* Your assessment is very important for improving the work of artificial intelligence, which forms the content of this project
Download Diffusion, Osmosis, and Movement across a Membrane
Membrane potential wikipedia , lookup
Cell nucleus wikipedia , lookup
Cytoplasmic streaming wikipedia , lookup
Extracellular matrix wikipedia , lookup
Cell encapsulation wikipedia , lookup
Cellular differentiation wikipedia , lookup
Cell culture wikipedia , lookup
Signal transduction wikipedia , lookup
Cell growth wikipedia , lookup
Organ-on-a-chip wikipedia , lookup
Cytokinesis wikipedia , lookup
Cell membrane wikipedia , lookup
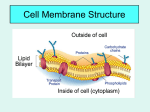
Name: _______________________________________________ Date: __________________________ Period: ______ Unit 5, Part 2 Notes – Cell Membrane and Transport Mrs. Krouse, AP Biology Structure of the Cell Membrane (the Fluid Mosaic Model) Phospholipids- give fluidity / flexibility to the membrane because they have unsaturated fatty acid tails that are bent to space the phospholipids apart in the membrane o Phospholipids form a double layer with the hydrophilic (polar / waterloving) heads facing the water on the outside and the inside of the cell and the hydrophobic (nonpolar / water fearing) tails on the inside of the membrane so they can separate themselves from the water on the outside and the inside of the cell o The membrane tends to be more flexible at higher temperatures, when the fatty acid tails have more carbon to carbon double bonds (i.e. when the fatty acid is MORE unsaturated), and when there are fewer cholesterol (a type of steroid) molecules stuck between the phospholipids Proteins- each cell membrane has a particular pattern of protein molecules scattered across the surface of the cell membrane like a mosaic painting. There are two types of membrane proteins based on their position within the membrane—integral proteins and peripheral proteins. o Integral- these membrane proteins are stuck all the way through the membrane (i.e. they are embedded in the membrane); the portion of the protein that comes into contact with the phospholipid heads is hydrophilic, and the portion of the protein that comes into contact with the phospholipid tails is hydrophobic. o Peripheral- these membrane proteins are stuck either on the inner or outer surface of the membrane (i.e. they are not “embedded” all the way through) Captions for pictures to the right… 1. This is an enzyme (used to speed up a chemical reaction that changes reactants into products) that just happens to be on the cell membrane 2. This is a carrier protein (used for facilitative diffusion) or protein pump (used for active transport) that must bind to the molecule it is transporting before it changes shape to release the molecule on the other side of the membrane 3. This is a glycoprotein (a protein with a carbohydrate chain attached) that is used to signify a cell’s identity (ex: a bone cell vs. a blood cell) and helps cells to recognize one another 4. These proteins are used to attach two cells to one another. 5. This is a protein channel (used in facilitated diffusion) that helps to transport charged particles or large, polar molecules 6. This protein helps to bind to and anchor the cytoskeleton. 1 Carbohydrates o Attach to phospholipid to form a glycolipid or a protein to form a glycoprotein on the outer surface of the cell membrane o These carbohydrate chains serve as markers to distinguish one cell from another (i.e. a red blood cell has different carbohydrate chains on its surface than a bone cell) and assist with recognition between cells Simple Diffusion Spontaneous movement of particles from an area of high concentration to an area of low concentration (i.e. particles move WITH / DOWN their concentration gradient… note: a concentration gradient means there is a difference in concentration across an area) Does not require energy so it is called a passive process Occurs via random kinetic movement (random movement of particles, which is faster at higher temperatures) Net diffusion stops when the concentration of particles on both sides is equal (if crossing a membrane) or when there is a uniform distribution of particles… this is called equilibrium o When equilibrium is reached, molecules continue to move, but no net (overall) change in concentration occurs in a particular area o Diffusion of one compound (ex: carbon dioxide) is independent from diffusion of other compounds (ex: oxygen); both will diffuse down their individual concentration gradients regardless of the concentration of the other compound Types of Molecules that can Move through Simple Diffusion across the Membrane Diffusion directly through lipid bilayer o The greater the lipid solubility of the diffusing particle (i.e. how nonpolar / hydrophobic the molecule is), the more permeable the membrane will be. (In other words, nonpolar / hydrophobic particles will diffuse more easily through the nonpolar tail region of the membrane.) o Smaller particles will typically diffuse more rapidly than larger particles o O2, H2O, CO2, N2, etc. rapidly diffuse across the lipid bilayer. (Note: Even though water is polar, it is small enough that it can pass through without a high degree of repulsion from the nonpolar tails.) Diffusion of charged particles or large, polar (aka hydrophilic) molecules across the membrane. o Plasma membrane is semipermeable (i.e. it only lets certain substances through) o Larger hydrophilic molecules, such as sugars, do not freely move through the lipid bilayer o Charged particles (ex: Na+ or Cl-) cannot diffuse through lipid bilayer o Channel or carrier proteins (see facilitated diffusion section below) are required to transport charged particles and larger, hydrophilic molecules through the membrane 2 Facilitated Diffusion Allows diffusion of large, polar compounds such as sugars and amino acids or charged particles (ex: Na+) Does not require energy (passive) Highly Selective – each membrane transport protein transports a specific type of particle Some transport proteins are channel proteins, which are tubes through the membrane that are always open or only open when a particular molecule binds to them. The latter type of channel are called ligand-gated ion channels, where the ligand is the molecule that must bind to the protein to open the “gate” of the channel. Though water can diffuse through the lipid bilayer, there are also channel proteins called aquaporins that allow water to pass through the membrane at a faster rate. Some proteins are carrier proteins that change shape when a particular molecule binds to them, causing the protein to release the molecule on the outside of the membrane Fully reversible - molecules may enter the cell AND leave the cell through the transport protein. Particles move from areas of high concentration to areas of low concentration. Movement rate of particle movement depends on the number of membrane transport proteins o Maximum rate limited by number of protein transporters o Once all transporters are operating at 100%, an increase in concentration of particles will not increase the rate of movement How to Cheat - Glucose Continually Enters the Cell by Facilitated Diffusion Glucose binds to transport protein Transporter changers conformation and glucose is released into cell Intracellular glucose is immediately phosphorylated (i.e. an energized phosphate group – perhaps from ATP – is added to the glucose molecule) o phosphorylated glucose does not diffuse out (remember that the transport protein is very specific) o internal glucose (unphosphorylated) concentration remains low so glucose will still diffuse from a higher concentration outside of the cell towards a lower concentration (of unphosphorylated glucose) inside the cell. In other words, glucose continues to enter the cell even though there is a high concentration of phosphorylated glucose inside the cell. 3 Regulation of Glucose Uptake by Insulin Insulin stimulates increase in number of glucose transporters at membrane surface of liver cells as membrane spheres called vesicles bring more glucose transporters to the cell membrane (exocytosis) o Increase in number of transporters increases glucose diffusion rate o Glucose gets phosphorylated once it enters the cell, so it will continue to enter by diffusion (see bottom of previous page for explanation) o Once in liver cells (i.e. removed from the bloodstream), glucose molecules are joined together to make an energy storage polysaccharide (glycogen) o This keeps the blood glucose level from getting too high after a meal. This is one of the ways that our body maintains homeostasis (stable internal conditions). Insulin is secreted in response to high blood glucose levels. Low insulin levels decrease the number of glucose transporters at membrane surface o Portions of membrane with transporters fold inward (endocytosis), trapping the transport protein in a vesicle that enters the cell o Vesicle cannot refuse (rejoin) with the membrane until insulin levels increase o This keeps the blood glucose levels from getting too low (additional glucose cannot enter liver cells from the blood stream if there are no transporters) o Another hormone, glucagon, stimulates the breakdown of glycogen in the liver cells into individual glucose monomers and the secretion of glucose from liver cells into the blood stream. Glucagon is released in response to low blood glucose levels Diabetes Type I - Juvenile Diabetes - cannot make insulin o Autoimmune disease (the body’s immune system attacks its own cells) o Insulin-secreting pancreatic cells destroyed Type II - Adult Onset Diabetes - loss of ability to respond to insulin o Membrane receptors for insulin on the surface of liver cells become desensitized. They no longer respond to insulin and stimulate the addition of more transport proteins to the cell membrane for bringing glucose into the cell. Osmosis, the Passive Transport of Water Osmosis = the diffusion of water across a semi-permeable membrane (semi-permeable / selectively permeable = only lets certain substances pass through) Plasma membrane is permeable to water but not to certain solutes o Solutes = dissolved particles o Solvent = liquid in which particles may be dissolved (in this case, water) Water moves from solution with lower concentration of dissolved particles (i.e. a high concentration of water) to solution with higher concentration of dissolved particles (i.e. a low concentration of water) Water moves from dilute solution (more water, less solute) to concentrated solution (less water, more solute) 4 Solution Types Based on Solute and Water Concentrations (Note: these terms could refer to solutions outside or inside the cell) Hypertonic Solution: o Hyper = more or higher (think hyperactive = high energy); Tonic = dissolved particles o This solution has a high concentration of solutes and a low concentration of water compared to another solution Hypotonic Solution: o Hypo = less or lower (think hypothermia = low energy); Tonic = dissolved particles o This solution has a low concentration of solutes and a high concentration of water compared to another solution Isotonic Solution: o This solution has the same concentration of solutes and water as the solution to which it is being compared Note: During osmosis, water always moves from a hypotonic solution (high water concentration) to a hypertonic solution (low water concentration) In freshman biology, you are taught the following definitions of hypotonic, hypertonic, and isotonic solutions: Hypotonic Solution – Has a high water and low solute concentration compared to the solution on the other side of the membrane Hypertonic Solution – Has a low water and high solute concentration compared to the solution on the other side of the membrane Isotonic Solution – Has the same water and solute concentrations as the solution on the other side of the membrane We are also taught that water should move from a high water concentration to a low water concentration. In freshman biology, we assume that the solution being described as hypotonic, hypertonic, or isotonic is on the OUTSIDE of the cell. Therefore, based on that assumption, we can use the following memory tricks… 1. When placed in a hypotonic solution, the cell is like a hippo, it drinks all the water and gets bigger. This happens because a hypotonic solution has a high water concentration, so water should move from a high water concentration outside the cell across the membrane towards a low water concentration inside the cell (see image to the right). In other words, the cell should gain water and swell. Note: The arrow shown in the image indicates the direction of water movement. 2. When placed in a hypertonic solution, the cell is very hyper. It moves around a lot and sweats (loses water). This happens because a hypertonic solution has a low water concentration, so water should move from a high water concentration inside the cell across the membrane towards a low water concentration outside the cell (see image to the right). In other words, the cell should lose water and shrink. 5 3. When placed in an isotonic solution, the cell says “I so happy!”… it does not want to gain or lose water. This happens because an isotonic solution has the same water concentration as the solution on the other side of the membrane, so there will be no net (overall) movement of water either into or out of the cell. Water WILL move across the membrane due to random molecular movement, but it will move into and out of the cell at the same rate (see image to the right). In other words, the cell should stay the same size In AP biology, we do NOT assume that the solution being described as hypotonic, hypertonic, or isotonic is on the outside of the cell. It could be inside the cell as well! Therefore, we cannot always use the same memory tricks, so we have to remember the definitions given at the top of the page for hypotonic, hypertonic, and isotonic solutions. The following scenarios and associated explanations may help you to better understand this new perspective. 1. If the solution outside a cell has a higher water concentration than the solution inside the cell, then the solution outside the cell can be described as hypotonic (high water and low solute concentrations), whereas the solution inside the cell can be described as hypertonic (low water and high solute concentration). Water should move from a high concentration outside the cell across the membrane towards a low water concentration inside the cell. (So… water is moving from a hypotonic solution to a hypertonic solution). In other words, the cell should gain water and swell. 2. If the solution inside the cell has a lower water concentration than the solution outside the cell, then (like in #1) the solution outside the cell can be described as hypotonic (high water and low solute concentrations), whereas the solution inside the cell can be described as hypertonic (low water and high solute concentration). Water should move from a high concentration outside the cell across the membrane towards a low water concentration inside the cell. (So… water is moving from a hypotonic solution to a hypertonic solution). In other words, the cell should gain water and swell. Note: The image at the above and to the right corresponds to BOTH scenarios 1 and 2 because they are the same scenario but with different given information. Scenario 1 identifies the water concentration of the solution outside the cell, whereas Scenario 2 identifies the water concentration of the solution inside the cell. 3. If the solution outside a cell has a lower water concentration than the solution inside the cell, then the solution outside the cell can be described as hypertonic (low water and high solute concentrations), whereas the solution inside the cell can be described as hypotonic (high water and low solute concentrations). Water should move from a high concentration inside the cell across the membrane towards a low water concentration outside the cell. (So… water is moving from a hypotonic solution to a hypertonic solution). In other words, the cell should lose water and shrink. 6 4. If the solution inside the cell has a higher water concentration than the solution outside the cell, then (like in #3) the solution outside the cell can be described as hypertonic (low water and high solute concentrations), whereas the solution inside the cell can be described as hypotonic (high water and low solute concentrations). Water should move from a high concentration inside the cell across the membrane towards a low water concentration outside the cell. (So… water is moving from a hypotonic solution to a hypertonic solution). In other words, the cell should lose water and shrink. Note: The image at the bottom right corner of the previous page corresponds to BOTH scenarios 3 and 4 because they are the same scenario but with different given information. Scenario 3 identifies the water concentration of the solution outside the cell, whereas Scenario 4 identifies the water concentration of the solution inside the cell. 5. If the solution outside the cell has the same water concentration as the solution inside the cell, then both solutions can be described as isotonic to one another. Because the water concentration is the same outside and inside the cell, there will be no net (overall) movement of water either into or out of the cell. Water WILL move across the membrane due to random molecular movement, but it will move into and out of the cell at the same rate. In other words, the cell should stay the same size. Let’s say we are trying to apply our understanding of hypotonic, hypertonic, and isotonic solutions to REAL animal and plant cells. See the descriptions of various scenarios directly below and an image summarizing all the scenarios on the next page. If an animal cell is in hypotonic solution (high water), the inside of the cell is hypertonic (low water) compared to the outside solution. Water should move from the outside solution into the cell. If too much water moves in, the animal cell could swell to the point of bursting the cell membrane. This is called lysis and the cell is said to be lysed. If a plant cell is in hypotonic solution, water will move into the plant cell, but it will not burst due to the cell wall. Pressure of the cell membrane on the cell wall due to water coming in is a good thing for the plant cell. This is called turgor pressure and keeps plant stems from wilting. A plant cell in this state is considered turgid. This is the optimal state for a plant cell. If an animal cell is in isotonic solution, the water concentration outside the cell is the same as the inside of the cell. Water will move into and out of the cell at the same rate, so there is no net (overall) movement of water into or out of the animal cell. In this situation the animal cell maintains the same size / shape. This is the optimal state for an animal cell. If a plant cell is in isotonic solution, water will move into and out of the cell at the same rate. Because there is not an overall movement of water into the cell, the cell membrane will not push against the cell wall, and the cell is considered flaccid. In this situation, a plant would begin to wilt slightly. 7 If an animal cell is in hypertonic solution (low water), the inside of the cell is hypotonic (high water) compared to the outside solution. Water should move from the inside of the cell to the outside solution. When water moves out of the cell, the cell shrinks like a raisin in the sun, and the cell is said to be shriveled. If a plant cell is in hypertonic solution, water will move out of the plant cell, and the cell membrane will pull away significantly from the cell wall as the size of the cytoplasm (which is mostly water) decreases. In this situation, the cell is considered plasmolyzed. and the plant as a whole will wilt considerably. Using Adaptations to Maintain Water Balance Paramecia, single-celled protists that live in freshwater, are constantly in an environment that is hypotonic (high water) compared to their cells. This means that water is always entering their cells. Paramecia solve the problem of excess water entering their cells by using an organelle called a contractile vacuole that can pump water out of the cell. You can alter the rate of contractile vacuole pumping by placing the Paramecium in increasingly hypotonic (high water) solutions Active Transport This is another type of transport across the membrane that involves movement of solutes UP / AGAINST the concentration gradient In other words, this type of transport moves solutes from an area of low concentration to an area of high concentration. Requires the use of membrane proteins that act as “pumps” to move solutes across the membrane after an input of energy (usually from ATP) o Can be saturated like facilitated diffusion proteins (in other words, once all the pumps are in use, the rate of transport cannot increase) o The energy requirement distinguishes active transport from facilitated diffusion 8 The Na+ / K+ Pump (i.e. the sodium potassium pump): An Example of Active Transport An integral membrane protein pumps K+ into the cell and pumps Na+ out of the cell (sometimes from a low to high concentration, requires energy from ATP) First, pump binds 3 Na+ inside cell ATP is hydrolyzed (bond between last two phosphate groups is broken by adding water, which releases energy) to become a single phosphate group (called inorganic phosphate or Pi) and ADP (adenosine diphosphate). The single phosphate group is transferred to the Na+ / K+ pump (this is called phosphorylation, which energizes the pump and allows it to change shape) when the pump is phosphorylated, its configuration (aka shape) changes and it opens up to release the Na+ on the outside of the cell The Na+ are released (the altered configuration of the pump does not favor the binding of more Na+ on the outside of the cell) Two K+'s from the outside now bind to the altered protein The binding of the K+ causes the protein to lose its phosphate group Now that the phosphate group is gone, the altered protein reverts back to its original shape, which was open to the inside of the cell The original shape does not favor the binding of K+, so these are released. Na+ then binds to the protein and the process is repeated The Na+ / K+ pump is highly important in the nervous system. It helps stop nerve signaling so that muscles are not perpetually stimulated by motor neurons (nerve cells that stimulate muscle cells) Other Active and Transport Mechanisms - The H+ / Sucrose Pump H+ is actively pumped out of the cell by a protein pump when ATP is hydrolyzed (broken by adding water) H+ accumulates outside the membrane, H+ binds to another membrane protein, but sucrose must also bind. When both are bound, the configuration changes, and the protein opens to the membrane interior. o This is known as cotransport as two molecules are moved across a membrane… the movement of H+ down (with) its 9 concentration gradient is used to power the movement of sucrose up (against) its concentration gradient o It is also known as a symport as both molecules are crossing in the same direction o If the molecules are moving in opposite directions it is known as an antiport The H+ / Sucrose Symporter is used to move sugar from leaf cells (where they are made during photosynthesis) across the leaf cell membrane into to the phloem (the tube-like tissue used to transport sugar from the top of the plant to the roots). Active Transport to Move Large Quantities across the Cell Membrane Note: There are two types of transport—endocytosis and exocytosis—that move large quantities of particles (i.e. bulk transport) into or out of the cell. Both endocytosis and exocytosis are considered active transport because they require energy to fold the membrane and move vesicles (see explanation below) and not necessarily because they move particles from an area of low concentration to an area of high concentration. Endocytosis- requires a vesicle (a membrane-surrounded sac), the cell takes large quantities of particles by forming new vesicles from the plasma membrane o Phagocytosis- “cell eating,” the cell membrane engulfs large quantities of solid particles to take into the cell o Pinocytosis- “cell drinking”, the cell membrane engulfs large quantities of liquid with dissolved solutes to take into the cell o Receptor-Mediated Endocytosis – triggered by a molecule binding to a receptor on the cell membrane, which initiates folding in of the membrane…nothing comes in unless the correct molecule binds to the receptor Exocytosis- reverse of endocytosis, cell secretes particles by fusing a vesicle with the membrane and spilling contents of the vesicle to outside Exocytosis Top Image: Phagocytosis Middle Image: Pinocytosis Bottom Image: ReceptorMediated Endocytosis 10