* Your assessment is very important for improving the workof artificial intelligence, which forms the content of this project
Download Analyses for Molecular Interactions in Living Cells
Magnesium transporter wikipedia , lookup
Cell encapsulation wikipedia , lookup
Confocal microscopy wikipedia , lookup
G protein–coupled receptor wikipedia , lookup
Protein (nutrient) wikipedia , lookup
Hedgehog signaling pathway wikipedia , lookup
Protein moonlighting wikipedia , lookup
Protein phosphorylation wikipedia , lookup
Signal transduction wikipedia , lookup
Nuclear magnetic resonance spectroscopy of proteins wikipedia , lookup
List of types of proteins wikipedia , lookup
Protein–protein interaction wikipedia , lookup
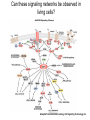
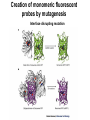
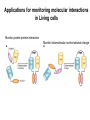
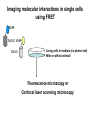
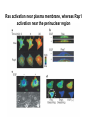
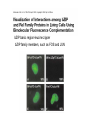
Analyses for Molecular Interactions in Living Cells Chi-Wu Chiang, Ph.D. Institute of Molecular Medicine College of Medicine National Cheng Kung University Can these signaling networks be observed in living cells? Adopted from 2003-2004 catalog, Cell Signaling Technology, Inc. Traditional methods to detect protein-protein interactions Yeast two-hybrid assay Mammalian two-hybrid assay Co-immunoprecipitation Affinity purification Co-localization by immunostaining Seeing is believing Fluorescent proteins Aequorea fluorescent protein (AFP) variants Green fluorescent protein (GFP) Enhanced green fluorescent protein (EGFP) Yellow fluorescent protein (YFP) Enhanced yellow fluorescent protein (EYFP) Cyan fluorescent protein (CFP) Enhanced cyan fluorescent protein (ECFP) From jellyfish Aequorea victoria Fluorescent probes Red fluorescent protein (RFP) from Discosoma genus, Reef coral DsRED, DsRED2, DsRED-express, mRFP1 Tended to be tetrameric DsRED-monomer is a new RFP (45 amino acid substitutions of DsRED) without property of forming tetramer Excitation and Emission Spectra of Fluorescent Proteins Protein ECFP EGFP EYFP DsRED2 Excitation Max nm Emission Max nm 439 484 476 512 529 563 592 510 The structure of GFP GFP is an 11-stranded b-barrel threaded by an a-helix running up the axis of the cylinder . The chromophore is attached to the a-helix and is buried almost perfectly in the center of the cylinder, which has been called a b-can Ser65, Tyr66, and Gly67 are key residues to form chromophore Creation of monomeric fluorescent probes by mutagenesis Interface disrupting mutation Compare the wtGFP to EGFP Mutations in several residues, such as Ser65, Ala206, Leu221, Phe223 Increase in stability and brightness Dimerization at high concentrations was overcome Compare EYFP to EGFP YFP was rationally designed on the basis of the GFP crystal structure to red-shift the absorbance and emission spectra with respect to EGFP and other green fluorescent variants YFP is much brighter than EGFP but is more sensitive to low pH and high halide concentrations Factors affect the efficiency using the Fluorescence probes Photostability -----caused by photobleaching PH sensitivity -----most of the first-generation probes are acid sensitive Oligomerizing property ----using AFPs with Ala206Lys mutation ----using newest DsRED varients, such as mRFP1 Perturbation of intracellular conditions ----introduction of the fluorescent probes may perturb the cellular component of interest Finding more fluorescent probes More mutated variants From Renilla mulleri Renilla mulleri GFP, with narrow excitation and emission spectrua From Anemonia sulcata dsFP593 Small molecule probes The biarsenical-tetracysteine system Based on: Membrane-permeant fluorescein derative with two As substitutents, named FIAsH Interaction of a single arsenic with a pair of thio groups is well known 1,2-ethanedithiol (EDT) as a 1,2-dithiol antidotes to prevent non-specific labeling in cells CCPGCC Nat. Rev. Mol. Cell Bio.3, 906-918 (2002). The biarsenical-tetracysteine system Analogues of FIAsH have different excitation and emission spectra CHoXAsH FIAsH ReAsH Current Methods in detecting protein-protein interactions in living cells Fluorescence resonance energy transfer (FRET) Bioluminescence resonance energy transfer (BRET) Biomolecular luminescence/fluorescence complementation Fluorescent protein complementation b-galactosidase/Luciferase complementation FRET Fluorescence (or Forster) Resonance Energy Transfer Energy transfer between two fluorophores within distance on nanometer scales FRET is the radiationless transfer of excited-state energy from an initially excited donor to an acceptor Emission Absorption Factors impact the rate of FRET Proper spectral overlap of the donor and acceptor The orientation factor, κ2, is given by κ2= (cosθT − 3cosθdcosθa) r6 r, the distance between the two fluorophores FRET is inversely proportional the distance between the fluorophores o < 10 nm or < 80 A apart Compare a small fluorescent moleculetagged FRET to a large fluorescent molecule-tagged FRET Small fluorescent chemicals Free orientation and FRET is only limited by the distance factor Larger fluorescent proteins Limited orientation and spatially restricted, however, FRET is sensitive to orientation, distance, and conformation of two interacting molecules The best pair of fluorophores are CFP and YFP Basic designs for analysis of molecular interactions by FRET CFP YFP Applications for monitoring molecular interactions in Living cells a. Intermolecular FRET-based indicators G protein subunits dissociation Transcription factor homo- and heterodimerization Ras and Rap1 activation b. Intramolecular FRET-based indicators Caspase activation Calcium flux sensor Kinase activation Applications for monitoring molecular interactions in Living cells Monitor protein-protein interaction Monitor intramolecular conformational change FRET applications Calcium sensor (Cameleon) M13, a peptide binds to calmodulin in calcium-dependent manner Kinase activation sensor Methods for FRET analysis in Living cells Analyses by Fluorescence Spectrophotometry Cells, treated or not treated, suspension In PBS Spectrofluorimeter measurement Spectrofluorimeter measurement Featured with Excitation light source, arc lamp, in UV or visible Emission detector, such as photon counter or charged coupled device (CCD) Scan full spectrum periodically, using filter sets and crystal counter< 1 second Detecting 14-3-3 dimerization in Living cells 430nm EYFP FRET ECFP 530nm 14-3-3 14-3-3 14-3-3 FRET1433ECFP/1433EYFP 160000 1433ECFP1 1433ECFP0.5/1433EYFP 2.5 1433ECFP0.75/1433EYF P2.2 1433ECFP1/1433EYFP2 120000 100000 80000 60000 1433EYFP2 40000 20000 pcDNA3 Wavelength 548 540 532 524 516 508 500 492 484 476 468 0 460 Emission intensity 140000 Imaging molecular interactions in single cells using FRET ECFP 14-3-3 EYFP 14-3-3 Living cells in medium (no phenol red) With or without stimuli Fluorescence microscopy or Confocal laser scanning microscopy FRET measured by Inverted Fluorescence Microscopy Featured with •Fluorescence illumination (HBO100 or HBO50) •Fluorescence optics (Plan-Neofluar 10x, 20x, 40x oil, 63x oil, 100x oil) •4 FRET filter cubes (CFP, YFP, FRET, Bleach), highly motorized •Digital camera •Computer •FRET image analysis software •Microscope Setup software Laser scanning confocal microscopy (1) optical fibers (7,8,9) secondary dichroic (2) collimators beam splitters (3) beam combination (10) pinhole diaphragm (4) main dichroic beam (11) emission filters splitter (12) photomultiplier tubes (5) scanner mirrors (13) neutral filters (6) scanning lens (14) monitor diode Spatio-temporal images of growth-factorinduced activation of Ras and Rap1 NAOKI MOCHIZUKI et al. Nature 411, 1065 - 1068 (2001); Ras, small G protein Activation of RAS by GTP binding Regulated by guanine nucleotide exchange factor (GEF), the activator And by GTPase activating protein (GAP), the inactivator Spatial: of the space Temporal: of the time Ras activation near plasma membrane, whereas Rap1 activation near the perinuclear region Bioluminescence Resonance Energy Transfer BRET Naturally, In jellyfish, blue-light emitting aequorin can promote GFP to excite green light In the current BRET system, In the presence of a substrate, bioluminescence from the luciferase excites the acceptor fluorophore 530 nm EYFP A 480 nm B Rluc Substate for Rluc Similar to FRET but avoid the photon excitation damage A bioluminescence resonance energy transfer (BRET) system: Application to interacting circadian clock proteins By Yao Xu et al. Whether bacterial circadian proteins form dimers to function? Proc. Natl. Acad. Sci. USA. 1999 January 5; 96 (1): 151–156 Biomolecular fluorescence complementation (BiFC) Origin from classical studies of intragenic complementation of the lacZ locus of E. coli, demonstrating that fragments of b-galactosidase that have no enzyme activity can associate spontaneously to generate an active complex GFP fragments fused to peptide sequences capable of producing an antiparallel coiled coil produced flurescent complexes in vitro and in E. coli Biomolecular luminescence/fluorescence complementation Fluorescence complementation Cut GFP GFPN GFPC (1-154) (155-238) A interacts with B? If Yes Protein A Protein B bZIP:basic region-leucine zipper bZIP family members, such as FOS and JUN Biomolecular luminescence complementation Luciferase complementation A interacts with B A B N Luc C Luc A B N LucC Luc luciferin light PNAS 101:12288-12293, 2004 The kinase mTOR is inhibited by FKBP in a rapamycindependent manner FRB: rapamycin-binding domain of the mTOR fused to NLuc FKBP:FK506-binding protein 12 fused to CLuc From Cell Biology to application in Biomedicine by FRET and BRET Uncover the molecular interactions in living cells and living animals in a spatial and temporal manner Molecular diagnosis Screening drugs in a high throughput way References Xu, Y. et al. A bioluminescence resonance energy transfer (BRET) system: Application to interacting circadian clock proteins. Proc. Natl. Acad. Sci. USA 96, 151-156 (1999) Mochizuki, N. et al. Spatio-temporal images of growth-factor-induced activation of Ras and Rap1. Nature 411, 1065-1068 (2001) Jin Zhang et al. Creating new fluorescent probes for cell biology. Nat. Rev. Mol. Cell Bio.3, 906-918 (2002). Hu, C. –D., Chinenov, Y., and Kerppola, T. K. Visualization of interactions among bZIP and Rel family proteins in living cells using biomolecular fluorescence complementation. Mole. Cell 9, 789-798, 2002 Jares-Erijman,E. A., & Jovin, T. M. FRET imaging. Nat. Biotechnol. 21, 1387-1395 (2003) Miyawaki, A. Visualization of the spatial and temporal dynamics of intracellular signaling. Develop. Cell 4, 295-305 (2003) Kathryn E. Luker et al. Kinetics of regulated protein–protein interactions revealed with firefly luciferase complementation imaging in cells and living animals. PNAS 101, 12288-12293 (2004)