* Your assessment is very important for improving the workof artificial intelligence, which forms the content of this project
Download Investigating the genetic and molecular mechanisms underlying
Survey
Document related concepts
Biochemical switches in the cell cycle wikipedia , lookup
Cell culture wikipedia , lookup
Cellular differentiation wikipedia , lookup
Organ-on-a-chip wikipedia , lookup
Cell growth wikipedia , lookup
Extracellular matrix wikipedia , lookup
Phosphorylation wikipedia , lookup
Endomembrane system wikipedia , lookup
Cytokinesis wikipedia , lookup
Signal transduction wikipedia , lookup
Protein phosphorylation wikipedia , lookup
Biochemical cascade wikipedia , lookup
Transcript
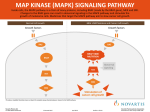
Award ID: RP160023 Project Title: Investigating the genetic and molecular mechanisms underlying RAS/ERK substrate network Award Mechanism: Individual Investigator Principal Investigator: Arur, Swathi Entity: The University of Texas M.D. Anderson Cancer Center Lay Summary: The RAS-Extracellular regulated kinase (ERK) pathway is critical for normal animal development, coordinating different processes like cell division, cell growth, cell death, and cellfate specification. Alterations in this pathway resulting in too much activity of RAS or ERK results in multiple types of cancers. ERK in turn, controls the different biological processes by controlling proteins, which it phosphorylates. These proteins are ERK targets that need to be phosphorylated to execute specific processes. However, during excessive ERK activation, some or all of these tissue specific ERK targets are also inappropriately phosphorylated, and result in mis-regulation of the processes, probably leading to the disease. These are likely the ‘drivers’ of the disease. Thus, understanding, when and where these regulators of ERK activity are phosphorylated during normal development is key to identifying when they come on aberrantly. These thus, form useful biomarkers for early detection of tumorigenesis. We have already shown that phosphorylation of these proteins by ERK is conserved in humans, as phosphorylated Dicer (identified by us as a target of ERK) antibody can be used to detect early stage endometrial tumors. We propose that understanding the genetic and molecular mechanisms governing how these phosphorylated proteins function in vivo, in a whole animal context, independently and together to regulate RAS-ERK dependent events such as cell growth, cell death and cell divisions is a critical first step to designing fruitful interventions. In so doing, our studies should accelerate our ability to detect, to treat, and, hopefully, ultimately to defeat different forms of cancer that are driven by mutations in oncogenic RAS pathway.