* Your assessment is very important for improving the workof artificial intelligence, which forms the content of this project
Download File - Biology with Radjewski
Nucleic acid analogue wikipedia , lookup
Ancestral sequence reconstruction wikipedia , lookup
Gene regulatory network wikipedia , lookup
Paracrine signalling wikipedia , lookup
Gene expression wikipedia , lookup
Expression vector wikipedia , lookup
Magnesium transporter wikipedia , lookup
G protein–coupled receptor wikipedia , lookup
Signal transduction wikipedia , lookup
Point mutation wikipedia , lookup
Ribosomally synthesized and post-translationally modified peptides wikipedia , lookup
Metalloprotein wikipedia , lookup
Peptide synthesis wikipedia , lookup
Protein purification wikipedia , lookup
Nuclear magnetic resonance spectroscopy of proteins wikipedia , lookup
Amino acid synthesis wikipedia , lookup
Biosynthesis wikipedia , lookup
Genetic code wikipedia , lookup
Western blot wikipedia , lookup
Two-hybrid screening wikipedia , lookup
Interactome wikipedia , lookup
Protein–protein interaction wikipedia , lookup
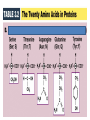
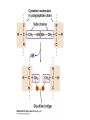
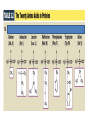
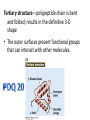
3.2 Proteins Mini Lecture Radjewski • Major functions of proteins: Enzymes—catalytic proteins Add to notes • Defensive proteins (e.g., antibodies) • Hormonal and regulatory proteins—control physiological processes • Receptor proteins—receive and respond to molecular signals • Storage proteins store amino acids • Structural proteins—physical stability and movement • Transport proteins carry substances (e.g., hemoglobin) • Genetic regulatory proteins regulate when, how, and to what extent a gene is expressed Protein monomers are amino acids. • Amino and carboxylic acid functional groups allow them to act as both acid and base. • The R group differs in each amino acid. PDQ 14 continued… and PDQ 17 • Only 20 amino acids occur extensively in the proteins of all organisms. • They differ by their R groups. – The R groups determines the shape of the protein, which determines the possible function of the protein • Oligopeptides or peptides—short polymers of 20 or fewer amino acids (some hormones and signaling molecules) • Polypeptides or proteins range in size from insulin, which has 51 amino acids, to huge molecules such as the muscle protein titin, with 34,350 amino acids. How to make a protein • Amino acids are linked in condensation reactions to form peptide linkages or bonds. Primary structure of a protein—the sequence of amino acids Secondary structure— regular, repeated spatial patterns in different regions, resulting from hydrogen bonding • • α (alpha) helix— right-handed coil • β (beta) pleated sheet—two or more polypeptide chains are extended and aligned Tertiary structure—polypeptide chain is bent and folded; results in the definitive 3-D shape • The outer surfaces present functional groups that can interact with other molecules. Interactions between R groups determine tertiary structure. • Disulfide bridges hold a folded polypeptide together • Hydrogen bonds stabilize folds • Hydrophobic side chains can aggregate • van der Waals interactions between hydrophobic side chains • Ionic interactions • Quaternary structure—two or more polypeptide chains (subunits) bind together by hydrophobic and ionic interactions, and hydrogen bonds. • These weak interactions allow small changes that aid in the protein’s function. Factors that can disrupt the interactions that determine protein structure (denaturing): • Temperature • Concentration of H+ • High concentrations of polar substances • Nonpolar substances PDQ 23 • Primary – peptide bonds • Secondary – hydrogen bonds • Tertiary – disulfide brides, hydrogen bonding, hydrophobic interactions, Van der Waals, Ionic interactions • Quaternary – same as tertiary