orthology on basis of domains or domain composition of whole protein?) -check also known domain architectures and their taxonomic limitations Using different approaches to functional annotation: Status for SPTR • Automatic annotation (RuleBase): 20% of all protein sequences/20% of all new sequences • Automatic classification (InterPro, CluSTr, Structure): 60% of all protein sequences/60% of all new sequences • Automatic characterisation (GO): 40% of all protein sequences/40% of all new sequences • Full annotation (SWISS-PROT style): 20% of all protein sequences/5% of all new sequences Using different approaches to functional annotation: Future for SPTR • Automatic annotation (RuleBase): 50% of all protein sequences in 2004 • Automatic classification (InterPro, CluSTr, Structure): 90% of all protein sequences in 2004 • Automatic characterisation (GO): 70% of all protein sequences in 2004 • Full annotation (SWISS-PROT style): 10% of all protein sequences in 2004 IMPORTANT TO NOTE: • DON’T COMPLETELY TRUST COMPUTER RESULTS • CHECK LITERATURE • CONFIRM WITH WETLAB WORK- mutational analysis gives valuable info about function • COMPROMISE BETWEEN OVER AND UNDERPREDICTIONS -overpredictions can be checked by curators, easier to delete than find missing info.